Abstract
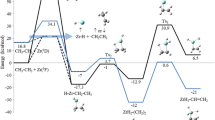
The two-state reaction mechanism of CpCo(C4H4) with isocyanate on the triplet and singlet potential energy surfaces has been investigated at the B3LYP level. A study is described for the computation of spin–orbit coupling of triplet state of the minimal energy crossing point (CP) with their singlet states and of the zero-field splitting (ZFS) parameters of the triplet states, including the full one- and two-electron terms of the Breit–Pauli Hamiltonian. There are two key crossing points along this two-state reaction pathway. The first crossing point—CP2 exists near 1B. The reacting system will change its spin multiplicity from the triplet state to the singlet state near this crossing region. Although the spin–orbit coupling interaction and ZFS D-tensor of the CP2 region are very strong, the reaction system will occur the reverse intersystem crossing from T1 to S0. Therefore, its spin-flip efficiency may be lower. The second crossing point, CP3 will again change its spin multiplicity from the singlet state to the triplet state in the Co–Cγ bond activation pathway, leading to a decrease in the barrier height of 1TS(CF) from 19.5 to 9.5 kcal/mol (1 cal = 4.182 J), and the efficiency of intersystem crossing from S0 to T1 is high because the larger spin–orbit coupling (SOC) matrix elements will result in the overpopulations of the three sublevels of T1 (3.30 × 10−1, 3.32 × 10−1, and 3.38 × 10−1, respectively).









Similar content being viewed by others
References
Braunstein P, Nobel D (1989) Transition-metal-mediated reactions of organic isocyanates. Chem Rev 89:1927–1945
Dazinger G, Schmid R, Kirchner K (2004) Mechanism of the ruthenium- catalyzed formation of pyrane-2-one and their sulfur and selen analogs from acetylene and CX2 (X = O, S, Se): a theoretical study. New J Chem 28:153–155
Xie Y, Geng LN, Qu F et al (2009) Preparation and characterization of 3-(triethoxysilyl) propyl isocyanate self-assembled monolayer on surface of chip. Chin Sci Bull 54:738–742
Wang QN, Yao ZG, Xu F et al (2012) Efficient synthesis of functionalized 2-pyridones by ytterbium chloride catalyzed tandem condensation. Chin Sci Bull 57:1612–1615
Hong P, Yamazaki H (1977) Synthesis of 2-oxo-1,2-didydropridines by the reaction of cobaltacylopentadiene complexes with isocyanates. Synthesis 1:51–52
Wakatsuki Y, Yamazaki H (1976) Cobaltocene catalyzed synthesis of pyridines. Synthesis 1:26–27
Dahy AA, Yamada K, Koga N (2009) Theoretical study on the reaction mechanism for the formation of 2-methylpyridine cobalt(i) complex from cobaltacyclopentadiene and acetonitrile. Organometallics 28:3636–3649
Wang Y, Kumar D, Yang CL et al (2007) Theoretical study of N-demethylation of substituted N, N-dimethylanilines by cytochrome P450: the mechanistic significance of kinetic isotope effect profiles. J Phys Chem B 111:7700–7710
Fiedler A, Schroder D, Shaik S et al (1994) Electronic structures and gas phase reactivities of cationic late-transition-metal oxides. J Am Chem Soc 116:10734–10741
Lü LL, Wang XF, Zhu YC et al (2012) Theoretical study of spin-orbit coupling and intersystem crossing in the two-state reaction between Nb(NH2)3 and N2O. Sci China Chem 55:158–166
Lü LL, Wang YC, Geng ZY et al (2009) Activation of C2 H6 by gas-phase Ta+: potential energy surfaces, spin–orbit coupling, spin-inversion probabilities, and reaction mechanisms. Organometallics 28:6160–6170
Lü LL, Wang XF, Wang YC et al (2008) A theoretical study of the proton transfer proton transfer process in the spin-forbidden reaction 1HNO + OH−1→3NO−+H2O. Chin Sci Bull 53:1489–1496
Lü LL, Wang YC, Wang Q et al (2010) Why is Pt4 + the least efficient cationic cluster in activating the C–H Bond in Methane? Two-state reaction computational investigation. J Phys Chem C 114:17610–17620
Schroder D, Shaik S, Schwarz H (2000) Two-state reactivity as a new concept in organometallic chemistry. Acc Chem Res 33:139–145
Hirao H, Kumar D, Que L et al (2006) Two-state reactivity in alkane hydroxylation by non-heme iron-oxo complexes. J Am Chem Soc 128:8590–8606
Dalton DM, Oberg KM, Yu RT et al (2009) Enantioselective rhodium-catalyzed [2+2+2] cycloadditions of terminal alkynes and alkenyl isocyanates: mechanistic insights lead to a unified model that rationalizes product selectivity. J Am Chem Soc 131:15717–15728
Agenet N, Gandon V, Vollhardt KPC et al (2007) Cobalt-catalyzed cyclotrimerization of alkynes: the answer to the puzzle of parallel reaction pathways. J Am Chem Soc 129:8860–8871
Hong FE, Weng CM (2011) DFT studies on the reaction of CpCo(PPh3)2 with diphenylphosphinoalkynes: formation of cobaltacycles and cyclobutadiene-substituted CpCoCb diphosphines. Organometallics 30:3740–3748
Harvey JN, Poli R, Smith KM (2003) Spin forbidden chemical reactions of transition metal compounds. New ideas and new computational challenges. Chem Soc Rev 32:1–8
Frisch MJ (2003) Gaussian 03, Revision-E.01. Gaussian, Pittsburgh
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–733
Yoshizawa K, Shiota Y, Yamabe T (1999) Intrinsic reaction coordinate analysis of the conversion of methane to methanol by an iron-oxo species: a study of crossing seams of potential energy surfaces. J Chem Phys 111:538–544
Harvey JN, Aschi M, Schwarz H et al (1998) The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor Chem Acc 99:95–99
Hess BA, Marian CM, Wahlgren U et al (1996) A mean-field spin-orbit method applicable to correlated wavefunctions. Chem Phys Lett 251:365–371
Neese F (2006) Importance of direct spin–spin coupling and spin-flip excitations for the zero-field splittings of transition metal complexes: a case study. J Am Chem Soc 128:10213–10222
Lü LL, Zhu YC, Wang XF et al (2013) Spin-orbit coupling and zero-field splitting of the high-spin ferric enzyme-substrate complex: protocatechuate 3,4-dioxygenase complexed with 3,4-dihydroxyphenylacetate. Chin Sci Bull 58:627–633
Neese F (2010) ORCA—An ab initio, density functional and semiempirical program package, Version 2.8. Max-Planck Institute for Bioinorganic Chemistry, Mülheim an der Ruhr
Harvey JN (2007) Understanding the kinetics of spin-forbidden chemical reaction. Phys Chem Chem Phys 9:331–343
Koseki S, Fedorov DG, Schmidt MW et al (2001) Spin-orbit splittings in the third-row transition elements: comparison of effective nuclear charge and full breit–pauli calculations. J Phys Chem A 105:8262–8268
Isobe H, Yamanaka S, Kuramitsu S et al (2008) Regulation mechanism of spin-orbit coupling in charge-transfer-induced luminescence of imidazopyrazinone derivatives. J Am Chem Soc 130:132–149
Havlas Z, Downing JW, Michl J (1998) Spin–orbit coupling in biradicals. 2. Ab initio methodology and application to 1,1-biradicals: carbene and silylene. J Phys Chem A 102:5681–5692
Schäfer A, Horn H, Ahlrichs R (1992) Fully optimized contracted gaussian basis sets for atoms Li to Kr. J Chem Phys 97:2571–2577
Neese F, Solomon EI (1998) Calculation of zero-field splittings, g-values, and the relativistic nephelauxetic effect in transition metal complexes. Application to high-spin ferric complexes. Inorg Chem 37:6568–6582
Levanon H, Norris JR (1978) The photoexcited triplet state and photosynthesis. Chem Rev 78:185
Rozenshtein V, Berg A, Stavitski E et al (2005) Electron spin polarization of functionalized fullerences. Reversed quartet mechanism. J Phys Chem A 109:11144
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21263022), University Research Fund of Gansu Province Financial Department, and “QingLan” Talent Engineering Funds of Tianshui Normal University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lü, L., Wang, X., Zhu, Y. et al. Theoretical study of spin–orbit coupling and zero-field splitting in the spin-forbidden two-state reaction between cobaltacyclopentadiene and isocyanate. Chin. Sci. Bull. 59, 286–296 (2014). https://doi.org/10.1007/s11434-013-0024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-013-0024-5