Abstract
While assessing the filamentous fungi associated with woody tissues of stem collar rots, necroses, and lesions of European ash trees (Fraxinus excelsior) presenting symptoms of ash dieback in Germany, Cryptostroma corticale was recovered from three different ash trees. These isolated strains were the first report of C. corticale on ash and the first proof of an association of this plant pathogen with woody tissues of other tree species than Acer spp. in Germany. To test the pathogenicity of C. corticale against F. excelsior and to fulfil Koch’s postulates, inoculation tests in planta with strains isolated from Acer pseudoplatanus and F. excelsior were conducted according to Henle–Koch’s postulates in a greenhouse located in Göttingen. The pathogenicity tests were performed with apparently healthy ash saplings from June 2021 until January 2022. After three and seven months, neither necroses or lesions due to C. corticale nor disease symptoms were observed. Mostly, the inoculation wounds healed over, and C. corticale could not be re-isolated from the ash woody tissue. In an attempt to re-isolate the inoculated strains, the filamentous fungal endophytes of the ash woody tissues were isolated and identified. A total of 32 taxa of the Ascomycota were found, where the most common species was Boeremia exigua. Most frequently observed orders were Pleosporales (58.4%), followed by Sordariales (13.5%), Hypocreales (9.4%), and Diaporthales (8.7%). On average, 3.7 endophytic species were recorded on each sapling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anamorphic fungus Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller (Ascomycota) is the causal agent of Sooty bark disease (SBD). This species was first isolated from Acer campestre L. in Ontario in 1889 and called Coniosporium corticale Ellis & Everh. (Ellis & Everhart 1889). Phylogenetic studies based on analyses of four genes (ITS nrDNA, actin, RPB2 and β-tubulin) revealed that C. corticale is a member of the Xylariaceae Tul. & C. Tul., Graphostromataceae M.E. Barr, J.D. Rogers & Y.M. Ju (Ju et al. 1998; Koukol et al. 2015). Koukol et al. (2015) illustrated the affinity of C. corticale to the genus Biscogniauxia with the closest relatives being B. bartholomaei (Peck) Lar.N. Vassiljeva and Graphostroma platystomum (Schwein.) Piroz. (Koukol et al. 2015).
Further cases of SBD were subsequently observed in Canada and the USA, and it is likely that C. corticale is originally native to North America (Enderle et al. 2020; Gregory and Waller 1951). Major hosts of the SBD pathogen are Acer species (Sapindaceae and Sapindales), such as A. pseudoplatanus L., A. saccharinum L. (Gregory and Waller 1951), as well as less commonly A. campestre L. (Moreau and Moreau 1954), A. platanoides L. (Bencheva 2014), and A. negundo L. (Young 1978). There is evidence to suggest the existence of additional potential host trees, such as Tilia spp. and Betula spp. (Cochard et al. 2015), as well as Aesculus hippocastanum L. (Young 1978). In Europe, first observations of the Sooty bark disease were made on A. pseudoplatanus in Wanstead Park Essex, UK, in the year 1945 (Gregory and Waller 1951). In Germany, the first report on sycamore (A. pseudoplatanus) firewood diseased by C. corticale dates to the year 1964 (Enderle et al. 2020; Plate and Schneider 1965).
Most likely C. corticale primarily infects its host tree through fresh wounds (Townrow 1953; Dickenson 1980). The warmth-loving, invasive anamorphic fungus is presumed to be an opportunistic latent pathogen switching from its endophytic lifestyle to pathogenic and saprophytic life stages when its host is stressed (Dickenson 1980). SBD of sycamore leads to mortality of the infected trees, triggered and accelerated by a tree stressor, such as drought (Gregory and Waller 1951; Dickenson and Wheeler 1981; Enderle et al. 2020). Typical disease symptoms, described in detail by Enderle et al. (2020), include the peeling of the outer stem or branch bark layer and the appearance of a brownish-black, sooty layer of conidia.. However, in years with normal temperatures and sufficient precipitation, especially in summer, the course of disease can proceed without any externally visible symptoms (Cech 2019). After infection with C. corticale via wounds or less likely natural openings of the tree (Dickenson 1980), the latent pathogen may survive as an endophyte. First, the fungal mycelium spreads via the xylem and later via the phloem through the wood into the heartwood (Young 1978; Dickenson and Wheeler 1981).
Due to severe drought conditions during several summers in the last two decades, outbreaks of SBD have been increasingly observed in mainland Europe (Koukol et al. 2015; Cochard et al. 2015; Bork 2018; Enderle et al. 2020). Until now, C. corticale is considered established in eleven European countries: Belgium (Cech 2004, 2019), Bulgaria (Bencheva 2014), Czech Republic (Koukol et al. 2015; Kelnarová et al. 2017), France (Moreau and Moreau 1951; 1954), Germany (Bork 2018; Delb et al. 2019; Rohde et al. 2019; Wenzel et al. 2019; Enderle et al. 2020), Italy (Oliveira Longa et al. 2016), the Netherlands (EPPO 2014), Norway (Spaulding 1961), the UK, and Switzerland (Cochard et al. 2015).
The conidia release of C. corticale poses a potential risk to human health. Persons who have intensive or occupational contact with masses of conidia can develop Pneumonitis due to C. corticale, also referred to as maple bark disease (MBD) or maple bark strippers’ lung. The latter denotes the opportunistic mycosis of the lungs caused by inhalation of C. corticale conidia while stripping the bark from maple logs (Emanuel et al. 1966). MBD in humans is a hypersensitivity pneumonitis (HP) causing symptoms similar to allergic asthma, COPD, flu-like infections, influenza, and interstitial pneumonia (Braun et al. 2021; Kespohl et al. 2022).
As part of the “FraxForFuture” demonstration project, the sub-network “FraxPath” (Peters et al. 2021; Langer et al. 2022) investigates the formation of stem collar necrosis associated with trees affected by ash dieback. The fungal community associated with stem collar rots, necroses, and lesions of European ash trees (Fraxinus excelsior L., Oleaceae, Lamiales) was assessed in several German forest plots (Peters et al. 2023). To this end, classical culture-based isolation methods, in planta inoculations and fungal identification by ITS-barcoding and morphological characteristics, were used according to Langer (2017), Bußkamp et al. (2020), and Langer and Bußkamp (2021). In the course of this in-depth investigation of stem collar necrosis and rots of common ash, fungi associated with woody tissues of 58 ash trees from nine localities in Germany were isolated including Cryptostroma corticale (Peters et al. 2023). According to previous authors (Petrini 1991; Saikkonen et al. 1998; Arnold and Lutzoni 2007), we consider those fungi as endophytes that spend at least a significant amount of their life cycle within the host woody tissue without causing symptoms.
European ash dieback caused by the invasive alien fungal pathogen Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz & Hosoya (syn. H. pseudoalbidus Queloz, Grünig, Berndt, T. Kowalski, T.N. Sieber & Holdenr., anamorph: Chalara fraxinea T. Kowalski, Ascomycota) was first observed in Poland at the beginning of the 1990s (Przybył 2002; Kowalski 2006). It is assumed that this fungus was introduced from Far East Asia to Europe (Gross et al. 2014; Drenkhan et al. 2014) and spreads very quickly (Timmermann et al. 2011; Enderle et al. 2019). Ash dieback and its impacts, for example stem collar necroses or root and butt rot, often have fatal consequences for the survival, growth and wood quality of F. excelsior (Langer et al. 2022). This disease, which is now widespread in Europe, is the most serious threat to European ash trees to date (Skovsgaard et al. 2017; Peters et al. 2021). In addition to the typical and eponymous crown symptoms of ash, such as shoot dieback and leaf necrosis, stem collar rot and necrosis were often observed on diseased trees (Husson et al. 2012; Langer 2017; Meyn et al. 2019; Enderle et al. 2017). In Germany, ash dieback has been present since 2002 at least (Schumacher et al. 2007) and is present in all regions where common ash grows (Langer 2017). To assess the impact of C. corticale on the health of European ash, pathogenicity tests were carried out according to the Henle–Koch postulates (Evans 1976), using the occurrence of necrosis as an indicator for pathogenicity.
Materials and methods
Fungal strains
All fungal strains used in our pathogenicity tests were identified based on morphology and ITS sequencing. The ITS sequences (containing ITS1, 5.8S and ITS2 regions) have been deposited in GenBank (Table 1), and all the strains are permanently stored in the NW-FVA strain collection. They were cultivated on malt yeast peptone agar (MYP), modified according to Langer (1994), containing 0.7% malt extract (Merck, Darmstadt, Germany), 0.05% yeast extract (Fluka, Seelze, Germany), 0.1% peptone (Merck), and 1.5% agar (Fluka).
Cryptostroma corticale isolates from Acer pseudoplatanus.
NW-FVA 5572 (2020-54-B2u-7), Germany, Lower Saxony, Universal Transverse Mercator (UTM) 32 U 602426 5808725, leg. P. Gawehn and R. Schlößer, 25.05.2020; isolated from woody stem tissue sampled by increment boring; Accession No. OP010049
NW-FVA 5889 (B41-1 2020-50-8), Germany, Saxony-Anhalt, UTM 32 U 667800 5697482, leg. P. Gawehn and R. Schlößer, 11.05.2020; isolated from conidia; Accession No. OP010050.
Cryptostroma corticale isolates from Fraxinus excelsior sampled from woody stem tissue of three different trees with stem collar rots (Peters et al. 2023)
NW-FVA 5932 (ES-2020-9-14), Germany, Lower Saxony, UTM 32 U 564973 5757894, leg. S. Peters and P. Gawehn, 20.10.2020; Accession No. OP023158
NW-FVA 6116 (ES-2021-53-33) Germany, Lower Saxony, UTM 32 U 579914 5757111, leg. S. Peters and P. Gawehn, 22.02.2021; Accession No. OP010051
NW-FVA 6181 (ES-2021-49-28) Germany, Lower Saxony, UTM 32 U 579913 5757147, leg. S. Peters and P. Gawehn, 22.02.2021; Accession No. OP010052.
Plant material
Seventy two-year-old Fraxinus excelsior saplings (assortment 50–80 cm) were purchased from the tree nursery Schlegel & Co., Riedlingen, Germany, originating from the provenance 8 11 02-Nordostdeutsches Tiefland and re-planted in 5 l pots (18.6 × 18.6 × 20 cm) containing potting compost (PROFI-LINIE Kleeschulte Topfsubstrat mineralisch: pH 6, salinity 1.5 g/l, N total: 320 mg/l, P2O5: 120 mg/l, K2O: 350 mg/l, Mg: 120 mg/l, Kleeschulte Erden GmbH & Co. KG, Briloner Straße 14, D-59602 Rüthen, Germany). At the beginning of the experiments, the plants were healthy without visible stem necroses or symptoms of ash dieback. Plant height above substrate was measured at harvest (Online Resource 1). A one-way-ANOVA (aov) was used to determine if there were any statistically significant differences for the saplings height between the treatments “inoculated with C. corticale”, “inoculated with MYP”, and “untreated”. Differences were considered as statistically significant if p-value was below the threshold of 0.01.
Pathogenicity tests
To estimate the impact of five different C. corticale test strains (NW-FVA 5572 and 5889 sampled from sycamore, NW-FVA 5932, 6116, and 6181 isolated from common ash) on European ash, pathogenicity tests were conducted in vivo according to Henle–Koch postulates (Evans 1976). The presence of necroses was used as an indicator of pathogenicity. The inoculation experiments were performed in a foil greenhouse at the NW-FVA in Göttingen, southern Lower Saxony, Germany (UTM 32 U 563091 5710663), from the 15 June 2021 until the 20 January 2022 (7 months).
For each tested strain, ten ash saplings were inoculated with a MYP-agar plug of a one-week-old culture of the fungus. The plugs were placed in wounds made with a sterile scalpel (1–5-mm diameter) on the stem at a height of 44 cm above ground. The removed bark was replaced on top of the plug, and then the stem was wrapped with Parafilm. A set of ten untreated controls, saplings which were not inoculated at all, and mock controls, prepared by inoculating ten saplings with a sterile pure culture medium plug of MYP, were established. The trees were arranged in equal distance from each other and watered to maintain the peat adequately moist.
After three months (13 September 2021), five saplings per treatment group were sampled to check infection success and necrosis formation. The bark around the area of inoculation was peeled away for visualisation and measurement of the extent of any necroses. Lesion lengths were measured with a ruler in the vertical direction to an accuracy of 1 mm. Fungi were re-isolated from discoloured and non-discoloured stem tissue at six loci adjacent to the point of inoculation (above: isolate 1 = right at the necrosis edge, isolate 2 = 1 cm above, and isolate 3 = 2 cm above; below: isolate 4 = right at the necrosis edge, isolate 5 = 1 cm below, and isolate 6 = 2 cm below). The resulting filamentous isolates were identified. The pathogenicity tests ended seven months (20 January 2022) after inoculation when the remaining test plants were sampled and evaluated as described above.
Determination of Fungi
Isolated endophytic strains were assigned to morphotypes (MT) and identified on the basis of micro-morphological characteristics according to Bußkamp et al. (2020) and Langer and Bußkamp (2021) and/or sequencing of the ITS region (White et al. 1990). At least one representative strain of each morphotype was submitted to molecular identification, involving DNA extraction from the mycelium. Mycelium was placed in 1.5-ml Eppendorf tubes with five glass beads (3 mm) and 150 μl of TE buffer (10 ml 1 mmol Tris HCl (pH 0.8), 2 ml 0.5 mmol EDTA; Carl Roth, Karlsruhe, Germany) and crushed in a Mixer Mill MM 200 (Retsch, Haan, Germany) with 25 vibrations per second for 90 s. Subsequently, genomic DNA was extracted following the protocol of Izumitsu et al. (2012).
The 5.8S nuclear ribosomal gene with the two flanking internal transcribed spacers ITS-1 and ITS-2 (ITS region) was amplified and sequenced using the primer pair ITS-1F (Gardes and Bruns 1993) and ITS-4 (White et al. 1990). The PCR mixture consisted of 1 μl of DNA and 19 μl mastermix, which contained 2.5 μl 10 × buffer (Carl Roth, Karlsruhe, Germany), 1 μl of each primer (10 mmol, 2.5 μl MgCl2 (25 mmol), 0.1 μl Taq polymerase (Carl Roth, Karlsruhe, Germany), and 2.5 μl of 2 mmol dNTPs (Biozym Scientific GmbH, Hessisch Oldendorf, Germany). Each reaction was topped up to a volume of 20 μl by adding sterile water.
A StepOnePlus™ PCR System (Applied Biosystems, Waltham, Massachusetts, USA) was used to carry out the DNA amplifications. The conditions for the amplification of the ITS region were set according to Bien et al. (2020). A 1% agarose gel was used to visualise the PCR products. The products were sent to Eurofins Scientific Laboratory (Ebersberg, Germany) for sequencing. Resulting sequences were checked and edited where necessary using BioEdit Sequence Alignment Editor (v. 7.2.5; Hall 1999) and submitted to GenBank subsequently.
For identification, the ITS sequences obtained were used in blastn searches in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/, Altschul et al. 1997). A similarity threshold of at least 98% was set for species-level identification. Blastn results were critically interpreted with emphasis on well-curated culture collections such as the Westerdijk Fungal Biodiversity Collection (CBS). Generally, morphological characteristics were used to confirm the results of molecular identification. In the case that no definite identification was possible to a specific taxonomic level, the taxon name was marked by cf. (confer) to indicate uncertainties.
At least one representative culture for each MT was stored in MYP slant tubes at 4 °C at the fungal culture collection of the NW-FVA. Frequency of isolated taxa, defined as the portion of the amount of isolated strains in relation to the total number of isolated filamentous strains, was calculated. Additionally, continuity of isolated taxa, defined as the number of trees from which the fungus was isolated in relation to the total number of trees, was calculated.
Results
Isolation of Cryptostroma corticale from ash stem collars
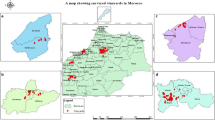
From three out of 58 European ash trees (Fraxinus excelsior), Cryptostroma corticale could be isolated from woody stem tissue (Fig. 1) at two locations in Lower Saxony.
Pathogenicity tests
At the end of the experiment, the height of the tested plants varied considerably independently from the harvest date. The mean plant height was 90.6 cm (min. 42, max. 148 cm), and stem diameter was 0.65 cm (min. 0.3 cm, max. 1.01 cm). One-way ANOVA showed that there were no significant differences for the saplings height between the different treatments for the incubation period of 13 and 31 weeks (Online Resource 1). No necroses were observed regardless of the treatment group studied. All plants were healthy, and calluses had formed over the loci of inoculation.
Re-isolation and isolated endophytic fungi
In total, 420 chips of stem tissue originating from 70 saplings were incubated. From these, 438 mycelial outgrowths were observed (Online Resource 2). Most filamentous fungi grew out between one and three weeks after incubation of the tissue sample. Some of the observed fungal mycelia were omitted due to obvious repetitions or contaminations. From 2.1% of all incubated segments, no outgrowth was detected, while 12.3% yielded yeasts. Cryptostroma corticale could not be re-isolated as an endophyte nor isolated from the mock- or untreated controls.
The resulting pure culture isolates were all Ascomycota assigned to 32 taxa, and all but one species (Ploettnerulaceae sp.) could be identified to genus or species level (Table 1). Most frequently observed orders were Pleosporales (58.4%), followed by Sordariales (13.5%), Hypocreales (9.4%), Diaporthales (8.7%), Xylariales (1.6%), Dothideales (0.7%), and Helotiales (0.2%). Between one and eight different species were found in the studied woody tissue per sapling (Table 2). On average, 3.7 species were recorded on each sapling. The most frequent taxa were Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley (26.5%), Dichotomopilus sp. (11.4%), Alternaria infectoria E.G. Simmons (10.0%), Alternaria sp.—alternata-Gr. (8.9%), and Diaporthe cf. rudis (5.7%). The most abundant species in respect to continuity was B. exigua, which was isolated from 71.4% of all studied ash saplings. The second most continuous species was A. infectoria (38.6%) followed by Dichotomopilus sp. (32.9%) and Diaporthe cf. rudis (30%).
Discussion
The main result of this study was that Koch’s postulates could not be fulfilled for Cryptostroma corticale on Fraxinus excelsior. Therefore, the species could not be proven as a causal agent of disease in F. excelsior, although it was found in association with stem collar necrosis. In the in planta experiments under the environmental conditions of our pathogenicity tests, C. corticale could not be re-isolated. This suggests that the fungus cannot infect sterile-wounded, healthy tissue of young ash trees, in contrast to its ability to enter and infect sycamore through fresh wounds (Townrow 1953; Dickenson 1980). May be the time of year plays a role in the success of infection (Dickenson 1980). Unpublished concurrent inoculation tests with sycamore saplings, using an identical methodology, were partially successful with inoculation with the strain NW-FVA 5932 (original host tree F. excelsior) and unsuccessful with inoculation with the strain NW-FVA 5889 (original host tree A. pseudoplatanus). Only one out of five sycamore saplings were successfully infected by the C. corticale strain isolated from ash. This suggests that the infection on 6 June 2022, i.e. in that early summer, was rather unfavourable for a successful infection under the environmental conditions prevailing at that time. Another explanation for the failure to infect the young stems could be to lack of sufficient nutrients to allow the fungal inoculum to break down cell walls, as in the case of leaf scars (Dickenson 1980).
All isolated endophytic species could be assigned to the Ascomycota which fits the results on endophytes of seedling woody tissues of other tree species, for example 2-year-old Pinus sylvestris (Blumenstein et al. 2021). Blumenstein et al. (2021) isolated 18 different endophytic Ascomycota species including Alternaria spp., Diaporthe spp., Epicoccum nigrum, Microsphaeropsis olivacea (Bonord.) Höhn., Sydowia polyspora (Bref. & Tavel) E. Müll., and Truncatella conorum-piceae (Tubeuf) Steyaert. The most frequently isolated fungus in the Scots pine seedlings was a species determined as Didymellaceae sp. The latter species occurred in all tested Scots pine trees and is identical with Didymella sp. found in this study. In another study, 18 filamentous endophytic fungal species were isolated from woody stem tissue of three Fagus sylvatica saplings (6–17-year-old, mean: 9.6 years old; Langer and Bußkamp 2021). With the exception of the white-rot fungus Coprinellus micaceus (Agaricaceae and Basidiomycota), all isolated beech endophytes of the aforementioned study were members of the Ascomycota. A reason for the absence or low frequency of endophytic Basidiomycota on ash might be the young age or the vigour of the plants. So far, the chronological genesis of the tree endophyte community of seedlings has not yet been fully elucidated. However, it is known that leaves of forest trees do not harbour endophytes at the time of budding (Toti et al. 1993; Scholtysik et al. 2013). Moreover, it is assumed that fungal tree endophytes are vertically (Rodriguez et al. 2009) or horizontally transmitted by spores (Wilson and Carroll 1994; Helander et al. 2007; Scholtysik et al. 2013). Horizontally transmitted endophytes are species-rich and tend to colonise tissues in a broad range of host species (Helander et al. 2007; Rodriguez et al. 2009; Suryanarayanan 2011). The composition of endophytic assemblages in forest trees differ between geographically distinct locations (Peršoh et al. 2010, 2013; Guerreiro et al. 2017, 2022), which indicates that these assemblages are influenced by environmental factors such as temperature and humidity (Zimmerman and Vitousek 2012). Furthermore, common ash leaves, shoots and stems have different assemblies of fungal endophytes (Unterseher et al. 2007). This explains why the species composition of ash endophytes differed in the study of Bilański and Kowalski (2022) compared to the results on woody tissues of ash stems. The latter authors isolated 97 different fungal taxa from asymptomatic leaf petioles of F. excelsior collected in southern Poland. Ascomycota accounted for 94.6% of these species, whereas 5.4% were Basidiomycota. The most abundant species was Nemania serpens (Pers.) Gray, which was isolated in 38.0% of the studied petioles followed by Diaporthe eres Nitschke (33.6%), Fraxinicola fraxini (Aderh.) Crous, M. Shen & Y. Zhang ter (26.4%), Diaporthe sp. 1 (20.4%), Alternaria sp. 1 (14.8%), Colletotrichum acutatum J.H. Simmonds (14.8%), Nemania diffusa (Sowerby) Gray (14.0%), Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. (12.4%), and Colletotrichum sp. (12.4%). Nemania serpens was also isolated in this study but significantly less frequently (0.2%), whereas Boeremia exigua (26.5%) and Dichotomopilus sp. (11.4%) were the most frequently observed species in the stems of the ash saplings.
The total amount of endophytic species isolated from woody ash tissue in this study (32) is comparable to the results of previous investigations (Butin and Kowalski 1986; Kowalski and Kehr 1992; Unterseher et al. 2005). In contrast to findings that Diaporthales dominate endophytic fungal communities in angiosperms (Sieber 2007), our results showed that the endophytic community of ash saplings from forest tree nurseries was dominated by Pleosporales (58.4%), followed by Sordariales (13.5%), Hypocreales (9.4%), and Diaporthales (8.7%). The predominance of Pleosporales in the endophytic community of the examined ash saplings is in agreement with the results of Blumenstein et al. (2021) and Bußkamp et al. (2021) on Scots pine seedlings. In addition to the differences in geography, tree age and tissue type between the above-mentioned studies, the origin of the trees could have an influence on the detected endophytic communities, since nursery trees were used for the isolation of endophytes in this study. Lade et al. (2022) found that the nursery origin had significant effects on the fungal community structure in graft and root tissues of grapevine. However, whether these findings can be transferred to forestry plants needs to be tested, considering that grapevines are intensively processed in the nursery.
Although the pathogenic behaviour of C. corticale on European ash could not be demonstrated in this study, the actual role of this fungus in the necrotic tissue of the sampled trees remains unknown and needs to be further investigated. Accidental colonisation in this newly discovered fungus-host association can be ruled out as it is highly unlikely that such a case would be observed three times in two different locations. In contrast, infection with Hymenoscyphus fraxineus and subsequent fungi in ash stem collar necrosis and the host weakness caused by these fungi may have provoked and facilitated the entry of the wound parasite C. corticale. However, as an increase in SBD severity on sycamore has been recognised in the recent years, due to extended drought events, the discovered fungus-host relationship with ash needs to be taken into account when predicting the spread of C. corticale, and SBD outbreaks in the near future in the face of climate change.
References
Adesemoye AO, Orrell T, Kodati S (2018) Effect of virulence of root rot pathogens and cultivar resistance on disease occurrence in dry beans. Plant Health Prog 19(3):237–241. https://doi.org/10.1094/PHP-06-18-0034-RS
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein databases search programs. Nucleic Acids Res 25:3389–3402. https://doi.org/10.1093/nar/25.17.3389
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88(3):541–549
Ban Y, Tang M, Chen H, Xu Z, Zhang H, Yang Y (2012) The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS ONE 7(10):e47968. https://doi.org/10.1371/journal.pone.0047968
Bencheva S (2014) First report of Cryptostroma corticale (Ellis & Everh.) P.H. Greg. & S. Waller on Acer platanoides L. in Bulgaria. Silva Balcania 15(2):101–104
Bien S, Kraus C, Damm U (2020) Novel collophorina-like genera and species from Prunus trees and vineyards in Germany. Persoonia - Mol Phylogeny Evol Fungi 45(1):46–67. https://doi.org/10.3767/persoonia.2020.45.02
Bilański P, Kowalski T (2022) Fungal endophytes in Fraxinus excelsior petioles and their in vitro antagonistic potential against the ash dieback pathogen Hymenoscyphus fraxineus. Microbiol Res 257:126961. https://doi.org/10.1016/j.micres.2022.126961
Blumenstein K, Bußkamp J, Langer GJ, Schlößer R, Parra Rojas NM, Terhonen E (2021) Sphaeropsis sapinea and associated endophytes in Scots Pine: Interactions and Effect on the Host Under Variable Water Content. Front For Glob Change 4
Bork K (2018) Rußrindenkrankheit an Ahorn: Erstfund in Bayern. AFZ - Wald 20:40–41
Braun M, Klingelhöfer D, Groneberg DA (2021) Sooty bark disease of maples: the risk for Hypersensitivity pneumonitis by fungal spores not only for woodman. J Occup Med Toxicol 16(1):2. https://doi.org/10.1186/s12995-021-00292-5
Bußkamp J, Blumenstein K, Terhonen E, Langer GJ (2021) Differences in the virulence of Sphaeropsis sapinea strains originating from Scots pine and non-pine hosts. For Pathol. https://doi.org/10.1111/efp.12712
Bußkamp J, Langer GJ, Langer EJ (2020) Sphaeropsis sapinea and fungal endophyte diversity in twigs of Scots pine (Pinus sylvestris) in Germany. Mycol Prog 19(9):985–999. https://doi.org/10.1007/s11557-020-01617-0
Butin H, Kowalski T (1986) Die natürliche Astreinigung und ihre biologischen Voraussetzungen. Eur J for Pathol 16(3):129–138. https://doi.org/10.1111/j.1439-0329.1986.tb01053.x
Cech TL (2004) Eine Pilzkrankheit als eigenartige Folge des Sommers 2003? http://www.stadtbaum.at/cpag/123.htm
Cech TL (2019) Rußrindenkrankheit bedroht Ahornbestände in Laubwäldern im Osten Niederösterreichs. Forstsch Aktuell 65:24
Cochard B, Crovadore J, Bovigny PY, Chablais R, Lefort F (2015) First reports of Cryptostroma corticale causing sooty bark disease in Acer sp. in Canton Geneva, Switzerland. New Dis Rep 31:8. https://doi.org/10.5197/j.2044-0588.2015.031.008
Crous P, Wingfield MJ, Schumacher RK, Akulov A, Bulgakov T, Carnegie A, Jurjević Ž, Decock C, Denman S, Lombard L, Lawrence D, Stack AJ, Gordon TR, Bostock R, Burgess T, Summerell B, Taylor P, Edwards J, Hou LW, Groenewald JZ (2020) New and interesting fungi. 3. Fungal Syst Evol 6:157–231. https://doi.org/10.3114/fuse.2020.06.09
Crous PW, Schumacher RK, Akulov A, Thangavel R, Hernández-Restrepo M, Carnegie AJ, Cheewangkoon R, Wingfield MJ, Summerell BA, Quaedvlieg W, Coutinho TA, Roux J, Wood AR, Giraldo A, Groenewald JZ (2019) New and interesting fungi. 2. Fungal Syst Evol 3:57–134. https://doi.org/10.3114/fuse.2019.03.06
Delb H, Grüner J, John R, Wußler J (2019) Waldschutzsituation 2018/2019 in Baden-Württemberg. AFZ - Wald 74:14–17
Dickenson S, Wheeler BEJ (1981) Effects of temperature, and water stress in sycamore, on growth of Cryptostroma corticale. Trans Br Mycol Soc 76(2):181–185. https://doi.org/10.1016/S0007-1536(81)80136-2
Dickenson SJ (1980) Biology of Cryptostroma corticale and the sooty bark disease of sycamore. Dissertation, University of Bath
Drenkhan R, Sander H, Hanso M (2014) Introduction of Mandshurian ash (Fraxinus mandshurica Rupr) to Estonia: Is it related to the current epidemic on European ash (F. excelsior L.)? Eur J for Res 133(5):769–781. https://doi.org/10.1007/s10342-014-0811-9
Emanuel DA, Wenzel FJ, Lawton BR (1966) Pneumonitis due to cryptostroma corticale (Maple-Bark Disease). N Engl J Med 274(25):1413–1418. https://doi.org/10.1056/NEJM196606232742504
Enderle R, Riebesehl J, Becker P, Kehr R (2020) Rußrindenkrankheit an Ahorn - Biologie, Pathologie und Entsorgung von Schadholz. In: Jahrbuch der Baumpflege 2020, 24th edn. Haymarket Media, Braunschweig, pp 85–100
Enderle R, Sander F, Metzler B (2017) Temporal development of collar necroses and butt rot in association with ash dieback. Iforest - Biogeosciences for 10:529–536. https://doi.org/10.3832/ifor2407-010
Enderle R, Stenlid J, Vasaitis R (2019) An overview of ash (Fraxinus spp.) and the ash dieback disease in Europe. CAB Rev 14(025):1–12
EPPO (2014) First report of Cryptostroma corticale in the Netherlands. EPPO Reporting Service no. 07 - 2014 Num. article: 2014/133. In: EPPO Glob. Database. https://gd.eppo.int/reporting/article-3226. Accessed 27 May 2021
Gross A, Holdenrieder O, Pautasso M, Queloz V, Sieber TN (2014) Hymenoscyphus pseudoalbidus the causal agent of European ash dieback. Mol Plant Pathol 15(1):5–21. https://doi.org/10.1111/mpp.12073
Guerreiro M, Brachmann A, Begerow D, Persoh D (2017) Transient leaf endophytes are the most active fungi in 1-year-old beech leaf litter. Fungal Divers. https://doi.org/10.1007/s13225-017-0390-4
Guerreiro MA, Kleetz J, Torres MR, Polle A, Peršoh D, Begerow D (2022) Interaction between growth environment and host progeny shape fungal endophytic assemblages in transplanted Fagus sylvatica. Fungal Ecol 60:101175. https://doi.org/10.1016/j.funeco.2022.101175
Haelewaters D, Dirks AC, Kappler LA, Mitchell JK, Quijada L, Vandegrift R, Buyck B, Pfister DH (2018) A preliminary checklist of fungi at the Boston Harbor Islands. Northeast Nat 25(sp9):45–76. https://doi.org/10.1656/045.025.s904
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. https://doi.org/10.14601/Phytopathol_Mediterr-14998u1.29
Hauptman T, Piškur B, de Groot M, Ogris N, Ferlan M, Jurc D (2013) Temperature effect on Chalara fraxinea: heat treatment of saplings as a possible disease control method. For Pathol 43(5):360–370. https://doi.org/10.1111/efp.12038
Helander M, Ahlholm J, Sieber TN, Hinneri S, Saikkonen K (2007) Fragmented environment affects birch leaf endophytes. New Phytol 175(3):547–553
Husson C, Caël O, Grandjean JP, Nageleisen LM, Marçais B (2012) Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol 61(5):889–895. https://doi.org/10.1111/j.1365-3059.2011.02578.x
Kelnarová I, Černý K, Zahradník D, Koukol O (2017) Widespread latent infection of Cryptostroma corticale in asymptomatic Acer pseudoplatanus as a risk for urban plantations. For Pathol 47(4):e12344. https://doi.org/10.1111/efp.12344
Kespohl S, Grüner J, Enderle R, Riebesehl J, Raulf M (2022) Exogen allergische Alveolitis (EAA) durch den Erreger der Rußrindenkrankheit (Cryptostroma corticale): Eine diagnostische Herausforderung. IPA-J 1:26–30
Koukol O, Kelnarová I, Černý K (2015) Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For Pathol 45(1):21–27. https://doi.org/10.1111/efp.12129
Kowalski T (2006) Chalara fraxinea sp. nov. associated with dieback of ash in Poland. For Pathol 36:264–270. https://doi.org/10.1111/j.1439-0329.2006.00453.x
Kowalski T, Kehr RD (1992) Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 44(2):137–168
Lade SB, Štraus D, Oliva J (2022) Variation in Fungal Community in Grapevine (Vitis vinifera) Nursery Stock Depends on Nursery. Variety and Rootstock J Fungi 8(1):47. https://doi.org/10.3390/jof8010047
Langer GJ (2017) Collar rots in forests of Northwest Germany affected by ash dieback. In: Enderle R, Pliura A, Vaisatis R (eds) Dieback of European Ash (Fraxinus spp.) - Consequences and Guidelines for Sustainable Management. SLU Uppsala, Uppsala, Balt For 23:4–19 pp 4–19
Langer GJ, Bußkamp J (2021) Fungi associated with woody tissues of European beech and their impact on tree health. Front Microbiol 12:702467. https://doi.org/10.3389/fmicb.2021.702467
Langer GJ, Fuchs S, Osewold J, Peters S, Schrewe F, Ridley M, Kätzel R, Bubner B, Grüner J (2022) FraxForFuture: research on European ash dieback in Germany. J Plant Dis Prot. https://doi.org/10.1007/s41348-022-00670-z
Lombard L (2017) Fungal Planet 712: Fusarium petersiae L. Lombard, sp. nov. Persoonia 39:456–457
Meyn R, Langer GJ, Gross A, Langer EJ (2019) Fungal colonization patterns in necrotic rootstocks and stem bases of dieback-affected Fraxinus excelsior L. For Pathol 49(4):e12520. https://doi.org/10.1111/efp.12520
Moreau C, Moreau M (1951) La , Suie‘ des Sycamores a Paris. Bull Société Mycol Fr 67:404–418
Moreau C, Moreau M (1954) Nouvelles observationes sur le deperissement des erables. Bull Société Linn Normandie 7:6–67
Oliveira Longa CM, Vai N, Maresi G (2016) Cryptostroma corticale in the northern Apennines (Italy). Phytopathol Mediterr 55(1):136–138. https://doi.org/10.14601/Phytopathol_Mediterr-17164
Panno L, Bruno M, Voyron S, Anastasi A, Gnavi G, Miserere L, Varese GC (2013) Diversity, ecological role and potential biotechnological applications of marine fungi associated to the seagrass Posidonia oceanica. New Biotechnol 30(6):685–694. https://doi.org/10.1016/j.nbt.2013.01.010
Patejuk K, Baturo-Ciesniewska A, Najberek K, Pusz W (2021) First Report of Fusarium lateritium Causing Shoot Dieback of Acer negundo in Europe. Plant Dis. https://doi.org/10.1094/PDIS-06-21-1294-PDN
Peršoh D, Melcher M, Flessa F, Rambold G (2010) First fungal community analyses of endophytic ascomycetes associated with Viscum album ssp. austriacum and its host Pinus sylvestris. Fungal Biol 114(7):585–596. https://doi.org/10.1016/j.funbio.2010.04.009
Peršoh D, Segert J, Zigan A, Rambold G (2013) Fungal community composition shifts along a leaf degradation gradient in a European beech forest. Plant Soil 362(1):175–186. https://doi.org/10.1007/s11104-012-1271-y
Peters S, Fuchs S, Bien S, Bußkamp J, Langer GJ, Langer EJ (2023) Fungi associated with stem collar necroses of Fraxinus excelsior affected by ash dieback. Mycol Prog (accepted with Minor Revision). https://doi.org/10.21203/rs.3.rs-2484538/v1
Peters S, Langer GJ, Kätzel R (2021) Eschentriebsterben - Kriterien zur Schadensbonitur an Eschen, 1st edn. Fachagentur für nachwachsende Rohstoffe (FNR), Gülzow-Prüzen
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews HJ, Hirano SS (eds) Microbial ecology of leaves. Springer-Verlag, New York and Berlin, pp 179–197
Przybył K (2002) Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For Pathol 32(6):387–394. https://doi.org/10.1046/j.1439-0329.2002.00301.x
Rodriguez RJ, White JF Jr, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182(2):314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
Rohde M, Langer G, Hurling R, Plašil P (2019) Waldschutzsituation 2018 in Nordwestdeutschland. AFZ - Wald 74:38–41
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29:319–343
Scholtysik A, Unterseher M, Otto P, Wirth C (2013) Spatio-temporal dynamics of endophyte diversity in the canopy of European ash (Fraxinus excelsior). Mycol Prog 12(2):291–304. https://doi.org/10.1007/s11557-012-0835-9
Schumacher J, Wulf A, Leonhard S (2007) Erster Nachweis von Chalara fraxinea in Deutschland - ein Verursacher neuartiger Schäden an Eschen. J Cultiv Plantsmie 59:121–123
Sieber TN (2007) Endophytic fungi in forest trees: are they mutualists? Fungal Biol Rev 21(2):75–89. https://doi.org/10.1016/j.fbr.2007.05.004
Skovsgaard JP, Wilhelm G, Thomsen I, Metzler B, Kirisits T, Havrdová L, Enderle R, Dobrowolska D, Cleary M, Clark J (2017) Silvicultural strategies for Fraxinus excelsior in response to dieback caused by Hymenoscyphus fraxineus. Forestry 00:1–18. https://doi.org/10.1093/forestry/cpx012
Spaulding P (1961) Foreign Diseases of Forest Trees of the World: an annotated list. Washington D. C.
Suryanarayanan TS (2011) Diversity of Fungal Endophytes in Tropical Trees. In: Pirttilä AM, Frank AC (eds) Endophytes of Forest Trees. Springer, Netherlands, Dordrecht, pp 67–80
Timmermann V, Børja I, Hietala AM, Kirisits T, Solheim H (2011) Ash dieback: pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway*. EPPO Bull 41(1):14–20. https://doi.org/10.1111/j.1365-2338.2010.02429.x
Toti L, Viret O, Horat G, Petrini O (1993) Detection of the endophyte Discula umbrinella in buds and twigs of Fagus sylvatica. Eur J for Pathol 23(3):147–152. https://doi.org/10.1111/j.1439-0329.1993.tb00954.x
Townrow JA (1953) The biology of cryptostroma corticale and the sooty bark disease of sycamore. In: Report on forest research. London, pp 118–120
Unterseher M, Otto P, Morawetz W (2005) Species richness and substrate specificity of lignicolous fungi in the canopy of a temperate, mixed deciduous forest. Mycol Prog 4(2):117–132. https://doi.org/10.1007/s11557-006-0115-7
Unterseher M, Reiher A, Finstermeier K, Otto P, Morawetz W (2007) Species richness and distribution patterns of leaf-inhabiting endophytic fungi in a temperate forest canopy. Mycol Prog 6(3):201–212. https://doi.org/10.1007/s11557-007-0541-1
van der Merwe R, Halleen F, van Dyk M, Jacobs VG, Mostert L (2021) Occurrence of Canker and Wood Rot Pathogens on Stone Fruit Propagation Material and Nursery Trees in the Western Cape of South Africa. Plant Dis 105(11):3586–3599. https://doi.org/10.1094/PDIS-10-20-2124-RE
Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi AMS, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92(1):135–154. https://doi.org/10.1016/j.simyco.2018.05.001
Wenzel A, Thiel J, Stürtz M (2019) Waldschutzsituation 2018/19 in Thüringen. AFZ- Wald 74:26–29
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18(1):315–322
Wilson D, Carroll GC (1994) Infection studies of Discula quercina, an endophyte of Quercus garryana. Mycologia 86(5):635–647. https://doi.org/10.1080/00275514.1994.12026463
Young CWT (1978) Sooty Bark Disease of Sycamore (Arboricultural Leaflet). Stationery Office Books, Department of Environment, London u.a
Zimmerman NB, Vitousek PM (2012) Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc Natl Acad Sci U S A 109(32):13022–13027. https://doi.org/10.1073/pnas.1209872109
Acknowledgements
The authors are grateful for the technical support from NW-FVA staff Peter Gawehn, Annette Ihlemann, Martina Hille, Ursula Rabel and Rebekka Schlößer during the project and thank Etta Starick for preparing the figures and Jan Tropf for performing the Anova analysis. Maia Ridley, as a native English speaker, improved the English language and grammar of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The FraxCollar project receives funding via the Waldklimafonds (WKF) funded by the German Federal Ministry of Food and Agriculture (BMEL) and Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) administrated by the Agency for Renewable Resources (FNR) under Grant Agreement No 2219WK22A4.
Author information
Authors and Affiliations
Contributions
SP sampled the Cryptostroma corticale strains from European ash. GL and JB planned and conducted the pathogenicity tests. GL performed the isolation of the endophytes, the necroses measurements, and the re-isolation of the fungi. SB performed the DNA-Isolation, PCR, and species identification. GL analysed the data and wrote the first draft. SB, JB, and SP contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they have no other conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Langer, G.J., Peters, S., Bußkamp, J. et al. Cryptostroma corticale and fungal endophytes associated with Fraxinus excelsior affected by ash dieback. J Plant Dis Prot (2023). https://doi.org/10.1007/s41348-023-00750-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-023-00750-8