Abstract
Antrodiella species (Agaricomycotina, Polyporales) are often growing on or near to the living, dead, or dying fruitbodies of pioneer wood-inhabiting fungi. Antrodiella citrinella always occurs on wood that previously has been decayed by the polypore Fomitopsis pinicola. However, the underlying mechanism remained unclear. Based on field observations, it has been assumed that the succeeding species is not only a highly competitive wood decomposer but also a mycoparasite feeding on the preceding species. To investigate the interaction between A. citrinella and the putative host F. pinicola, the species were grown in dual cultures at different temperatures (5–25 °C). The interaction tests were complemented with qualitative enzymatic tests for both species and microscopic examination of the interaction zone. In the dual cultures, A. citrinella replaced F. pinicola only at low temperature (5 °C); at higher temperatures (25 °C), it was vice versa. Light microscopy revealed preferential growth of A. citrinella toward F. pinicola, hyphal contact, and finally death of F. pinicola hyphae. Enzymatic tests showed that A. citrinella is capable to degrade extracellular proteins, chitin, cellulose, and lignin. We interpret the interaction as mycoparasitism, as we suggest that A. citrinella is capable to recognize, kill, and feed from F. pinicola, beside its ability to degrade woody substrates. The results are discussed in an ecological context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In deadwood, there is usually a succession of wood-inhabiting fungi, where pioneer species are successively replaced by secondary species. Patterns in fungal succession have been investigated in various studies using observational data (Renvall 1995; Jönsson et al. 2008; Ottosson et al. 2014), in vitro studies (e.g., Holmer et al. 1997; Hiscox et al. 2018), and deadwood experiments (e.g., Olsson et al. 2011; Weslien et al. 2011). Generally, the fungal community is shaped by biotic and abiotic conditions (Boddy 2000; Hiscox and Boddy 2017) and affected by the identity of dominant pioneer species (Dickie et al. 2012; Hiscox et al. 2015). One conspicuous observation is that some fungi, termed “successor species,” seem to strongly depend on the presence of other preceding species, the “predecessor species,” while other wood-inhabiting species seem to appear and disappear by chance (e.g., Pouska et al. 2013). Thus, beside general successional pathways, some species seem to follow a stricter kind of succession (Niemelä et al. 1995; Halbwachs et al. 2021). Although various explanations for this phenomenon exist (Jahn 1967; Niemelä et al. 1995; Gams et al. 2004), i.e., pioneer effects, dominance effects, competition, commensalism, and parasitism, to our knowledge, no replacement mechanism has been proven for any strict successor yet. In this study, the relationship between the two polypore species Antrodiella citrinella Niemelä & Ryvarden and Fomitopsis pinicola (Sw.) P. Karst. will be examined.
Antrodiella citrinella gained some attention in nature conservation issues in recent years because of its indicator function for old-growth forests (e.g., Blaschke et al. 2009; Holec et al. 2018; Braunisch et al. 2020). While the knowledge of its overall distribution (Niemelä and Ryvarden 1983; Vlasak 1990; Grosse-Brauckmann and Luschka 1991; Pieri et al. 2000; Ryvarden and Melo 2014), habitat requirements (Niemelä and Ryvarden 1983; Bässler and Müller 2010), cultural characteristics (David and Tortic 1986; Wieners et al. 2016), and phenology (Wieners et al. 2016; Holec et al. 2018) is growing, not much is known about the biotic interaction with the preceding polypore F. pinicola. In literature, the explanations for the strict succession of A. citrinella range from commensalism by means of resource complementary use (Holmer et al. 1997) over competition (Gams et al. 2004) to parasitism (Niemelä and Ryvarden 1983).
Antrodiella species are often growing on or near to the living, dead, or dying fruitbodies of pioneer wood-inhabiting fungi, as summarized in Table 1. The association of Antrodiella species with their predecessor species is always specific, in most cases at the species or genus level, and in one case at the family level (e.g., Niemelä et al. 1995; Johannesson et al. 2000; Miettinen et al. 2006). This suggests that the interaction between successor and predecessor has a long evolutionary trajectory, and that the association may indicate an advantageous strategy to gain and maintain resources. When Niemelä and Ryvarden (1983) described A. citrinella, they considered mycoparasitism sensu Rayner and Todd (1979) and Lumsden (1981) as explanation for the strong successional link to F. pinicola. Interestingly, Vampola (1991) suggested for A. parasitica, which grows on living and dead basidiocarps of Trichaptum spp., that this species is a mycoparasite as well. However, a parasitic interaction for A. citrinella (or related taxa) has not been experimentally proven yet (Holec et al. 2018).
The aim of this study is to test experimentally whether A. citrinella has two modes of nutrition, a saprotrophic and a mycoparasitic one. Here, we use the term mycoparasitism as defined by Jeffries and Young (1994): a parasite can obtain nutrition in a parasitic manner from its host. To test this hypothesis, dual culture tests were complemented with tests for enzymatic activity and light microscopic examination of the interaction zone. Dual cultures were used to assess the combative abilities of both species. Since the polypore is already known to grow mainly in the cool season (Wieners et al. 2016; Holec et al. 2018), the dual cultures were incubated at different temperatures. Extracellular enzymes such as proteases and chitinase were investigated because they are involved in cell wall degradation and nutrition of mycoparasitic fungi (e.g., Gams et al. 2004; Hiscox and Boddy 2017). The interaction zone was studied to characterize the fungal interaction. This approach attempted to facilitate the interpretation of the results in an ecological context.
Material and methods
Origin of isolates, reference material, and culture maintenance
Four A. citrinella and two F. pinicola isolates were used to test the hypothesis. Four dikaryotic strains (SBUG-M 1723, SBUG-M 1724, SBUG-M 1737, and SBUG-M 1738) were collected as part of the mycological inventory of the core zone of the Black Forest National Park (Germany) (Scholler & Popa 2021). One dikaryotic strain (Acit1) originated from the Bavarian Forest National Park (Germany). And one monokaryotic strain (Acit5) was obtained from germinated basidiospores of an in vitro fructification of SBUG-M 1737. The four isolates SBUG-M 1723, SBUG-M 1724, SBUG-M 1737, and SBUG-M 1738 were deposited in the fungus collections of the Department of Bacterial Physiology SBUG, University of Greifswald (Greifswald, Germany). The isolate SBUG-M 1723 was additionally deposited in the German Collection of Microorganisms and Cell Cultures (DSMZ) under the number DSM 108506. Basidiocarps for strain isolation (SBUG-M 1737: KR-M-0049005; SBUG-M 1738: KR-M-0049209) and A. citrinella isolate reference material (KR-M-0049247 to KR-M-0049250) were air dried (40 °C) and stored in the fungus collections of the Natural History Museum Karlsruhe (Karlsruhe, Germany) (KR). All isolates were maintained at 20 °C and 70(± 5) % relative humidity on 2% malt extract agar (MEA: 20 g/l malt extract, 20 g/l agar) and regularly transferred to new MEA plates (9 cm in diameter). Circular inocula (1 cm in diameter) were cut out of the active growth zone using a cork borer and then used for the experiments.
Dual culture and hyphal interaction tests
To characterize the interspecific interaction, the effect of temperature (5 °C; 15 °C; 25 °C) on the competitive abilities of A. citrinella and F. pinicola was investigated by using dual cultures on MEA. For the dual cultures, inocula (1 cm) of both species were placed in the same petri dish (9 cm) at opposing sites with a distance of 6 cm. All possible combinations of A. citrinella and F. pinicola isolates were tested, and three technical replicates per combination were used. The dual cultures were documented photographically in a weekly interval for ten weeks. The mycelia of both species were distinguished by their appearance; F. pinicola formed dense, white aerial mycelium, while the mycelium of A. citrinella was less dense, and more cottony.
In a preliminary test, no gross mycelial contact, but hyphal interaction was observed on nutrient-poor substrata. For the light-microscopical documentation of the interaction between A. citrinella and F. pinicola, dual cultures according to Helfer (1991) were prepared. Sterilized microscope slides were placed in empty petri dishes and then covered with 2% MEA to create a medium layer as thin as possible. Here, a nutrient-rich substrate (2% MEA) was used instead of a nutrient-poor substrate (0.5% MEA) to ensure sufficient mycelial growth. Inocula of A. citrinella and F. pinicola were placed at opposing sites and incubated at 15 °C and observed up to four weeks. The interaction was documented using a Zeiss Primo Star and a Zeiss Imager.Z1 light microscope (Germany, Oberkochen). The hyphae of both species were distinguished by diameter and wall thickness. Also, the hyphae of the monokaryon had septa without clamps.
Enzymatic activity
To gain qualitative information on the mode of nutrition of A. citrinella and F. pinicola, chemical detection reactions were carried out to characterize the physiological-biochemical properties of the study organisms. The production of proteases (F19), cellulase (F22), polyphenol oxidase (F28), laccase (F31), and peroxidase (F34) was investigated according to Kreisel and Schauer (1987). For the detection of chitinase, an enzyme test according to Helfer (1991) was adapted and performed. For the protease test, an agar medium turbid with gelatin was prepared. Similarly, chitin agar was prepared for the chitinase test. A clear zone around the inoculum indicated a positive test. The other tests were chemical detection reactions conducted with the whole culture. Positive results were indicated by color changes.
Results
Mycelial interaction
The outcome of interactions of A. citrinella and F. pinicola was strongly dependent on the temperature (Table 2). At 5 °C, all A. citrinella isolates were more competitive than the F. pinicola isolates, and a slow and steady replacement process was observed (Fig. 1A). Different outcomes were documented at 15 °C (Fig. 1B): mostly, A. citrinella was partly or completely overgrown by F. pinicola. Generally, the F. pinicola isolate SBUG-M 1738 was slightly more competitive than the isolate SBUG-M 1724. And at 25 °C, both F. pinicola isolates replaced A. citrinella within less than two weeks (Fig. 1C). At 5–15 °C, the hyphae of F. pinicola were stained brownish in the interaction zone. At 15 °C, the complete agar medium was darkened. And at 25 °C, no change in color of the agar medium was observed. Samples taken from the interaction zone and dying, dead, or lysed hyphae were observed under the microscope.
Dual cultures of A. citrinella (top) and F. pinicola (bottom) at different temperatures. A: At 5 °C. A. citrinella slowly replaced F. pinicola. The interaction zone was stained brownish. B: Deadlock at 15 °C. The complete agar medium was darkened. C: At 25 °C F. pinicola replaced A. citrinella within two weeks. Diameter: 9 cm
Hyphal interaction
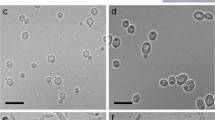
Light microscopy revealed hyphal interaction of A. citrinella and F. pinicola (Fig. 2). In some cases, the hyphae of A. citrinella conducted only short contact via infection hypha (Fig. 2A and B); in other cases, the hyphae of A. citrinella grew along the hyphae of F. pinicola. In both cases, preferential growth of A. citrinella toward F. pinicola was observed. After physical contact, death of single F. pinicola hyphal segments was regularly observed (Fig. 2C and D). This process took less than 2 h. Dead hyphal segments (single cells) were recognized by the darker color and a granular lumen. Hyphal death was often followed by cell lysis (Fig. 2E–H). Death of single hyphae was observed immediately after hyphal contact; after 48 h, the hyphae were almost completely lysed (Fig. 2A–H). In some regions, F. pinicola formed chlamydospores that were also attacked by A. citrinella. No penetration and no growth inside F. pinicola hyphae were observed.
Hyphal interaction of A. citrinella and F. pinicola. A, B: A. citrinella (Ac) and F. pinicola (Fp) before contact in close proximity. C, D: Formation of infection hypha. A. citrinella is growing toward F. pinicola hypha and contacts it (arrow). Death of F. pinicola hypha is recognized by dark granular cell lumen. E–H: Lysis of F. pinicola hypha. After two days, the hypha is almost completely dissolved. A. citrinella hypha including infection hypha is alive. Bar: 20 μm
Enzymatic activity
Both species, F. pinicola and A. citrinella, had enzymes for cellulose degradation. The latter also produced polyphenol oxidase, laccase, and peroxidase, three enzymes that are involved in the degradation of lignin. In addition to the enzymes directly involved in wood degradation, the production of chitinase and proteases was investigated. It could be shown that A. citrinella forms chitinase and proteases. The tests for F. pinicola were both negative.
Discussion
In this study, we provided experimental results that suggest a mycoparasitic interaction between A. citrinella and F. pinicola. The results of this study indicate that A. citrinella can recognize, kill, and feed on F. pinicola, which are key features of necrotrophic parasites (Gams et al. 2004). The ability of A. citrinella to produce chitinase and proteases generally supports this interpretation. In the following, the different aspects of the fungal interaction are outlined in more detail.
Outcome of interactions
Based on field observations, especially the occurrence of A. citrinella only in the cold season (Wieners et al. 2016; Holec et al. 2018), we expected that the temperature has an effect on the outcome of interactions in dual cultures. The results of this study show that A. citrinella was more competitive than F. pinicola at 5 °C; at 25 °C, it was vice versa. At 15 °C, F. pinicola was slightly more competitive. Based on single culture growth rates at different temperatures, Wieners et al. (2016) already assumed that replacement only takes place at low temperatures, namely, below 10 °C. The results of the current study point into the same direction. Growth rates and competitive abilities of wood-inhabiting fungi have been reported to correlate well (Fryar et al. 2002; Hiscox et al. 2016). This relation seems to apply for A. citrinella and F. pinicola, too. Thus, the results indicate a realized niche of A. citrinella at low temperature.
Parasitic interaction
As the fruiting bodies of A. citrinella usually occur on or close to the dead fruiting bodies of F. pinicola, we expected a mycoparasitism that is mediated by hyphal contact. By using the experimental setup described by Helfer (1991), we studied the replacement mechanism and identified the following three steps: (1) A. citrinella made physical contact with F. pinicola. It was regularly observed that the former species made short contact or grew along the hyphae of the later. (2) Many hyphae of F. pinicola died after contact with A. citrinella. This step was recognized by the protoplasmic destruction of the dead hyphae. And (3) subsequently, nutrients from F. pinicola were exploited by A. citrinella. The last step was concluded from the lysis of the dead hyphae together with the production of chitinase and proteases.
Rayner and Webber (1984) distinguish between two basic replacement mechanisms following hyphal contact that are mycoparasitism and hyphal interference. The latter is very common in antagonistic interactions between wood decaying basidiomycetes (Rayner & Boddy 1988). Hyphal interference is mediated by non-enzymatic, diffusible metabolites and leads to growth inhibition at close proximity (< 50 μm) or after contact. It may also cause the death of involved hyphal compartments, followed by protoplasmic destruction. In contrast, mycoparasitism is a trophic interaction, where the parasite obtains nutrition in a parasitic manner from its host. In this study, system physical contact and death of single hyphal segments, but no growth inhibition in dual cultures, were observed. Also, lysis of F. pinicola hyphae after contact with A. citrinella was observed, which was also reported for the mycoparasitism of Trametes spp. by Lenzites betulina (Rayner et al. 1987). Thus, we suggest that mycoparasitism can explain the findings of this study better than hyphal interference.
There are two types of necrotrophic mycoparasites that are characterized by the host-parasite-interface, contact necrotrophs, and invasive necrotrophs (Jeffries and Joung 1994). In dual cultures, protoplasmic destruction and cell lysis were observed regularly. But specialized interfaces, i.e., haustoria, or the penetration of hyphae by appressoria or the invasion into the complete thallus, known from other mycoparasitic genera (Jeffries and Young 1994; Gams et al. 2004), were not observed. Accordingly, we assume a contact necrotrophic interaction. This would be a rather unspecific mode of interaction and only the first evolutionary step of a true mycoparasite. But even though the association of A. citrinella with F. pinicola seems to be rather specific, dual culture experiments showed that A. citrinella is an overall strong competitor in natural and artificial species-species combinations (Holmer et al. 1997).
Ecological implications
From an ecological perspective a mycoparasitic, wood-inhabiting polypore may use its ability to specifically replace a dominant pioneer species as a strategy for secondary resource capture (Rayner et al. 1987). This consideration would suggest that the presence of the pioneer species is more important than the host tree species. For A. citrinella, this seems to be the case: although it is mainly encountered on conifers (Picea, Abies, and Pinus), which constitute the main substrate of F. pinicola, the polypore follows F. pinicola also on Fagus, Betula, and Populus (Bässler and Müller 2010; Ryvarden and Melo 2014; Holec et al. 2018). Also, the explanation provided by Holmer et al. (1997), who assumed a rather unspecific link and therefore suggested resource complementary use (a white rotter follows a brown rotter), seems unsatisfying. The observation that nine out of ten Antrodiella species with known associations to other wood decomposers follow a white rotting predecessor strengthen the point that the successional link is rather specific and mainly mediated by the species identity of the predecessor species, not the tree species identity or decay capability.
Potential reasons for the relative rarity of A. citrinella are of interest to nature conservation. Despite the occurrence of F. pinicola throughout coniferous forests in Europe, the distribution of A. citrinella is disjunct. One reason for this might be that A. citrinella occurs only at high host abundances: Bässler and Müller (2010) found in their threshold analysis that A. citrinella mainly occurs in forest stands with more than 180 F. pinicola basidiocarps per hectare. Such high abundances are very unlikely in managed forests. Another factor to consider is microclimate. The species seems to be restricted to moist habitats (Niemelä and Ryvarden 1983; Ryvarden and Melo 2014). And based on the results of this study, we suggest that A. citrinella is capable to parasitize and thereby replace F. pinicola only at low temperatures. Nevertheless, we expect that A. citrinella can build stable populations wherever those conditions are met, as particularly in protected forest areas with high deadwood amounts in Central European mountains or in North Europe.
Cautionary notes
In this study, we presented results that indicate a parasitic mode of nutrition for the polypore A. citrinella. Nevertheless, we are aware of some shortcomings of the applied set of qualitative methods, which motivated us to formulate some steps to further investigate the interaction of A. citrinella and F. pinicola: to gain a deeper, more mechanistic understanding of the interaction, methods from the OMICs toolbox could be applied (i.e., gene expression in interaction zone, quantitative enzyme assays in time series, and screening of the genomes for functionally relevant enzymes). Also, stable isotope analysis or staining of living cells with fluorescent dyes could be used to trace nutrients that are transferred from one organism to the other. With this, we hope to stimulate much more exiting research with the study organisms A. citrinella and related taxa.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bässler C, Müller J (2010) Importance of natural disturbance for recovery of the rare polypore Antrodiella citrinella Niemelä & Ryvarden. Fungal Biol 114(1):129–133. https://doi.org/10.1016/j.funbio.2009.11.001
Bernicchia A, Ryvarden L, Gibertoni TB (2007) Antrodiella semistipitata (Basidiomycetes, Polyporales), a new species from Italy. Mycotaxon 99:231–238
Blaschke M, Helfer W, Ostrow H, Hahn C, Loy H, Bußler H, Krieglsteiner L (2009) Naturnähezeiger –Holz bewohnende Pilze als Indikatoren für Strukturqualität im Wald. Natur Und Landschaft 84(12):560–566
Boddy L (2000) Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol Ecol 31(3):185–194. https://doi.org/10.1111/j.1574-6941.2000.tb00683.x
Braunisch V, Hauck F, Dalüge N, Hoschek M, Ballenthien, E, Winter MB, Michels HG (2020) Waldzielartenkonzept und Waldnaturschutz-Informationssystem: Instrumente zur Artenförderung im Staatswald von Baden-Württemberg. standort.wald 51:53–76
David A, Tortic M (1986) Contribution à l’étude de quatre polypores européens peu connus. Cryptogam, Mycol 7(1):1–13
Dickie IA, Fukami T, Wilkie P, Allen RB, Buchanan PK (2012) Do assembly history effects attenuate from species to ecosystem properties? A field test with wood-inhabiting fungi. Ecol Lett 15:133–141. https://doi.org/10.1111/j.1461-0248.2011.01722.x
Fryar SC, Kirby GC, Hyde KD (2002) Interspecific competitive ability of homokaryotic and heterokaryotic wood decay basidiomycetes. Austral Ecol 27(3):343–349. https://doi.org/10.1046/j.1442-9993.2002.01186.x
Gams W, Diederich P, Põldmaa K (2004) Fungicolous fungi. In: Mueller GM, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring methods. Elsevier Academic Press, San Diego, pp 343–392
Grosse-Brauckmann H, Luschka N (1991) Vier interessante Aphyllophorales-Arten aus dem Bayerischen Wald: Junghuhnia fimbriatella, Antrodiella citrinella (Poriaceae), Hymochnicium cymosum und Resinicium furfuraceaum (Corticiaceae). Hoppea 50:519–525
Halbwachs H, Ryvarden L, Bässler C (2021) Larger basidiomycetes growing on poroid lignicolous fungi show rot type related colonization patterns. Asian Journal of Mycology 4(2):19–28
Helfer W (1991) Pilze auf Pilzfruchtkörpern – Untersuchungen zur Ökologie, Systematik und Chemie. Libri Botanici 1, IHW-Verlag, Eching. p 160
Hiscox J, Boddy L (2017) Armed and dangerous – chemical warfare in wood decay communities. Fungal Biol Rev 31(4):169–184. https://doi.org/10.1016/j.fbr.2017.07.001
Hiscox J, Savoury M, Müller CT, Lindahl BD, Rogers HJ, Boddy L (2015) Priority effects during fungal community establishment in beech wood. ISME J 9(10):2246–2260. https://doi.org/10.1038/ismej.2015.38
Hiscox J, Clarkson G, Savoury M, Powell G, Savva I, Lloyd M, Shipcott J, Choimes A, Cumbriu XA, Boddy L (2016) Effects of pre-colonisation and temperature on interspecific fungal interactions in wood. Fungal Ecol 21:32–42
Hiscox J, O’Leary J, Boddy L (2018) Fungus wars: basidiomycete battles in wood decay. Stud Mycol 89:117–124. https://doi.org/10.1016/j.simyco.2018.02.003
Holec J, Běťák J, Pouska V, Dvořák D, Zíbarová L, Kout J, Adam D (2018) Old-growth forest fungus Antrodiella citrinella – distribution and ecology in the Czech Republic. Czech Mycol 70(2):127–143
Holmer L, Renvall P, Stenlid J (1997) Selective replacement between species of wood-rotting basidiomycetes, a laboratory study. Int J STD AIDS 101(6):714–720
Jahn H (1967) Trametes hoehnelii (Bres.) und Gloeoporus dichrous (Fr.) als Nachfolger von Inonotus-Arten. Westfälische Pilzbriefe 6:159–162
Jeffries P, Young TWK (1994) Interfungal parasitic relationships. CAB, Wallingford. p 288
Johannesson H, Renvall P, Stenlid J (2000) Taxonomy of Antrodiella inferred from morphological and molecular data. Mycol Res 104(1):92–99
Jönsson MT, Edman M, Jonsson BG (2008) Colonization and extinction patterns of wood-decaying fungi in a boreal old-growth Picea abies forest. J Ecol 96(5):1065–1075. https://doi.org/10.1111/j.1365-2745.2008.01411.x
Kout J, Vlasák J, Spirin V (2014) Contribution to the Antrodiella americana species complex (Basidiomycota, Polyporales). Czech Mycol 66(1):53–60
Kreisel H, Schauer F (1987) Methoden des mykologischen Laboratoriums. VEB Gustav Fischer Verlag, Jena
Lumsden R (1981) Ecology of mycoparasitism. In: Wicklow D, Carroll G (ed) The fungal community: its organization and role in the ecosystem, 1st edn. Mycology series 2. Marcel Dekker, Inc., New York, Basel, p 295–318
Miettinen O, Niemelä T, Spirin W (2006) Northern Antrodiella species: the identity of A. semisupina, and type studies of related taxa. Mycotaxon 96:211–240
Niemelä T, Ryvarden L (1983) Antrodiella citrinella: a new polypore species. Karstenia 23:26–30. https://doi.org/10.29203/ka.1983.220
Niemelä T, Renvall P, Penttilä R (1995) Interactions of fungi at late stages of wood decomposition. Ann Bot Fenn 32:141–152
Olsson J, Jonsson BG, Hjältén J, Ericson L (2011) Addition of coarse woody debris–the early fungal succession on Picea abies logs in managed forests and reserves. Biol Cons 144(3):1100–1110. https://doi.org/10.1016/j.biocon.2010.12.029
Ottosson E, Nordén J, Dahlberg A, Edman M, Jönsson M, Larsson KH, Olsson L, Penttila R, Stenlid J, Ovaskainen O (2014) Species associations during the succession of wood-inhabiting fungal communities. Fungal Ecol 11:17–28. https://doi.org/10.1016/j.funeco.2014.03.003
Pieri M, Rivoire B, Gannaz M (2000) Antrodiella citrinella Niem. & Ryv. Polypore nouveau pour la France. Bulletin Trimestriel De La Fédération Mycologique Dauphiné-Savoie 159:45–47
Pouska V, Svoboda M, Lepš J (2013) Co-occurrence patterns of wood-decaying fungi on Picea abies logs: does Fomitopsis pinicola influence the other species? Pol J Ecol 61(1):119–134
Rayner ADM, Todd N (1979) Population and community structure and dynamics of fungi in decaying wood. In: Woolhouse H (ed) Advances in botanical research, vol 7. Academic Press, London, pp 333–420
Rayner ADM, Webber JF (1984) Interspecific mycelial interactions - an overview. In Jennings DH, Rayner ADM (Eds) The Ecology and Physiology of the Fungal Mycelium. British Mycological Society Symposium 8. Cambridge University Press. pp 383-417
Rayner ADM, Boddy L, Dowson CG (1987) Temporary parasitism of Coriolus spp by Lenzites betulina: a strategy for domain capture in wood decay fungi. FEMS Microbiology Letters 45(1):53–58
Rayner ADM, Boddy L (1988) Fungal decomposition of wood: its biology and ecology. John Wiley & Sons Ltd. p 587
Renvall P (1995) Community structure and dynamics of wood-rooting Basidiomycetes on decomposing conifer trunks in Northern Finland. Karstenia 35:1–51. https://doi.org/10.29203/ka.1995.309
L Ryvarden I Melo (2014) Poroid fungi of Europe. Fungiflora, Oslo. p 455
Scholler M, Popa F (ed) (2021) Die Pilze des ehemaligen Bannwalds Wilder See im Nationalpark Schwarzwald unter besonderer Berücksichtigung der mit Abies alba (Weiß-Tanne) vergesellschafteten Arten. Forschung Im Nationalpark Schwarzwald 1:1–480
Spirin WA, Zmitrovich IV (2003) Notes on some rare polypores, found Russia. I: Genera Antrodiella, Gelatoporia, Irpex Oxyporus, Pilatoporus, and Porpomyces. Karstenia 43(2):67–82
Vampola P (1991) Antrodiella parasitica, a new species of polypore. Czech Mycol 45:10–14
Vampola P, Pouzar Z (1996) Contribution to the knowledge of the Central European species of the genus Antrodiella. Czech Mycol 49:21–33
Vlasak J (1990) Antrodiella citrinella, a new record: a new polypore for Czechoslovakia. Czech Mycol 44:238–239
Weslien J, Djupström LB, Schroeder M, Widenfalk O (2011) Long-term priority effects among insects and fungi colonizing decaying wood. J Anim Ecol 80(6):1155–1162. https://doi.org/10.1111/j.1365-2656.2011.01860.x
Wieners M, Reinhard A, Förschler M, Scholler M (2016) The rare polypore Antrodiella citrinella and its special phenology in the Black Forest National Park (Germany). Journal of Biodiversity & Endangered Species 4(2):1–5. https://doi.org/10.4172/2332-2543.1000168
Acknowledgements
This study was carried out as part of a master thesis. MW is grateful for the supervision by Prof. Dr. Siegfried Fink (University of Freiburg) and Prof. Dr. Michael Scherer-Lorenzen (University of Freiburg). We thank Sabine Diener, Susanne Röske, Anne Reinhard, and Luna Relinque Gantert for technical support, the Black Forest National Park administration for collection permits and Prof. Dr. Irmgard Greilhuber (University of Vienna) for providing an additional A. citrinella isolate from the Bavarian Forest National Park.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was carried out as part of a master thesis at the University of Freiburg. During the preparation of the manuscript, Max Wieners was supported by the German Federal Environment Foundation (DBU) under the grant number AZ 20019/616.
Author information
Authors and Affiliations
Contributions
Conceptualization: Max Wieners and Markus Scholler; methodology: Max Wieners, Claus Bässler, and Markus Scholler; formal analysis and investigation: Max Wieners; writing—original draft preparation: Max Wieners; writing—review and editing: Claus Bässler and Markus Scholler.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Meike Piepenbring
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wieners, M., Bässler, C. & Scholler, M. Mycoparasitism of Fomitopsis pinicola (Sw.) P. Karst. by Antrodiella citrinella Niemelä & Ryvarden. Mycol Progress 22, 55 (2023). https://doi.org/10.1007/s11557-023-01906-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-023-01906-4