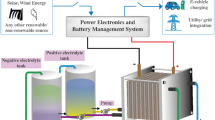
Abstract
Vanadium redox flow batteries have the advantages of long life, flexible design, safety, and reliability, so they have broad development prospects in the field of energy storage. Borides are extensively used in electrochemistry due to their excellent electrical conductivity. In this program, HfB2 was used as catalyst for the V3+/V2+ pair. The catalytic effect was verified by electrochemical impedance spectroscopy and cyclic voltammetry. The result shows that the cell using HfB2 as negative catalyst can exhibit excellent rate capability when the current density is 50 ~ 125 mA cm−2. At 125 mA cm−2, the discharge capacity of the modified cell is 68.7 mA h, which is much higher than pristine cell (22.8 mA h). And the energy efficiency of pristine cells is 11.2% lower than that of modified cells. These consequences suggest that HfB2 is a catalyst with promising prospects to enhance electrochemical activity of V3+/V2+ redox reaction in VRFB.










Similar content being viewed by others
References
Yang SN, Zhang MS, Wu XW, Wu XS, Zeng FH, Li YT, Duan SY, Fan DH, Yang Y, Wu XM (2019) The excellent electrochemical performances of ZnMn2O4/Mn2O3: the composite cathode material for potential aqueous zinc ion batteries. J Electroanal Chem 832:69–74. https://doi.org/10.1016/j.jelechem.2018.10.051
He CM, Ma ZL, Wu Q, Cai YZ, Huang YG, Liu K, Fan YJ, Wang HQ, Li QY, Qi JH, Li QK, Wu XW (2020) Promoting the ORR catalysis of Pt-Fe intermetallic catalysts by increasing atomic utilization and electronic regulation. Electrochim Acta 330:135119. https://doi.org/10.1016/j.electacta.2019.135119
Zhong M, Guan JD, Feng QJ, Wu XW, Xiao ZB, Zhang W, Tong S, Zhou N, Gong DX (2018) Accelerated polysulfide redox kinetics revealed by ternary sandwich-type S@Co/N-doped carbon nanosheet for high-performance lithium-sulfur batteries. Carbon 128:86–96. https://doi.org/10.1016/j.carbon.2017.11.084
Liu ZZX, Qin LP, Lu BG, Wu XW, Liang SQ, Zhou J (2022) Issues and opportunities facing aqueous Mn2+/MnO2− based batteries. Chemsuschem 15:e202200348. https://doi.org/10.1002/cssc.202200348
Li B, Xue J, Han C, Liu N, Ma KX, Zhang RC, Wu XW, Dai L, Wang L, He ZX (2021) A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J Colloid Interface Sci 599:467–475. https://doi.org/10.1016/j.jcis.2021.04.113
Tang F, Gao JY, Ruan QY, Wu XW, Wu XS, Zhang T, Liu ZX, Xiang YH, He ZQ, Wu XM (2020) Graphene-wrapped MnO/C composites by MOFs-derived as cathode material for aqueous zinc ion batteries. Electrochim Acta 353:136570. https://doi.org/10.1016/j.electacta.2020.136570
Li FZ, Li JS, Feng QJ, Yan J, Tang YG, Wang HY (2018) Significantly enhanced oxygen reduction activity of Cu/CuNxCy co-decorated ketjenblack catalyst for Al–air batteries. J Energy Chem 27:419–425. https://doi.org/10.1016/j.jechem.2017.12.002
Xie XS, Liang SQ, Gao JW, Guo S, Guo JB, Wang, Xu GY, Wu XW, Chen G, Zhou J (2020) Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ Sci 13:503–510. https://doi.org/10.1039/C9EE03545A
Xiang YH, Li J, Wu XW, Xiong LZ, Yin ZL (2015) Synthesis and characterization of manganese-rich transition metal carbonate precursor in the presence of ethanol. Adv Powder Technol 26:1712–1718. https://doi.org/10.1016/j.apt.2015.10.012
Jiang Z, Li YH, Han C, Huang ZQ, Wu XW, He ZX, Meng W, Dai L, Wang L (2020) Raising lithium storage performances of NaTi2(PO4)3 by nitrogen and sulfur dual-doped carbon layer. J Electrochem Soc 167:020550. https://doi.org/10.1149/1945-7111/ab6c5c
Jiang YQ, Liu ZH, Lv YR, Tang A, Dai L, Wang L, He ZX (2022) Perovskite enables high performance vanadium redox flow battery. Chem Eng J 443:136341. https://doi.org/10.1016/j.cej.2022.136341
Zhang DY, Li CC, Lin NZ, Xie BS, Chen J (2022) Mica-stabilized polyethylene glycol composite phase change materials for thermal energy storage. Int J Miner Metall Mater 29:168–176. https://doi.org/10.1007/s12613-021-2357-4
Yu LH, Lin F, Xiao WD, Ling Xu, Xi JY (2019) Achieving efficient and inexpensive vanadium flow battery by combining CexZr1−xO2 electrocatalyst and hydrocarbon membrane. Chem Eng J 356:622–631. https://doi.org/10.1016/j.cej.2018.09.069
Liu YB, Yu LH, Liu L, Xi JY (2021) Tailoring the vanadium/proton ratio of electrolytes to boost efficiency and stability of vanadium flow batteries over a wide temperature range. Applied Energy. 301:https://doi.org/10.1016/j.apenergy.2021.117454
Parasuraman A, Lim TM, Menictas C, Skyllas-Kazacos M (2013) Review of material research and development for vanadium redox flow battery applications. Electrochim Acta 101:27–40. https://doi.org/10.1016/j.electacta.2012.09.067
Yan Y, Li B, Guo W, Pang H, Xue HG (2016) Vanadium based materials as electrode materials for high performance supercapacitors. J Power Sources 329:148–169. https://doi.org/10.1016/j.jpowsour.2016.08.039
Yang CM, Wang HN, Lu SF, Wu CX, Liu YY, Tan QL, Liang DW, Xiang Y (2015) Titanium nitride as an electrocatalyst for V(II)/V(III) redox couples in all-vanadium redox flow batteries. Electrochim Acta 182:834–840. https://doi.org/10.1016/j.electacta.2015.09.155
Dai JC, Ding TL, Dong YC, Teng XG (2021) Amphoteric nafion membrane with tunable cationic and anionic ratios for vanadium redox flow battery prepared via atom transfer radical polymerization. Ionics 27:2127–2138. https://doi.org/10.1007/s11581-021-03980-8
Zhao LJ, Ma Q, Xu Q, Su HN, Zhang WQ (2021) Performance improvement of non-aqueous iron-vanadium flow battery using chromium oxide–modified graphite felt electrode. Ionics 27:4315–4325. https://doi.org/10.1007/s11581-021-04222-7
Kong P, Zhu L, Li FR, Xu GB (2019) Self-supporting electrode composed of SnSe nanosheets, thermally treated protein, and reduced graphene oxide with enhanced pseudocapacitance for advanced sodium-ion batteries. ChemElectroChem 6:5642–5650. https://doi.org/10.1002/celc.201901517
Sun PZ, Lu P, Xu JC, Qiang Ma, Zhang WQ, Shah AA, Su H, Yang WW, Xu Q (2021) The influence and control of ultrasonic on the transport and electrochemical properties of redox couple ions in deep eutectic solvent (DES) for redox flow battery application. Electrochim Acta 394:139140. https://doi.org/10.1016/j.electacta.2021.139140
Cheng R, Xu JC, Wang XY, Ma Q, Su HN, Yang WW, Xu Q (2020) Electrochemical characteristics and transport properties of V(II)/V(III) redox couple in a deep eutectic solvent: Magnetic field effect. Frontiers in Chemistry. 8:https://doi.org/10.3389/fchem.2020.00619
Xiang Y, Daoud WA (2019) Binary NiCoO2-modified graphite felt as an advanced positive electrode for vanadium redox flow batteries. J Mater Chem A 7:5589–5600. https://doi.org/10.1039/c8ta09650c
Hassan A, Tzedakis T (2019) Enhancement of the electrochemical activity of a commercial graphite felt for vanadium redox flow battery (VRFB), by chemical treatment with acidic solution of K2Cr2O7. Journal of Energy Storage 26:100967. https://doi.org/10.1016/j.est.2019.100967
Yang ZF, Wei YG, Zeng YK, Yuan YP (2021) Effects of in-situ bismuth catalyst electrodeposition on performance of vanadium redox flow batteries. J Power Sources 506:230–238. https://doi.org/10.1016/j.jpowsour.2021.230238
Li B, Zhang XT, Wang TT, He ZX, Lu BG, Liang SQ, Zhou J (2021) Interfacial engineering strategy for high-performance Zn metal anodes. Nano-Micro Letters 14:6. https://doi.org/10.1007/s40820-021-00764-7
Mehboob S, Mehmood A, Lee JY, Shin HJ, Hwang J, Abbas S, Ha HY (2017) Excellent electrocatalytic effects of tin through in situ electrodeposition on the performance of all-vanadium redox flow batteries. J Mater Chem A 5:17388–17400. https://doi.org/10.1039/c7ta05657e
Shen JX, Liu SQ, He Z, Shi L (2015) Influence of antimony ions in negative electrolyte on the electrochemical performance of vanadium redox flow batteries. Electrochim Acta 151:297–305. https://doi.org/10.1016/j.electacta.2014.11.060
Yang XF, Liu T, Xu C, Zhang HZ, Li XF, Zhang HM (2017) The catalytic effect of bismuth for VO2+/VO2+ and V3+/V2+ redox couples in vanadium flow batteries. J Energy Chem 26:1–7. https://doi.org/10.1016/j.jechem.2016.09.007
Lv YR, Yang CM, Wang HN, Zhang J, Xiang Y, Lu SF (2020) Antimony-doped tin oxide as an efficient electrocatalyst toward the VO2+/VO2+ redox couple of the vanadium redox flow battery. Catal Sci Technol 10:2484–2490. https://doi.org/10.1039/c9cy01793c
Lee W, Jo C, Youk S, Shin HY, Lee J, Chung Y, Kwon Y (2018) Mesoporous tungsten oxynitride as electrocatalyst for promoting redox reactions of vanadium redox couple and performance of vanadium redox flow battery. Appl Surf Sci 429:187–195. https://doi.org/10.1016/j.apsusc.2017.07.022
Mehboob S, Ali G, Shin HJ, Hwang J, Abbas S, Chung KY, Ha HY (2018) Enhancing the performance of all-vanadium redox flow batteries by decorating carbon felt electrodes with SnO2 nanoparticles. Appl Energy 229:910–921. https://doi.org/10.1016/j.apenergy.2018.08.047
Tseng TM, Huang R, Huang C, Liu C, Hsueh KL, Shieu FS (2014) Carbon felt coated with titanium dioxide/carbon black composite as negative electrode for vanadium redox flow battery. J Electrochem Soc 161:A1132–A1138. https://doi.org/10.1149/2.102406jes
Yao C, Zhang HM, Liu T, Li XF, Liu ZH (2012) Carbon paper coated with supported tungsten trioxide as novel electrode for all-vanadium flow battery. J Power Sources 218:455–461. https://doi.org/10.1016/j.jpowsour.2012.06.072
Zhou H, Shen Y, Xi J, Qiu X, Chen L (2016) ZrO2-nanoparticle-modified graphite felt: bifunctional effects on vanadium flow batteries. ACS Appl Mater Interfaces 8:15369–15378. https://doi.org/10.1021/acsami.6b03761
Chen Q, Du YY, Li KM, Xiao HF, Wang W, Zhang WM (2017) Graphene enhances the proton selectivity of porous membrane in vanadium flow batteries. Mater Des 113:149–156. https://doi.org/10.1016/j.matdes.2016.10.019
Park M, Ryu J, Kim Y, Cho J (2014) Corn protein-derived nitrogen-doped carbon materials with oxygen-rich functional groups: a highly efficient electrocatalyst for all-vanadium redox flow batteries. Energy Environ Sci 7:3727–3735. https://doi.org/10.1039/C4EE02123A
Xue J, Jiang YQ, Zhang ZX, Zhang TX, Han C, Liu YG, Chen ZS, Xie ZB, Zhang GL, Dai L, Wang L, He ZX (2021) A novel catalyst of titanium boride toward V3+/V2+ redox reaction for vanadium redox flow battery. J Alloy Compd 875:159915. https://doi.org/10.1016/j.jallcom.2021.159915
Guo ZL, Zhou J, Sun ZM (2017) New two-dimensional transition metal borides for Li ion batteries and electrocatalysis. Journal of Materials Chemistry A 5:23530–23535. https://doi.org/10.1039/C7TA08665B
Qi S, Fan Y, Zhao L, Li W, Zhao M (2021) Two-dimensional transition metal borides as highly efficient N2 fixation catalysts. Appl Surf Sci 536:147742. https://doi.org/10.1016/j.apsusc.2020.147742
Shin EK, Anggia E, Parveen AS, Park JS (2019) Optimization of the protonic ceramic composition in composite electrodes for high-performance protonic ceramic fuel cells. Int J Hydrogen Energy 44:31323–31332. https://doi.org/10.1016/j.ijhydene.2019.09.247
Huang RJ, Liu SQ, He Z, Zhu WW, Ye GY, Su YK, Deng WW, Wang J (2022) Electron‐deficient sites for improving V2+/V3+ redox kinetics in vanadium redox flow batteries. Advanced Functional Materials. 2111661.https://doi.org/10.1002/adfm.202111661
Dinesh MM, Huang TZ, Feng HY (2019) Electrochemical impacts of sheet-like hafnium phosphide and hafnium disulfide catalysts bonded with reduced graphene oxide sheets for bifunctional oxygen reactions in alkaline electrolytes. RSC Adv 9:2599. https://doi.org/10.1039/C8RA09598A
Duan Y, Li S, Tan Q, Chen Y, Zou K, Dai X, Bayati M, Xu B, Dala L, Liu T (2021) Cobalt nickel boride nanocomposite as high-performance anode catalyst for direct borohydride fuel cell. Int J Hydrogen Energy 46:15471–15481. https://doi.org/10.1016/j.ijhydene.2021.02.064
Kumar N, Noh W, Daly SR, Girolami GS, Abelson JR (2009) Low temperature chemical vapor deposition of Hafnium nitride−boron nitride nanocomposite films. Chem Mater 21:5601–5606. https://doi.org/10.1021/cm901774v
Ling W, Deng Q, Ma Q, Wang H, Zhou C, Xu J, Yin Y, Wu X, Zeng X, Guo Y (2018) Hierarchical carbon micro/nanonetwork with superior electrocatalysis for high-rate and endurable vanadium redox flow batteries. Adv Sci(Weinh) 5:1801281. https://doi.org/10.1002/advs.201801281
Funding
This work was financially supported by National Natural Science Foundation of China (Nos. 51872090, 51772097), Hebei Natural Science Fund for Distinguished Young Scholar (No. E2019209433), Youth Talent Program of Hebei Provincial Education Department (No. BJ2018020), Natural Science Foundation of Hebei Province (No. E2020209151), and Science and Technology Project of Hebei Education Department (SLRC2019028).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, J., Yang, Y., Ren, Y. et al. A novel hafnium boride catalyst for vanadium redox flow battery. Ionics 28, 4273–4282 (2022). https://doi.org/10.1007/s11581-022-04656-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04656-7