Abstract
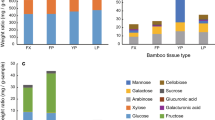
Ship timber beetles (Coleoptera: Lymexylidae) grow symbiotic fungi of the genus Alloascoidea in wood. The female adults possess fungus-carrying organs (mycetangia) and deposit the symbiont onto wood during oviposition. The larvae acquire the symbiont, excavate a tunnel into wood, and feed on the symbiont growing on the tunnel walls. As lymexylids use wood as a larval habitat, it is possible that the fungal symbionts can utilize indigestible wood-associated sugars. However, their abilities to assimilate wood-associated carbon sources remain unknown. In addition, no lymexylid-associated fungal communities have been reported except for Elateroides dermestoides. Here, I report that the ship timber beetle E. flabellicornis originating from Japan harbored five fungal species. When microbial isolation was conducted from mycetangia of a female adult of E. flabellicornis, colonies of filamentous fungi and yeasts were recovered. DNA analyses revealed that they were Alloascoidea sp., Ambrosiozyma llanquihuensis, Ambrosiozyma sp., Cyberlindnera sp., and Saccharomycopsis sp. When wood-associated carbon assimilation abilities of four of the five fungal species: Alloascoidea sp., Am. llanquihuensis, Cyberlindnera sp., and Saccharomycopsis sp., were tested, the abilities were variable among them. Only Saccharomycopsis sp. assimilated galactose and galacturonic acid. Alloascoidea sp. and Cyberlindnera sp. strongly assimilated xylan from corn. Saccharomycopsis sp. and Cyberlindnera sp. assimilated cellobiose. All of the fungi assimilated glucose, mannose, and xylose. These results suggest that the association with multiple fungal species with various carbon assimilation abilities may help the larvae of E. flabellicornis to achieve efficient nutrient intake in space-limited tunnels within nutrient-poor wood.


Similar content being viewed by others
Data availability
All DNA sequences reported in this study are available in the GenBank nucleotide collection database (accession numbers: LC586257–LC586261, LC586282–LC586286); all other datasets in the current study are available from the corresponding author on reasonable request.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Batra LR, Francke-Grosmann H (1961) Contributions to our knowledge of ambrosia fungi. I. Ascoidea hylecoeti sp. nov. (Ascomycetes). Am J Bot 48:453–456
Batra LR, Francke-Grosmann H (1964) Two new ambrosia fungi: Ascoidea asiatica and A. africana. Mycologia 56:632–636
Biedermann PHW, Vega FE (2020) Ecology and evolution of insect-fungus mutualisms. Annu Rev Entomol 65:431–455
Bibikova II, Fateeva MV, Mamayev BM (1988) The yeast flora of Elateroides dermestoides L. in the Amour River. Mikol Fitopatol 22:289–292 (in Russian)
Breznak JA, Brune A (1994) Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol 39:453–487
Brown ES (1954) The biology of the coconut pest Melittomma insulare and its control in the Seychelles. Bull Entomol Res 45:1–66, pls 1–6
Cuccodoro G (2007) Lymexyloidea. In: Löbl I, Smetana A (eds) Catalogue of Palaearctic Coleoptera Vol. 4. Elateroidea - Derodontoidea - Bostrichoidea - Lymexyloidea - Cleroidea - Cucujoidea. Apollo Books, Stenstrup, pp. 362–364
Davis TS (2015) The ecology of yeasts in the bark beetle holobiont: a century of research revisited. Microb Ecol 69:723–732
de Hoog GS, Smith MTh (2011) Ascoidea Brefeld & Lindau (1891). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 325–328
Francke-Grosmann H (1967) Ectosymbiosis in wood-inhabiting insects. In: Henly SM (ed) Symbiosis: associations of invertebrates, birds, ruminants, and other biota. Academic Press, New York, pp 141–205
Grünwald S, Pilhofer M, Höll W (2010) Microbial associations in gut systems of wood- and bark-inhabiting longhorned beetles [Coleoptera: Cerambycidae]. Syst Appl Microbiol 33:25–34
Haack RA, Slansky F (1987) Nutritional ecology of wood-feeding Coleoptera, Lepidoptera, and hymenoptera. In: Slansky F, Rodriguez JG (eds) Nutritional ecology of insects, mites, and spiders. Wiley, New York, pp 449–486
Hausner G, Reid J, Klassen GR (1993) On the subdivision of Ceratocystis s. 1, based on partial ribosomal DNA sequences. Can J Bot 71:52–63
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kurosawa Y (1985) Lymexylidae. In: Kurosawa Y, Hisamatsu S, Sasaji H (eds) the Coleoptera of Japan in color Vol. III. Hoikusha, Osaka, pp 167–169, pl 27 (in Japanese)
Kurtzman CP (2011) Lindnera Kurtzman, Robnett & Basehoar-Powers (2008). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 521–543
Kurtzman CP, Robnett CJ (2003) Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res 3:417–432
Kurtzman CP, Robnett CJ (2013a) Relationships among genera of the Saccharomycotina (Ascomycota) from multigene phylogenetic analysis of type species. FEMS Yeast Res 13:23–33
Kurtzman CP, Robnett CJ (2013b) Alloascoidea hylecoeti gen. nov., comb. nov., Alloascoidea africana comb. nov., Ascoidea tarda sp. nov., and Nadsonia starkeyi-henricii comb. nov., new members of the Saccharomycotina (Ascomycota). FEMS Yeast Res 13:423–432
Kurtzman CP, Smith MTh (2011) Saccharomycopsis Schiönning (1903). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 751–763
Lachance M-A, Boekhout T, Scorzetti G, Fell JW, Kurtzman CP (2011) Candida Berkhout (1923). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 987–1278
Lawrence JF (2020) The Australian Lymexylidae (Coleoptera: Tenerionoidea) with one new genus and two genera new to Australia. Zootaxa 4895:211–238
Lehenberger M, Biedermann PHW, Benz JP (2019) Molecular identification and enzymatic profiling of Trypodendron (Curculionidae: Xyloterini) ambrosia beetle-associated fungi of the genus Phialophoropsis (Microascales: Ceratocystidaceae). Fungal Ecol 38:89–97
Li Y, Bateman CC, Skelton J, Jusino MA, Nolen ZJ, Simmons DR, Hulcr J (2017) Wood decay fungus Flavodon ambrosius (Basidiomycota: Polyporales) is widely farmed by two genera of ambrosia beetles. Fungal Biol 121:984-989
Nakase T, Jindamorakot S, Am-In S, Ninomiya S, Kawasaki H, Limtong S (2011) Candida maleeae sp. nov., a novel anamorphic yeast species in the Ambrosiozyma clade found in Thailand. J Gen Appl Microbiol 57:253–258
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics: proceedings of an international symposium, Newport, Oregon, 4–7 August 1992. CAB International, Wallingford, pp 225–233
Paulus HF (2004) Urtea graeca nov. gen. et nov. spec., der erste Vertreter der tropischen Atractocerinae in Europa sowie eine Beschreibung von Hymaloxylon aspoecki nov. spec. aus Yunnan (China) (Coleoptera, Cucujiformia, Lymexylidae, Atractocerinae nov. status). Denisia 13:277–290
Smith MTh (2011) Ambrosiozyma van der Walt (1972). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts, a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 311–317
Suh S-O, Marshall CJ, McHugh JV, Blackwell M (2003) Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol Ecol 12:3137–3145
Suh S-O, McHugh JV, Pollock DD, Blackwell M (2005) The beetle gut: a hyperdiverse source of novel yeasts. Mycol Res 109:261–265
Tanahashi M, Kubota K, Matsushita N, Togashi K (2010) Discovery of mycangia and the associated xylose-fermenting yeasts in stag beetles (Coleoptera: Lucanidae). Naturwissenschaften 97:311–317
Wheeler QD (1986) Revision of the genera of Lymexylidae (Coleoptera: Cucujiformia). Bull Am Mus Nat Hist 183:113–210
Yamamoto S (2019) Fossil evidence of elytra reduction in ship-timber beetles. Sci Rep 9:4938
Acknowledgments
I wish to thank Motoyuki Shimizu for helpful information on the carbon assimilation test and Hiroshi Ikeda for DNA experiments. I also thank two anonymous reviewers for their useful comments on this manuscript.
Funding
This study was partly supported by a KAKENHI Grant (18 K14473) from the Japan Society for the Promotion of Science (JSPS) and the Institute for Fermentation, Osaka (G-2018-1-034).
Author information
Authors and Affiliations
Contributions
The author contributed to the study conception and design, material preparation, data collection and analysis, and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toki, W. A single case study of mycetangia-associated fungi and their abilities to assimilate wood-associated carbon sources in the ship timber beetle Elateroides flabellicornis (Coleoptera: Lymexylidae) in Japan. Symbiosis 83, 173–181 (2021). https://doi.org/10.1007/s13199-021-00745-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-021-00745-9