Abstract
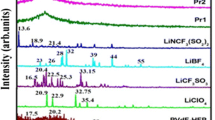
This paper reports the comparative studies on polymer gel electrolytes (PGEs) comprising liquid electrolytes of the triflate salts of Li, Na, and Mg in tetraethylene glycol dimethyl ether (TEGDME) solvent and poly(vinylidene fluoride-hexafluoropropylene) and poly(vinylpyrroliddone), i.e., PVdF(HFP)/PVP polymer blend matrix. The effect of different cations is investigated using various structural, thermal, and electrochemical techniques. The ionic conductivity and the ion-transport behavior are investigated using electrochemical impedance spectroscopy (EIS) over wide range of frequency. The XRD studies indicate the prominent structural variation after the immobilization of Li, Na, and Mg triflate salts in the PVdF(HFP)/PVP/TEGDME matrix. The Li+ ion conducting PGE composition displays the maximum room temperature ionic conductivity of ~ 6.5 × 10−3 S cm−1. Further, it exhibits a high dielectric constant value and superior ion-dynamics as compared to Na+ and Mg2+ based electrolytes. The PGEs display translational ion-dynamics and conductivity relaxation clubbed with polarizing effects and long-range mobility/migration of the cations (Na+, Mg2+, Li+). The morphological and structural studies reveal that Li+ ion conducting PGE offers a porous structure with smooth surface facilitating faster ionic motion. The PGEs possess considerable electrochemical stability window (≥ 4.0 V) and thermal stability, which prove them worthy for developing ion-batteries, super-capacitors and other next-generation electrochemical devices.










Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Lai YY, Li X, Zhu Y (2020) Polymeric active materials for redox flow battery application. ACS Appl Polym Mater 2(2):113–128. https://doi.org/10.1021/acsapm.9b00864
Patel M, Mishra K, Banerjee R, Chaudhari J, Kanchan DK, Kumar D (2023) Fundamentals, recent developments and prospects of lithium and non-lithium electrochemical rechargeable battery systems. J Energy Chem 81:221–259. https://doi.org/10.1016/j.jechem.2023.02.023
Syali MS, Kumar D, Mishra K, Kanchan DK (2020) Recent advances in electrolytes for room-temperature sodium-sulfur batteries: A review. Energy Storage Mater 31:352–372. https://doi.org/10.1016/j.ensm.2020.06.023
Review A (2023) D. Kasprzak, C.C.M. -Martinez, and M. Pumera, Sustainable and flexible energy storage devices. Energy Fuels 37(1):74–97. https://doi.org/10.1021/acs.energyfuels.2c03217
Liang B, Jiang Q, Tang S, Li S, Chen X (2016) Porous polymer electrolytes with high ionic conductivity and good mechanical property for rechargeable batteries. J Power Sources 307:320–328. https://doi.org/10.1016/j.jpowsour.2015.12.127
Guo J, Hou H, Cheng J, Wang C, Wang Q, Sun H, Chen X (2021) Microporous bayberry-like nano-silica fllers enabling superior performance gel polymer electrolyte for lithium metal batteries. J Mater Sci Mater Electron 32(1):81–93. https://doi.org/10.1007/s10854-020-04645-4
Poonam K, Sharma A, Arora SK (2019) Tripathi, Review of supercapacitors: Materials and devices. J Energy Storage 21:801–825. https://doi.org/10.1016/j.est.2019.01.010
Ye F, Liao K, Ran R, Shao Z (2020) Recent advances in filler engineering of polymer electrolytes for solid-state li-ion batteries: A review. Energy Fuels 34(8):9189–9207. https://doi.org/10.1021/acs.energyfuels.0c02111
Dai J, Zhao H, Lin X, Liu S, Fei T, Zhang T (2020) Design strategy for ultrafast-response humidity sensors based on gel polymer electrolytes and application for detecting respiration. Sens Actuators B Chem 304:127270. https://doi.org/10.1016/j.snb.2019.127270
Xin S, Yin YX, Guo YG, Wan LJ (2014) A high-energy room temperature sodium-sulfur battery. Adv Mater 26:1261–1265. https://doi.org/10.1002/adma.201304126
Lee DJ, Park JW, Hasa I, Sun YK, Scrosati B, Hassoun J (2013) Alternative materials for sodium ion-sulphur batteries. J Mater Chem A 1:5256. https://doi.org/10.1039/c3ta10241f
Pellegrini V, Bodoardo S, Brandell D, Edström K (2019) Challenges and perspectives for new material solutions in batteries. Solid State Commun 303–304:113733. https://doi.org/10.1016/j.ssc.2019.113733
Xia S, Wu X, Zhang Z, Cui Y, Liu W (2019) Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 5(4):753–785. https://doi.org/10.1016/j.chempr.2018.11.013
Ghotbi MY (2019) Solid state electrolytes for electrochemical energy devices. J Mater Sci Mater Electron 30(15):13835–13854. https://doi.org/10.1007/s10854-019-01749-4
Hou M, Liang F, Chen K, Dai Y, Xue D (2020) Challenges and perspectives of NASICON-type solid electrolytes for all-solidstate lithium batteries. Nanotechnol 31:132003. https://doi.org/10.1088/1361-6528/ab5be7
Wang S, Fang R, Li Y, Liu Y, Xin C, Richter FH, Nan CW (2021) Interfacial challenges for all-solid-state batteries based on sulfde solid electrolytes. J Materiomics 7(2):209–218. https://doi.org/10.1016/j.jmat.2020.09.003
Song JY, Wang YY, Wan CC (2000) Conductivity study of porous plasticized polymer electrolytes based on poly(vinylidenefuoride) a comparison with polypropylene separators. J Electrochem Soc 147(9):3219. https://doi.org/10.1149/1.1393886
Alipoori S, Mazinani S, Aboutalebi SH, Sharif F (2020) Review of PVA-based gel polymer electrolytes in fexible solid state supercapacitors: Opportunities and challenges. J Energy Storage 27:101072. https://doi.org/10.1016/j.est.2019.101072
Chauhan AK, Kumar D, Mishra K, Singh A (2021) Performance enhancement of Na+ ion conducting porous gel polymer electrolyte using NaAlO2 active fller. Mater Today Commun 26:101713. https://doi.org/10.1016/j.mtcomm.2020.101713
Shui Z, Chen Y, Zhao W, Chen X (2022) Flexible aluminum-air battery based on ionic liquid-gel polymer electrolyte. Langmuir 38(35):10791–10798. https://doi.org/10.1149/1.1393886
Feuillade G, Perche P (1975) Ion-conductive macromolecular gels and membranes for solid lithium cells. J Appl Electrochem 5(1):63–69. https://doi.org/10.1007/BF00625960
Nagajothi AJ, Kannan R, Rajashabala S (2018) Lithium ion conduction in plasticizer based composite gel polymer electrolytes with the addition of SiO2. Mater Res Innov 22(4):226–230. https://doi.org/10.1080/14328917.2017.1300725
Li W, Pang Y, Liu J, Liu G, Wang Y, Xia Y (2017) A PEObased gel polymer electrolyte for lithium ion batteries. RSC Adv 7(38):23494–23501. https://doi.org/10.1039/C7RA02603J
Maheshwaran C, Kanchan DK, Mishra K, Kumar D, Gohel K (2020) Effect of active MgO nano-particles dispersion in small amount within magnesium-ion conducting polymer electrolyte matrix. Nano Struct Nano Objects 24:100587. https://doi.org/10.1016/j.nanoso.2020.100587
Yahata Y, Kimura K, Nakanishi Y, Marukane S, Sato T, Tsujii Y, Ohno K (2019) Control of phase separation in polystyrene/ionic liquid-blended films by polymer brush-grafted particles. Langmuir 35(10):3733–3747. https://doi.org/10.1021/acs.langmuir.8b03891
Chauhan AK, Mishra K, Kumar D, Singh A (2021) Enhancing sodium ion transport in a PEO-based solid polymer electrolyte system with NaAlO2 active fllers. J Electron Mater 50(9):5122–5133. https://doi.org/10.1007/s11664-021-09051-y
Li T, Xu J, Wang C, Wu W, Su D, Wang G (2019) The latest advances in the critical factors (positive electrode, electrolytes, separators) for sodium-sulfur battery. J Alloys Compd 792:797–817. https://doi.org/10.1016/j.jallcom.2019.03.343
Liu J, Ahmed S, Khanam Z, Wang T, Song S (2020) Ionic liquid incorporated zn-ion conducting polymer electrolyte membranes. Polymers 12(8):1755. https://doi.org/10.3390/polym12081755
Barbosa JC, Dias JP, Méndez SL, Costa CM (2018) Recent advances in poly(vinylidene fluoride) and its copolymers for lithium-ion battery separators. Membranes 8(3):45. https://doi.org/10.3390/membranes8030045
Angulakshmi N, Stephan AM (2015) Effcient electrolytes for lithium-sulfur batteries. Front Energy 3:1. https://doi.org/10.3389/fenrg.2015.00017
Tachikawa N, Yamauchi K, Takashima E, Park JW, Dokko K, Watanable M (2011) Reversibility of electrochemical reaction of sulfur supported on inverse opel carbon in glyme-Li salt molten complex electrolytes. Chem Commun 47:8157. https://doi.org/10.1039/c1cc12415c
Wang H, Matsui M, Takeda Y, Yamamoto O, Im D, Lee D, Imanishi N (2013) Interface properties between lithium metal and a composite polymer electrolyte of PEO18Li(CF3SO2)2N-tetraethylene glycol dimethyl ether. Membranes 3(4):298–310. https://doi.org/10.3390/membranes3040298
Natarajan A, Stephan AM, Chan CH, Kalarikkal N, Thomas S (2017) Electrochemical studies on composite gel polymer electrolytes for lithium sulfur-batteries. J Appl Polym Sci 134(11):44594. https://doi.org/10.1002/app.44594
Gamal R, Sheha E, Shash N, El-Shaarawy MG (2015) Efect of tetraethylene glycol dimethyl ether on electrical, structural and thermal properties of PVA-based polymer electrolyte for magnesium battery. Acta Phys Pol A 127(3):803–810. https://doi.org/10.12693/APhysPolA.127.803
Arya A, Sharma AL (2017) Polymer electrolytes for lithium ion batteries: a critical study. Ionics 23(3):497–540. https://doi.org/10.1007/s11581-016-1908-6
Tripathi SK, Jain A, Gupta A, Mishra M (2012) Electrical and electrochemical studies on magnesium ion-based polymer gel electrolytes. J Solid State Electrochem 16(5):1799–1806. https://doi.org/10.1007/s10008-012-1656-0
Chen S, Lan R, Humphreys J, Tao S (2020) Perchlorate based “oversaturated gel electrolyte” for an aqueous rechargeable hybrid Zn-Li battery. ACS Appl Energy Mater 3(3):2526–2536
Macdonald JR (2005) Impedance spectroscopy: models, data fitting, and analysis. Solid State Ionics 176(2536):1961–2536. https://doi.org/10.1016/j.ssi.2004.05.035
Sundari GS, Kumar KV, Kumar NK, Reddy PA (2013) Structural and A.C. conductivity studies of (PVdF + NaClO4) solid polymer electrolyte system for an electrochemical cell applications. Asian J Chem 25:S459. https://asianpubs.org/index.php/ajchem/article/view/25_Supplementary%20Issue_128
Castillo J, Santiago A, Judez X, Garbayo I, Clemente JAC, Miñana MCM, Villaverde A, Marcos JAG, Zhang H, Armand M, Li C (2021) Safe, fexible, and high-performing gel-polymer electrolyte for rechargeable lithium metal batteries. Chem Mater 33(22):8812–8821. https://doi.org/10.1021/acs.chemmater.1c02952
Jin J, Wen Z, Liang X, Cui Y, Wu X (2012) Gel polymer electrolyte with ionic liquid for high performance lithium sulfur battery. Solid State Ionics 225:604–607. https://doi.org/10.1016/j.ssi.2012.03.012
Wang Y-X, Zhang B, Lai W, Xu Y, Chou S-L, Liu H-K, Dou S-X (2017) Room temperature sodium-sulfur batteries: a comprehensive review on research progress and cell chemistry. Adv Energy Mater 7(24):1602829. https://doi.org/10.1002/aenm.201602829
Song S, Duong HM, Korsunsky AM , Hu N, Lu L (2016) A Na+ Superionic conductor for room-temperature sodium batteries. Sci Rep 6:32330. https://doi.org/10.1038/srep32330
Park C-W, Ahn J-H, Ryu H-S, Kim K-W, Ahn H-J (2006) Room-temperature solid state Sodium∕Sulfur battery. Electrochem Solid State Lett 9(3):A123–A125. https://doi.org/10.1149/1.2164607
Zhou D, Chen Y, Li B, Fan H, Cheng F, Shanmukaraj D, Rojo T, Armand M, Wang G (2018) A stable quasi-solid-state sodium-sulfur battery. Angew Chem 57(32):10168–10172. https://doi.org/10.1002/anie.201805008
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all-solid-state battery applications: An overview. J Phys D Appl Phys 41:223001. https://doi.org/10.1088/0022-3727/41/22/223001
Xue Z, He D, Xie X (2015) Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J Mater Chem A 3(38):19218–19253. https://doi.org/10.1016/j.matpr.2017.10.043
Mahato DK (2018) Ac conductivity analysis of nanocrystallite MgFe2O4 ferrite. Mater Today Proc 5(3):9191–9195. https://doi.org/10.1016/j.matpr.2017.10.043
Aziz SB, Abidin ZHZ (2013) Electrical conduction mechanism in solid polymer electrolytes: new concepts to Arrhenius equation. J Soft Matt 2013:323868. https://doi.org/10.1155/2013/323868
Padmasree KP, Kanchan DK, Kulkarni AR (2006) Impedance and Modulus studies of the solid electrolyte system 20CdI2–80[xAg2O–y(0.7V2O5–0.3B2O3)], where 1 ≤x/y ≤ 3. Solid State Ionics 177(5–6):475–482. https://doi.org/10.1016/j.ssi.2005.12.019
Jonscher AK (1977) The ‘universal’ dielectric response. Nature 167(5613):673–679. https://doi.org/10.1038/267673a0
Woo HJ, Majid SR, Arof AK (2012) Dielectric properties and morphology of polymer electrolyte based on poly (ɛ-caprolactone) and ammonium thiocyanate. Mater Chem Phys 134:755. https://doi.org/10.1016/j.matchemphys.2012.03.064
Rathika R, Suthanthiraraj SA (2016) Ionic interactions and dielectric relaxation of PEO/PVDF-Mg[(CF3SO2)2N2] blend electrolytes for magnesium ion rechargeable batteries. Macromol Res 24(5):422–428. https://doi.org/10.1007/s13233-016-4053-1
Al-Gunaid MQA, Saeed AMN (2018) Efects of the electrolyte content on the electrical permittivity, thermal stability, and optical dispersion of poly(vinyl alcohol)–cesium copper oxide–lithium perchlorate nanocomposite solid-polymer electrolytes. J Appl Polym Sci 135(8):45852. https://doi.org/10.1002/app.45852
Pritam A, Arya AL (2019) Sharma, Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J Mater Sci 54(9):7131–7155. https://doi.org/10.1007/s10853-019-03381-3
Singh P, Gupta PN, Saroj AL (2020) Ion dynamics and dielectric relaxation behavior of PVA-PVP-NaI-SiO2 based nano-composites polymer blend electrolytes. Physica B 578:411850. https://doi.org/10.1016/j.physb.2019.411850
Aziz SB, Abidin ZHZ (2015) Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: electrical and dielectric analysis. J Appl Polym Sci 132(15):41774. https://doi.org/10.1002/app.41774
Nagajothi AJ, Kannan R, Rajashabala S (2018) Lithium ion conduction in plasticizer based composite gel polymer electrolytes with the addition of SiO2. Mater Res Innov 22: 226-230. https://doi.org/10.1080/14328917.2017.1300725
Su NC, Noor SAM, Roslee MF, Mohamed NS, Ahmad A, Yahya MZA (2019) Potential complexes of NaCF3SO3-tetraethylene dimethyl glycol ether (tetraglyme)-based electrolytes for sodium rechargeable battery application. Ionics 25(2):541–549. https://doi.org/10.1007/s11581-018-2718-9
Mohammadi F, Rabiee A (2011) Solution casting, characterization, and performance evaluation of perfluorosulfonic sodium type membranes for chlor-alkali application. J Appl Poly Sci 120(6):3469–3476. https://doi.org/10.1002/app.33526
Shafe AH, Khiar ASA (2018) Characterization of chitosan-starch blend based biopolymer electrolyte doped with ammonium nitrate. AIP Conf Pro 1972:030011. https://doi.org/10.1063/1.5041232
Sharma P, Kanchan DK, Gondaliya N, Pant M, Jayswal MS (2013) Conductivity relaxation in Ag+ ion conducting PEOPMMA-PEG polymer blends. Ionics 19(2):301–307. https://doi.org/10.1007/s11581-012-0738-4
Gondaliya N, Kanchan DK, Sharma P, Joge P (2012) Effects of silicone dioxide and poly(ethylene glycol) on the conductivity and relaxation dynamics of poly(ethylene oxide)-silver trifate solid polymer electrolyte. J Appl Polym Sci 125(2):1513–1520. https://doi.org/10.1002/app.36372
Chen L, Venkatram S, Kim C, Batra R, Chandrasekaran A, Ramprasad R (2019) Electrochemical stability window of polymeric electrolytes. Chem Mater 31(12):4598–4604. https://doi.org/10.1021/acs.chemmater.9b01553
Aravindan V, Karthikaselvi G, Vickraman P, Vickraman P, Naganandhini SP (2009) Polyvinylidene fluoride-based novel polymer electrolytes for magnesium-rechargeable batteries with Mg(CF3SO3)2. J Appl Polym Sci 112(5):3024–3029
Bhatt P, Pathak N, Mishra K, Kanchan DK, Kumar D (2022) Effect of different cations on ion-transport behavior in polymer Gel electrolytes intended for application inflexible electrochemical devices. J Electron Mater 51:1371–1384. https://doi.org/10.1007/s11664-021-09398-2
Dimri MC, Kumar D, Aziz SB, Mishra K (2021) ZnFe2O4 nanoparticles assisted ion transport behavior in a sodium ion conducting polymer electrolyte. Ionics 27(3):1143–1157. https://doi.org/10.1007/s11581-020-03899-6
Aldalur I, Zhang H, Piszcz M, Oteo U, Rodriguez-Martinez LM, Shanmukaraj D, Rojo T, Armand M (2017) J Power Sources 347:37–46. https://doi.org/10.1016/j.jpowsour.2017.02.047
Huang HJ, Ding F, Zhong H, Li H, Zhang WG, Liu XJ, Xu Q (2018) J Mater Chem A 6(20):9539–9549. https://doi.org/10.1039/C8TA03061H
Zhu T, Dong X, Liu Y, Wang Y-G, Wang C, Xia Y-Y (2019) An all-solid-statesodium–sulfur battery using a sulfur/carbonized polyacrylonitrile composite cathod. ACS Appl Energy Mater 2(7):5263–5271. https://doi.org/10.1021/acsaem.9b00953
Li H, Kuai Y, Yang J, Hirano S, Nuli Y, Wanga J (2022) A new flame-retardant polymer electrolyte with enhanced Li-ion conductivity for safe lithium-sulfur batteries. J Energy Chem 65:616–622. https://doi.org/10.1016/j.jechem.2021.06.036
Simoes RD, Job AE, Chinaglia DL, Zucolotto V, Camargo-Filho JC, Alves N, Giacometti JA, Oliveira ON, Constantino CJL (2005) Structural characterization of blends containing both PVDF and natural rubber latex. J Raman Spectrosc 36(12):1118–1124. https://doi.org/10.1002/jrs.1416
Karmakar A, Ghosh A (2011) Charge carrier dynamics and relaxation in (polyethylene oxide-lithium-salt)-based polymer electrolyte containing 1-butyl-1-methylpyrrolidinium bis(trifuoromethylsulfonyl)imide as ionic liquid. Phys Rev E 84:051802. https://doi.org/10.1103/PhysRevE.84.051802
Kumar D, Gohel K, Kanchan DK, Mishra K (2020) Dielectrics and battery studies on flexible nanocomposite gel polymer electrolyte membranes for sodium batteries. J Mater Sci Mater Electron 31(16):13249–13260. https://doi.org/10.1007/s10854-020-03877-8
Acknowledgements
The authors warmly credit the Electronics and Mechanical Engineering School, Ministry of Defence, Government of India. Authors also thank Dr. Neeladri Das, Department of Chemistry, IIT Patna, India for his support in structural investigations.
Funding
Dr. Deepak Kumar gratefully acknowledges financial support from the Science and Engineering Research Board, Government of India under Core Research Grant (CRG) Scheme vide File No. CRG/2022/008719.
Author information
Authors and Affiliations
Contributions
Rajkumar Singh: Investigation, writing–original draft; Kuldeep Mishra: Investigation, writing and editing; D.K. Kanchan: Investigation; Deepak Kumar: Investigation, writing and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, R., Mishra, K., Kanchan, D.K. et al. Comparative investigations on polymer gel electrolytes comprising triflate salts of Li, Na, Mg in TEGDME solvent and PVdF-HFP/PVP blend matrix. Ionics (2024). https://doi.org/10.1007/s11581-024-05557-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05557-7