Abstract
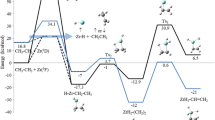
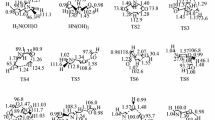
The dynamics and kinetics of the reaction of CH3+ cation with atomic oxygen in its lowest triplet and singlet states in the presence of N2 molecules as the bath gas is theoretically investigated. The potential energies over both surfaces at the CCSD(T)/aug-cc-pVTZ level of theory are explored. Both singlet and triplet surfaces characterize by barrierless initiation step. The entrance channel to form the energized adducts are governed by the capture probability once the centrifugal barrier is surmounted. Multiwell-multichannel mechanisms are found for both singlet and triplet potential energy surfaces. One-dimensional chemical master equation is solved to explore the dynamics and kinetics of the title reaction. The fractional populations of the stationary points as function of time are analyzed to determine the role of the energized intermediates on the dynamics of the title reaction. No significant temperature or pressure dependence for the title reaction was observed over a wide range of temperature (300–3000 K) and pressure (0.1–4.5 atm). The results indicate cis and trans-HCOH+, CH2O+, and H atom are the major products for both surfaces. CVT method was used to explore the importance of tunneling in hydrogen transfer isomerization reactions of CH2O+ to trans-HCOH+ and HOC+ to HCO+ using small-curvature approximation.
















Similar content being viewed by others
References
T. Wesley, Huntress, Jr., Laboratory studies of bimolecular reactions of positive ions in interstellar clouds, in comets, and in planetary atmospheres of reducing composition. Astrophys. J. Suppl. Ser. 33, 495–514 (1977)
M. Oppenheimer, J. Ap. 196, 251 (1975)
V.G. Anicich, W.T. Huntress, Jr., A survey of bimolecular ion-molecule reactions for use in modeling the chemistry of planetary atmospheres, cometary comae, and interstellar clouds. Astrophys. J. Suppl. Ser. 62, 553–672 (1986)
J.L. Beauchamp, Ann. Rev. Phys. Chem. 22, 527 (1971)
D.K. Bohme, Chemical ionization in flames, in Kinetics of Ion-Molecule Reactions NATO Advanced Study Institutes Series (Series B: Physics), ed. by P. Ausloos, vol 40 (Springer, Boston, MA, 1979)
V.G. Anicich, W.T. Huntress, J.H. Futrell, Chem. Phys. Lett. 40, 233 (1976)
S.E. Buttrill, J.K. Kim, W.T. Huntress, P. LeBreton, A. Williamson, J. Chem. Phys. 61, 2122 (1974)
L.A. Capone, R.C. Whitten, J. Dubach, S.S. Prasad, W.T. Huntress, Icarus 28, 367 (1976)
A. Dalgarno, J.H. Black, Rept. Progr. Phys. 39, 573 (1976)
A. Dalgarno, R.A. McCray, J. Ap. 181, 95 (1973)
F.C. Fehsenfeld, D.B. Dunkin, E.E. Ferguson, D.L. Albritton, Ap. J. (Lett.) 183, L25 (1973)
D. Schroder, H. Schwarz, Gas-phase activation of methane by ligated transition-metal cations. PNAS 105, 18114–18119 (2008)
Y. Tong, J.H. Lunsford, Mechanistic and kinetic studies of the reactions of gas-phase methyl radicals with metal oxides. J. Am. Chem. Soc. 113, 4741–4746 (1991)
X. Zhao, G.K. Koyanagi, D.K. Bohme, Reactions of methyl fluoride with atomic transition-metal and main-group cations: gas-phase room-temperature kinetics and periodicities in reactivity. J. Phys. Chem. A 110, 10607–10618 (2006)
J.C. Védrine, Heterogeneous catalysis on metal oxides. Catalysts 7, 341 (2017)
A.A. Viggiano, R.A. Morris, J.F. Paulson, E. Ferguson, Deuterated methyl cation reactions with atomic oxygen. Chem. Phys. Lett. 148, 296–298 (1988)
E.E. Ferguson Atomic data nuclear data tables, ed. by P. Ausloos. Proc. First NATO Conference on lon-Molecule Interactions, Biarritz, France, vol 12, 24 June–6 July 1973
E. Herbst, D.K. Bohme, J.D. Payzant, H. Schiff, I. Ap. J. 201, 603 (1975)
F.C. Fehsenfeld, Ion reactions with atomic oxygen and atomic nitrogen of astrophysical importance. Astrophys. J. 209, 638–639 (1976)
A. Dalgarno, T. DeJong, M. Oppenheimer, J.H. Black, Ap. J. (Lett.), 192, L37 (1974)
V.G.J. Anicich, Phys. Chem. Ref. Data 22, 1469 (1993)
E. Herbst, Annu. Rev. Phys. Chem. 46, 27 (1995)
J.M.C. Rawlings, S.D. Taylor, D.A. Williams, MNRAS 313, 461 (2000)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb et al., Gaussian 09Wrevision A. 02 (Gaussian Inc, Wallingford, 2009)
T.J. Lee, P.R. Taylor, A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem 23, 199–207 (1989)
I.M. Alecu, D.G. Truhlar, Computational study of the reactions of methanol with the hydroperoxyl and methyl Radicals. 1. Accurate thermochemistry and barrier heights. J. Phys. Chem. A 115, 2811–2829 (2011)
Active Thermochemical Tables. http://atct.anl.gov/. Accessed 20 Oct 2018
J.C. Rienstra-Kiracofe, W.D. Allen, H.F. Schaefer, The C2H5 + O2 reaction mechanism: high-level ab initio characterizations. J. Phys. Chem. A 104, 9823 (2000)
J. Peiro-Garcia, I. Nebot-Gil, Ab initio study on the mechanism of the atmospheric reaction OH + O3→HO2 + O2. Chem. Phys. Chem 4, 843–847 (2003)
J. Peiro-Garcia, I. Nebot-Gil, Ab Initio study of the mechanism of the atmospheric reaction: NO2 + O3→ NO3 + O2. J. Comput. Chem. 24, 1657–1663 (2003)
M. Martinez-Avila, J. Peiro-Garcia, V.M. Ramírez-Ramírez, I. Nebot-Gil. Ab initio study on the mechanism of the HCO + O2→HO2 + co reaction. Chem. Phys. Lett. 370, 313–318 (2003)
N. Lambert, N. Kaltsoyannis, S.D. Price, J. Zabka, Z. Herman, Bond-forming reactions of dications with molecules: a computational and experimental study of the mechanisms for the formation of HCF2 + from CF2 + and H2. J. Phys. Chem. A 110, 2898–2905 (2006)
S.R. Miller, N.E. Schultz, D.G. Truhlar, D.G. Leopold, A study of the ground and excited states of Al3 and Al3–. II. Computational analysis of the 488 nm anion photoelectron pectrumand a reconsideration of the Al3 bond dissociation energy. J. Chem. Phys. 130, 024304–024326 (2009)
S.H. Robertson, M.J. Pilling, L.C. Jitariu, I.H. Hillier, Master equation methods for multiple well systems: application to the 1-, 2-pentyl system. Phys. Chem. Chem. Phys. 9, 4085–4097 (2007)
S.H. Robertson, D.R. Glowacki, C.-H. Liang, C. Morley, R. Shannon, M. Blitz, P.W. Seakins, M.J. Pilling, MESMER (Master Equation Solver for Multi-Energy Well Reactions), an object oriented C + + program implementing master equation methods for gas phase reactions with arbitrary multiple wells 2008–2013. http://sourceforge.net/projects/mesmer. Accessed 26 Nov 2018
D.R. Glowacki, C.-H. Liang, C. Morley, M.J. Pilling, S.H. Robertson, MESMER: an open-source master equation solver for multi-energy well reactions. J. Phys. Chem. A 116, 9545–9560 (2012)
R.J. Shannon, R.L. Caravan, M.A. Blitz, D.E. Heard, A combined experimental and theoretical study of reactions between the hydroxyl radical and oxygenated hydrocarbons relevant to astrochemical environments. Phys. Chem. Chem. Phys. 16, 3466–3478 (2014)
J.A. Miller, S.J. Klippenstein, Master equation methods in gas phase chemical kinetics. J. Phys. Chem. A 110, 10528–10544 (2006)
J.T. Bartis, B. Widom, Stochastic models of the inter conversion of three or more chemical species. J. Chem. Phys. 60, 3474–3482 (1974)
R.G. Gilbert, S.C. Smith. Theory of Unimolecular and Recombination Reactions (Blackwell Scientific, Oxford, 1990)
P.J. Robinson, K.A. Holbrook. Unimolecular reactions. (Wiley-Interscience, New York, 1972)
W. Forst, Theory of Unimolecular Reactions (Elsevier, Amsterdam, 2012)
T. Baer, W.L. Hase, Unimolecular reaction dynamics: theory and experiments (Oxford University Press, Oxford, 1996)
J.W. Davies, N.J.B. Green, M.J. Pilling. The testing of models for unimolecular decomposition via inverse laplace transformation of experimental recombination data. Chem. Phys. Lett. 126, 373–379 (1986)
L.A. Curtiss, L.D. Kock, Energies of CH2OH, CH3O, and related compounds. J. Chem. Phys. 95, 4040–4043 (1991)
P.C. Burgers, A.A. Mommers, J.L. Holmes, Ionized, Oxycarbenes, [COH]+, [HCOH]+,[C(OH),]+, [HCO2]+, and [COOH]+, their generation, identification, heat of formation, and dissociation characteristics. J. Am. Chem. Soc. 105, 5977–5979 (1983)
J.C. Corchado, Y.Y. Chuang, E.L. Coitino, D.G. Truhlar, GAUSSRATE, version 9.1/P9.1-G03/G98/G94; Department of Chemistry and Supercomputer Institute (University of Minnesota, Minneapolis, 2003)
D. Lu, T.N. Truong, V.S. Melissas, G.C. Lynch, Y.-P. Liu, B.C. Garrett, R. Steckler, A.D. Isaacson, S.N. Rai, G. Hancock, J.G. Lauderdale, T. Joseph, D.G. Truhlar, POLYRATE 4: a new version of a computer program for the calculation of chemical reaction rates for polyatomics. Comp. Phys. Comm. 71, 235 (1992)
J.A. Miller, Faraday discuss, combustion chemistry: elementary reactions to macroscopic processes. R. Soc. Chem. 119, 461–475 (2001)
J.R. Barker, R.E. Weston, J. Phys. Chem. A 114, 10619–10633 (2010)
K.L. Gannon, M.A. Blitz, C.H. Liang, M.J. Pilling, P.W. Seakins, D.R. Glowacki, J. Phys. Chem. A 114, 9413–9424 (2010)
Acknowledgements
The financial support from the Research Council of Shiraz University is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13738_2018_1558_MOESM1_ESM.docx
In Supporting Information file the IRC graphs for the 15 corresponding reactions are included. The variation of vibrations along the reaction coordinate and also corresponding MEPs and Ground-state vibrationally adiabatic potentials VGa(s) for reactions R7, R9, and R11 are included. (DOCX 459 KB)
Rights and permissions
About this article
Cite this article
Mousavi, S., Mousavipour, S.H. A theoretical study on the dynamics of gas-phase reaction of methyl cation with atomic oxygen. J IRAN CHEM SOC 16, 807–825 (2019). https://doi.org/10.1007/s13738-018-1558-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1558-x