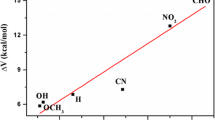
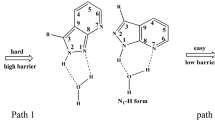
Abstract
The spin-forbidden reaction 1HNO(1A′)+OH−→3NO−(3Σ−)+H2O has been extensively explored using various CASSCF active spaces with MP2 corrections in several basis sets. Natural bond orbital (NBO) analysis, together with the NBO energetic (deletion) analysis, indicates that the two isomers have nearly equal total energy and could compete with each other in the title reaction. More significantly, the singlet/triplet surface crossing regions have been examined and the spin-orbit coupling (SOC) and energetics have been computed. The computational results indicate that the SOC is very large at the crossing point T1/S0 trans (ca. 40.9 cm−1). Moreover, the T1/S0 trans has a low energy of 10.67 kcal/mol relative to that of trans-S0. Therefore, the surface crossing to the triplet state seems much more efficient at the T1/S0 trans region along the minimum energy path (MEP), However, The values of single (P 1 ISC) and double (P 2 ISC) passes estimated at T1/S0 trans show that the ISC occurs with a little probability.
Similar content being viewed by others
References
Wong P Y, Hyun S J, Fukuto J M, et al. Reaction between S-nitrosothiols and thiols: Generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry, 1998, 37(16): 5362–5371
Adak S Q, Wang D J. Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. J Biol Chem, 2000, 275(10): 33554–33561
Miranda K M, Espey M G. Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem, 2001, 276(2): 1720–1727
Kirsch M, de Grooi H. Formation of peroxynitrite from reaction of nitroxyl anion with molecular oxygen. J. Biol Chem, 2002, 277(4): 13379–13388
Miranda M, Dutton A S, Ridnour L A, et al. Mechanism of aerobic decomposition of angeli’s salt (sodium trioxodinitrate) at physiological pH. J Am Chem Soc, 2005, 127(2): 722–731
Liochev S I, Fridovich I. Nitroxyl (NO−): A substrate for superoxide dismutase. Arch Biochem Biophys, 2002, 402(2): 166–171
Shafirovich V, Lymar S V. Spin-forbidden deprotonation of aqueous nitroxyl (HNO). J Am Chem Soc, 2003, 125(21): 6547–6552
Armentrout P B. Chemistry of excited electronic states. Science, 1991, 251(11): 175–179
Yarkony D R. Current issues in nonadiabatic chemistry. J Phys Chem, 1996, 100(48): 18612–18628
Schröder D, Schwarz H, Shaik S. In: Meunier B, ed. Metal-oxo and Metal-peroxo Species in Catalytic Oxidations. Berlin: Springer Verlag, 2000. 91, 123
Glendening E D, Badenhoop J K, Reed A E, et al. NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison, 2001
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 03 (Revision-B.01). Pittsburgh PA: Gaussian Inc., 2003
Bearpark M J, Mebel M A. A direct method for the location of the lowest energy point on a potential surface crossing. Chem Phys Lett, 1994, 223(3): 269–274
Ragazos I N, Robb M A, Bernardi M, et al. Optimization and characterization of the lowest energy point on a conical intersection using an MC-SCF Lagrangian. Chem Phys Lett, 1992, 197(3): 217–223
Wilsey S, Bernardi F, Olivucci M, et al. The thermal decomposition of 1,2-dioxetane revisited. J Phys Chem A, 1999, 103(11): 1669–1677
Celani P, Robb M A, Garavelli M, et al. Relaxation paths from a conical intersection: The mechanism of product formation in the cyclohexadiene/hexatriene photochemical interconversion. J Phys Chem A, 2002, 101(11): 2023–2032
Bader R F W. A quantum theory of molecular structure and its applications. Chem Rev, 1991, 91(5): 893–928
Koseki S, Schmidt M W, Gordon M S. MCSCF/6-3 1 G(d.p) calculations of one-electron spin-orbit coupllng constants in diatomic molecules. J Phys Chem, 1992, 96(26): 10768–10772.
Koseki S, Schmidt M W, Gordon M S. Main group effective nuclear charges for spin-orbit calculations. J Phys Chem, 1995, 99(34): 12764–12772
Merchan M, Robb M A, Biancafort L. Triplet-state formation along the ultrafast decay of excited singlet cytosine. J Am Chem Soc, 2005, 127(6): 1820–1825
Danovich D, Shaik S. Spin-orbit coupling in the oxidative activation of H-H by FeO+: Selection rules and reactivity effects. J Am Chem Soc, 1997, 119(7): 1773–1786
Rue C, Armentrout P B. Kinetic-energy dependence of competitive spin-allowed and spin-forbidden reactions: V++CS2. J Chem Phys, 1999, 110(16): 7858–7870
Reed A L, Curtiss A, Weinhold F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev, 1986, 88(6): 899–926
Kovacs A, Szabo A, Nemcsok D, et al. Blue-shifting C-H···X (X = O, Halogen) hydrogen bonds in the dimers of formaldehyde derivatives. J Phys Chem A, 2002, 10(23): 5671–5678
Sosa G L, Peruchena N M, Contreras R H, et al. Topological and NBO analysis of hydrogen bonding interactions involving C-H···O bonds. J Mol Struct (Theochem), 2002, 577(3): 219–228
Turro N J. Modern Molecular Photochemistry. Sausalito, CA: University Science Books, 1991
Reguero M M, Olivucci F, Bernandi M A, et al. Excited-state potential surface crossings in acrolein: A model for understanding the photochemistry and photophysics of a,p-enones. J Am Chem Soc, 1994, 116(5): 2103–2114
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Natural Science Education Foundation of Gansu Province, China (Grant No. 021-22)
About this article
Cite this article
Lü, L., Wang, X., Wang, Y. et al. A theoretical study of the proton transfer process in the spin-forbidden reaction 1HNO(1A′) + OH−→3NO−(3Σ−) + H2O. Chin. Sci. Bull. 53, 1489–1496 (2008). https://doi.org/10.1007/s11434-008-0094-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-008-0094-y