Abstract
Purpose
Biological nitrogen (N) fixation (BNF) of understory acacia species presents a potential way for effectively restoring N in forest systems. This study aimed to quantify the impact of acacia species and biochar application rates on BNF and soil mineral N in a suburban native forest of subtropical Australia in the first 4–5 years after prescribed burning.
Method
Plant growth values and BNF were measured to assess the impact of biochar rates at 0, 5, and 10 t ha−1 on different acacia species. Soil NH4+-N and NO3−-N along with their N isotope composition (δ15N) were determined to investigate soil–plant interactions in response to acacia species and biochar application.
Results
The application of 10 t ha−1 biochar significantly enhanced the growth of acacia species, and concurrently reduced the loss of NO3−-N at soil depths of 0–5 and 5–10 cm. Compared with Acacia disparimma (percentage of N derived from the atmosphere or %Ndfa: 78.2%), A. leiocalyx demonstrated significant higher BNF ability (%Ndfa: 91.3%). Similarly, A. leiocalyx had better growth, in terms of height (269.1 cm versus 179.6 cm), diameter at ground level (2.62 cm versus 1.94 cm), basal area (6.49 cm2 versus 3.43 cm2) and volume (692.2 cm3 versus 258.0 cm3). This was associated with its ability to promote organic matter mineralization, resulting in the accumulation of 15N-depleted NH4+-N. NH4+-N, acting as a substrate, was transformed into NO3−-N through nitrification. From regression analysis, the efficient absorption of NH4+-N by A. leiocalyx significantly mitigated NH4+-N leaching with increasing soil moisture concentration (SMC), resulting in lower δ15N of NH4+-N, which was more negatively related to SMC (R2 = 0.401), compared to that of A. disparimma (R2 = 0.250) at soil depth of 0–5 cm. The production of NO3−-N was reduced, leading to lower NO3−-N concentrations of A. leiocalyx than A. disparimma at soil depth of 0–5 cm (8.06 µg N g−1 versus 9.61 µg N g−1) and that of 5–10 cm (8.24 µg N g−1 versus 9.21 µg N g−1) respectively.
Conclusions
As an effective soil amendment, biochar exhibited promise in reducing mineral N loss and stimulating plant growth in long-term applications of exceeding three years. Higher BNF capacity and greater plant growth were observed with A. leiocalyx, compared with those of A. disparimma. The retention and utilisation of mineral N by A. leiocalyx can be considered as strategy to restore forest soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, climate factors and land management have highly affected soil labile carbon (C) and nitrogen (N) pools (Wang et al. 2019). It has been reported that precipitation affects soil organic matter decomposition and N turnover processes by changing soil moisture content (SMC) (Jackson et al. 2011; Li et al. 2022a). Both ammonia N (NH4+-N) and nitrates N (NO3−-N) are highly soluble and able to cause soil N losses via N leaching, denitrification, and even nitrous oxide (N2O) emission (Kasper et al. 2019; Taresh et al. 2021; Li et al. 2022a). Furthermore, changes in SMC also alter microbial activity and BNF (Warshan et al. 2016). In order to effectively reflect plant photosynthesis and water availability, foliar C isotope composition (δ13C) was used to indicate water use efficiency (WUE) (Huang et al. 2008; Taresh et al. 2021). Prescribed burning as a management measure to reduce wildfires in Australia forests also depletes soil C and N stocks (Reverchon et al. 2020; Yang et al. 2023).
N deposition and biological N fixation (BNF) are two crucial pathways for N input in ecosystems (Cusack et al. 2009; Bai et al. 2012, 2015a). As an effective source of N, BNF is particularly important for soil ecosystems in nature (Bai et al. 2015a, b; Reverchon et al. 2020). The process is primarily realized through a symbiotic relationship between legumes and rhizobia (Farhangi-Abriz et al. 2021a, 2022). This relationship transforms atmospheric N2 into forms directly assimilable by plants such as NH4+-N, NO3−-N, and organic N (Franche et al. 2008). These N compounds are partially released into the soil via plant root exchange (Iannetta et al. 2016; Yoseph and Shanko 2017). This process further attracts microbial growth, thereby promoting soil N mineralization (Adjesiwor and Islam 2016; Abdalla et al. 2019). Therefore, BNF is considered as a pollution-free pathway in enhancing soil fertility and supporting sustainable development.
The widespread acacia species in Australia are crucial for promoting the health of soil ecosystems by increasing litter and N2 fixation (Witt et al. 2017; Reverchon et al. 2020). The symbiosis between acacia species and rhizosphere microorganisms results in the fixation of atmospheric N2 into organisms and soil through root nodules (Farhangi-Abriz et al. 2021a, 2022). The acacia species not only promotes N cycling and maintains biodiversity but also contributes a lot to the C cycle (Seymour and Huyser 2008; Hosseini Bai et al. 2013). This contribution is closely linked to plant WUE and the N supplied by BNF for photosynthesis, potentially enhancing plant biomass accumulation (Kiers et al. 2003; Hosseini Bai et al. 2013). These species exhibit strong tolerance with environmental stress, beneficial for forest ecosystem recovery (Yang et al. 2009; Reverchon et al. 2012).
As a C-rich material, biochar addition can effectively increase the stable organic C content in soil and improves soil conditions (Blanco-Canqui 2017; Nguyen et al. 2017; Swagathnath et al. 2019; Nessa et al. 2021). This addition potentially lead to net N mineralization, thereby increasing the mineral N availability for plant uptake (Nelson et al. 2011; Wang et al. 2012; Asadyar et al. 2021). Furthermore, biochar significantly enhances the size and BNF of legumes under different climatic conditions, demonstrating its broad applicability (Macil et al. 2020; Farhangi-Abriz et al. 2021b; Das et al. 2022). Biochar with high porosity and high cation exchange capacity (CEC) has high adsorption capacity, which helps in the reduction of mineral N loss and enhance SMC and soil nutrient effectiveness (Dempster et al. 2012; Mukherjee et al. 2014; Sika and Hardie 2014; Chen et al. 2019). This includes the direct adsorption of NH4+ and highly mobile NO3− iron, extending their availability time for plant and microbial use (Mukherjee et al. 2014; Bai et al. 2015a). However, Liang et al. (2006) and Thies et al. (2015) have noted that biochar might limit the assimilation of NH4+ by microbes or plants. Consequently, the impact of biochar-soil interactions on N mineralization and the growth of leguminous plants remains limited.
N isotope composition (δ15N) is a critical indicator of N losses in both soil and plant systems, closely linked to microbial-mediated N transformations, such as N mineralization, nitrification as well as denitrification (Craine et al. 2009; Wang et al. 2015, 2020; Nessa et al. 2021). It is influenced by factors affecting microbial processes, which are soil moisture and plant species (Collins et al. 2019). Fractionation of NH4+-N and NO3−-N occurs in the absorption of plant rhizosphere, which affects the distribution of N in the plant and the isotopic differences in nitrogenous exudates (Ariz et al. 2011; Gauthier et al. 2013; Yousfi et al. 2013). However, the mechanisms of natural 15N variations in plants under complex environmental conditions is not fully understood (Tcherkez 2010; Succarie et al. 2020, 2022).
In the past, the relationship between plant BNF and soil inorganic N availability has been relatively underexplored. This study focused on the impact of understory acacia species on soil mineral N in the soil profiles with the addition of biochar. We selected Acacia leiocalyx and A. disparimma in this study to assess soil NH4+-N and NO3−-N concentrations, along with their δ15N, after 5 years of prescribed burning in the Toohey Forest. At the same time, the long-term impact of biochar application on plants and soil interactions over 3.5 years was explored.
2 Materials and methods
2.1 Study site and experiment design
This research was conducted at Toohey Forest (27°32′53"S; 153°03′21"E) in Brisbane, Southeast Queensland, Australia. Spanning an extensive area of approximately 680 hectares, this forest ecosystem is located in the subtropical climate zone, with an annual average precipitation of 1350 mm (Bai et al. 2012). Prescribed burning has been in effect in this forest since 1993 until the most recent burn at the study site in August 2017 (Reverchon et al. 2020). The study plots were established in May 2019, and field trials were conducted at Site 7 (S7). A mixed cover of understory acacia species, including Acacia leiocalyx and A. disparimma, and overstorey Eucalyptus psammitica are typical of the area.
To delineate the study plot, each plot was demarcated with an area of 4 m2 (2 m × 2 m). The trial employed a randomized complete block design with four circular blocks, each covering an area of 500 m2 containing 13 plots (Reverchon et al. 2020). These plots comprised two understory acacia species, A. leiocalyx and A. disparimma, with six individuals of each, and one E. psammitic as a reference plant. The biochar derived from pine wood (Pinus radiata) used in this study was produced with a pyrolysis temperature of 600 °C. Different rates of pine biochar, namely control 0 t ha−1, biochar 5 t ha−1, and biochar 10 t ha−1, were applied to the soil of A. leiocalyx and A. disparimma. This addition was artificially conducted on the plot surface in May 2019. E. psammitica, serving as a reference plant, received no biochar applied. According to Bruckman et al. (2015), these application rates were chosen to support sustainable development in forest systems at optimal and financially feasible levels. The physicochemical properties of biochar, including pH, total C, total N, δ13C, and δ15N, were measured, as reported by Yang et al. (2023).
2.2 Soil and foliage sample collection
After 42 months of field treatment establishment, soil samples were collected from three soil depths (0–5, 5–10 and 10–20 cm) using soil cores from various locations in each plot in November 2022. Subsequently, soil was sieved and homogenized evenly and stored at 4 °C to extract and analyze soil physicochemical properties in one week.
Foliage samples were collected and oven-dried thoroughly at 60 °C over 72 h. The dried foliage samples were then ground using the RocklabsTM ring grinder before being weighed for mass spectrometry analyses. The total C, total N and their isotope compositions (δ13C and δ15N) were evaluated following the method of Xu et al. (2000).
2.3 Measurements of foliage physicochemical properties
Approximately 6–7 mg of the foliage powder was weighed and transferred into tin capsules. Samples were analysed for their total C, total N, δ13C and δ15N using a high-precision isotope ratio mass spectrometer (IRMS, Elementar, Langenselbold, Hesse, Germany) follow procedure (Bai et al. 2015a).
δ13C and δ15N values would be determined by the following formula as reported previously (Sun et al. 2024):
where, R = the isotope ratio, Rsample = the ratio of 13C/12C and 15N/14N of sample respectively, RVPDB = the ratio of 13C/12C of the international standard (Vienna Pee Dee. Belemnite (VPDB)), Rstd = the ratio of 15N/14N of the international standard (atmospheric N2).
The percentage of N derived from atmospheric N2 (%Ndfa) was determined using the following formula (Bai et al. 2012):
where δ15Nref and δ15Nacacia are the δ15N values of the reference plants and acacia species respectively.
B value: Isotopic abundance of acacia species growing without N.
B values in previous extensive research have been reported within a range of -2.9 ‰ to 1.0 ‰ for woody species (Boddey et al. 2000; Bai et al. 2012). Various B values were assessed from -1.5 ‰ to 1.0 ‰ to confirm a suitable B value for acacia species based on this trial design. For the purpose of this study, we employed a B value of -1.5 ‰ to present the BNF results.
Freshly collected field soil was oven dried at 105 °C for 24 h to determine the soil moisture content (SMC). The SMC values were calculated using the formula below (Voroney 2019):
2.4 Measurements of soil properties
The concentration of NH4+-N and NO3−-N, along with their δ15N in soil samples, were determined by microdiffusion technique (Stark and Hart 1996). Fresh soil samples (about 8 g dry weight) and 2 M KCl (40 ml) solution were mixed at a ratio of 1:5 (w/w). After centrifuging the mixture, 10 ml of supernatant was extracted, and NH4+-N was released as NH3 by adding 100 µl of (NH4)2SO4 spiked solution and 0.4 g of MgO. The filter paper discs were added with 2.5 M KHSO4 to absorb the NH3 for seven days, and then dried in concentrated H2SO4 for 28 days. To the same solution, 100 µl of standard KNO3 spiking solution and 0.2 g of Devarda’s alloy were added. Filter paper discs were prepared in the same manner for NO3−-N collection.
Two batches of filter paper discs were encapsulated in tin capsules, and the 15N atom% in NH4+-N and NO3−-N was determined by mass spectrometry, respectively (Zhang et al. 2018). The NH4+-N and NO3−-N in the samples were converted into NH3 and absorbed by the filter paper discs.
2.5 Statistical analyses
The statistical analyses involved the application of a two-way analysis of variance (ANOVA) to investigate the significant impact of acacia species and biochar application rates on foliar total C, total N, δ13C, δ15N, BNF, as well as plant height, diameter at ground level (DGL), basal area (BA) and volume. These analyses were executed utilizing the statistical software SPSS 26.0 (IBM SPSS Statistics Inc., Chicago, USA). The same software was employed to perform a three-way ANOVA to evaluate the effects of different species, biochar rates, soil depths and their interactions on soil NH4+-N, NO3−-N, δ15N of NH4+-N and δ15N of NO3−-N. The threshold for statistical significance was established at α = 0.05.
Multiple regression was conducted to investigate the relationships between foliage δ13C and total N, as well as between SMC and soil mineral N using the software Origin Pro 9.0 (OriginLab, Northampton, MA, USA).
3 Results
3.1 Initial chemical and physical properties of plant samples
Significant differences were observed in foliar total C with biochar application with increases for 5 t ha−1 (49.16%) and 10 t ha−1 (49.64%) compared to the control (48.23%) (P < 0.05) (Table 1). Significantly differences in total C, δ13C and δ15N were noted between two understory acacia species. The foliar total C of A. disparimma (50.03%) was significantly higher than A. leiocalyx (48.66%) (Table 1). A. leiocalyx displayed a notably lower foliar δ13C value (-33.03 ‰) in comparison to A. disparimma (-32.31 ‰), and had a significantly lower foliar δ15N (-0.98 ‰) in contrast to A. disparimma (-0.20 ‰) (Table 1). It is worth noting that A. leiocalyx exhibited a significantly greater %Ndfa (nitrogen derived from the atmosphere) at 91.30% compare to A. disparimma at 78.22% (Table 1).
With the increase in biochar application rates, plant diameter at ground level (DGL), basal area (BA), and volume increased. With 10 t ha−1 of biochar treatment, DGL (2.97 cm), BA (7.90 cm2), and volume (835.0 cm3) exhibit the highest level of significance (P < 0.05) (Table 2). A similar trend was observed for plant height, although the increase was not statistically significant (P > 0.05) (Table 2).
Furthermore, distinct differences in growth data were observed between two acacia species. A. leiocalyx exhibited significantly greater plant height (269.1 cm), DGL (2.62 cm), BA (6.49 cm2), and volume (692.2 cm3) compared to those of A. disparimma (P < 0.05) (Table 2).
3.2 Soil mineral N and its δ15N
NH4+-N values in the 0–5 and 10–20 cm soil depth were 16.4 µg g−1 and 16.3 µg g−1, significantly higher than that in the 5–10 cm depth (14.0 ug g−1) (P < 0.05) (Table 3). Soil NO3−-N values were 8.91 µg g−1 and 8.93 µg g−1 in the 0–5 cm and 5–10 cm depth respectively, and significantly higher values were observed in the 10–20 cm depth (9.93 ug g−1) (P < 0.05) (Table 3). The δ15N of NH4+-N at the 10–20 cm soil (13.3 ‰) was significantly higher than the other two depths (P < 0.05) (Table 3). The δ15N of NO3−-N values in the 5–10 cm and 10–20 cm soil depth were 10.5 ‰ and 11.9 ‰ respectively, significantly higher than the 6.21 ‰ in the 0–5 cm layer (P < 0.05) (Table 3). With the 5 t ha−1 biochar application rate, NO3−-N (9.91 µg g−1) was significantly higher than that of 10 t ha−1 (8.66 µg g−1) (P < 0.05) (Table 3). The δ15N of NO3−-N at 10 t ha−1 was significantly lower (P < 0.05) (Table 3).
The A. leiocalyx had significantly lower NO3−-N values (8.74 µg g−1) compared to that of A. disparimma (9.58 µg g−1). The δ15N of NH4+-N values of A. leiocalyx (8.58 ‰) were significantly lower than that of A. disparimma (11.7 ‰) (P < 0.05) (Table 3). Conversely, δ15N of NO3−-N values were significantly higher for A. leiocalyx (11.9 ‰) compared to that of A. disparimma (7.94 ‰) (P < 0.05) (Table 3).
After three and half years of biochar application, in the 0–5 cm soil, soil NH4+-N with biochar rate of 5 t ha−1 (17.5 µg g−1) was higher than that with 10 t ha−1 (14.9 µg g−1) (P < 0.05) (Table 4). Soil NO3−-N was significantly higher in the soil with application rate of 5 t ha−1 (10.5 µg g−1) compared to 0 and 10 t ha−1 biochar rates (8.55 µg g−1 and 7.86 µg g−1) (P < 0.05) (Table 4). Soils without biochar application had higher δ15N of NO3−-N values than those with biochar application (0.05 < P < 0.10) (Table 4). In the 5–10 cm soil, the δ15N of NO3−-N value without biochar application were significantly higher than that of 10 t ha−1 (P < 0.05) (Table 4).
In the 0–5 cm and 5–10 cm soil depths, NO3−-N concentration of A. leiocalyx (8.06 µg g−1 and 8.24 µg g−1) were significantly lower than those of A. disparimma (9.61 µg g−1 and 9.21 µg g−1). Conversely, δ15N of NO3−-N values of A. leiocalyx (10.5 ‰ and 13.7 ‰) were significantly higher than those of A. disparimma (3.70 ‰ and 8.14 ‰) (P < 0.05) (Table 4). Furthermore, in the 0–5 cm soil, soil δ15N of NH4+-N of A. leiocalyx (7.88 ‰) was significantly lower than that of A. disparimma (11.7 ‰) (P < 0.05) (Table 4). In the 10–20 cm soil, soil NH4+-N of A. leiocalyx (14.0 ‰) was significantly lower than that of A. disparimma soil (18.6 ‰) (P < 0.05) (Table 4).
3.3 Relationships between plant physiological variables and soil properties
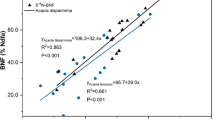
Plant foliar total N and δ13C showed stronger relationship in A. leiocalyx (R2 = 0.352, P < 0.05) than that of A. disparimma (R2 < 0.001, p = 0.986) (Fig. 1). Soil δ15N of NH4+-N was negatively related with SMC (R2 = 0.228, P = 0.001) at 0–5 cm depth (Fig. 2). Under different biochar treatments, increases in the biochar application rates led to a more significant decline in δ15N of NH4+-N with SMC at 0–5 cm depth, as indicated by the negative slopes for each regression equation (Fig. 3). At biochar application rate of 5 t ha−1, the relationship between SMC and δ15N of NH4+-N was significant, and it approached significance at 10 t ha−1 (Fig. 3). However, the effect was not significant when no biochar was applied (0 t ha−1) (Fig. 3).
A significant statistical relationship (P < 0.05) was observed between SMC and soil δ15N of NH4+-N under two acacia species at 0–5 cm depth (Fig. 4). Among them, A. leiocalyx showed a higher relationship (R2 = 0.401) compared to that of A. disparimma (R2 = 0.250) (Fig. 4). It was also noticeably observed that the values of soil δ15N of NH4+-N with A. leiocalyx were generally lower than those with A. disparimma (Fig. 4). At 5–10 cm soil depth, the concentration of NH4+-N was significantly positively related with SMC (R2 = 0.308, P = 0.002) (Fig. 5).
SMC was more closely related to soil NH4+-N concentration in A. disparimma (R2 = 0.373, P = 0.035) compared to that of A. leiocalyx (R2 = 0.228, P = 0.115) at 5–10 cm soil (Fig. 6a). Conversely, SMC was significantly positively related with soil NO3−-N concentration in A. leiocalyx, while in A. disparimma, the relationship was not significant (R2 = 0.098, P = 0.166) (Fig. 6b). At a depth of 10–20 cm, the regression for A. leiocalyx between SMC and NH4+-N concentration (R2 = 0.624, P < 0.001), as well as the regression between SMC and δ15N of NH4+-N (R2 = 0.467, P = 0.006 < 0.05), both demonstrated significant quadratic relationships (Fig. 7). With increasing SMC, both NH4+-N and δ15N of NH4+-N initially increased to a peak value, followed by a decline (Fig. 7).
4 Discussions
4.1 The effect of biochar application on acacia plants
Previous studies have noted an increase in growth in leguminous species with biochar application (Xiao et al. 2020; Farhangi-Abriz et al. 2021b). Our results showed foliar total C values and growth-related measurements increased with the amount of biochar applied (Tables 1 and 2). This supports the notion that biochar addition significantly improved rhizosphere soil conditions and nutrient availability (Schulz et al. 2013; Das et al. 2022). Biochar has been reported to enhance nodule growth and BNF levels to some extent (Güereña et al. 2015; Farhangi-Abriz et al. 2022). While, in this study, %Ndfa was not observed to be statistically significant with increasing biochar application. The reason is that biochar properties change over time, leading to the diminishing effect of enhanced BNF (Mia et al. 2017, 2018).
The BNF is a strategy for plant growth at the cost of consuming a large amount of photosynthetically accumulated C, especially under N limitation (Hosseini Bai et al. 2013). The foliar total N values measured for both species were within the range observed for acacia species (1.66–2.38N%) (Niinemets et al. 2009). A. leiocalyx exhibited significantly higher %Ndfa in this study. This is consistent with the findings of studies by Hosseini Bai et al. (2013) and Taresh et al. (2021), supporting that A. leiocalyx is a species with strong N fixation ability. N is an crucial element of C-fixing enzymes in photosynthesis (Wilson et al. 2000; Evans 2001). BNF can supply N for photosynthesis, supporting plant biomass accumulation and rapid growth (Bai et al. 2012). The lower foliar total C values and larger plant size of A. leiocalyx can therefore be attributed to more C being converted to biomass than accumulated in the foliage (Kiers et al. 2003). At the same time, high BNF leads to high photosynthesis rates, enabling faster C fixation and allocation to underground organs (such as roots and nodules) of A. leiocalyx (Bai et al. 2012). This in turn provided additional energy and C for the BNF process.
The δ13C of plant is generally regarded as an index of WUE, and higher δ13C values usually indicate higher WUE (Xu et al. 2000). Previous studies have demonstrated that A. leiocalyx exhibited higher δ13C values compared with those of A. disparimma, reflecting a better WUE and strategy under drought stress (El Amin and Luukkanen 2006; Bai et al. 2012; Hosseini Bai et al. 2013). However, in this study, it was observed that A. leiocalyx exhibited greater growth, yet its foliar δ13C value was significantly lower. It suggested that WUE was not the determining factor for plant growth in Toohey forest soil. Lower δ13C has been shown to support adequate water and N supply for the growth of A. leiocalyx (Whitehead et al. 2011). The significant regression observed between foliar δ13C and soil total N in A. leiocalyx can be attributed to the crucial role of N as a limiting resource for plant growth (Farooq et al. 2021). A. leiocalyx exhibited a high water demand to support vigorous growth and metabolic activities, and this demand was limited by soil N supply. While soil total N is not a limiting factor for A. disparimma due to its slow growth.
Previous studies indicated that this forest is located near a busy highway, experiencing prolonged high N deposition (Bai et al. 2012; Sun et al. 2024). Both acacia species had negative foliar δ15N values in this study, indicating their capacity to meet N requirements by directly absorbing deposited N from the canopy (Craine et al. 2015). Our results indicated that foliar δ15N value of A. leiocalyx is significantly more negative than those of A. disparrima. This is consistent with the study of Ma et al. (2015) in Toohey Forest and our study after applying biochar for 2 years (Sun et al. 2024). In general, plants tend to select N forms that are more readily available from the soil, thereby reducing their dependence on BNF (Regus et al. 2017). However, unexpectedly, high %Ndfa in both acacia species suggested that the available N concentration in the soil was insufficient. A possible explanation is the N volatilization loss from this forest due to prescribed burning conducted five years earlier (May and Attiwill 2003; Reverchon et al. 2011). Moreover, it has been reported that persistent heavy rainfall in the south-eastern Queensland in 2022 resulted in multiple floods (Bureau of Meteorology 2023). N leaching caused by precipitation, and nitrous oxide (N2O) emissions via denitrification further contribute to soil N depletion (Cameron et al. 2013; Di and Cameron 2016). This causes plants to take longer to recycle N into the soil system (Bai et al. 2012). The lower foliar δ15N values of A. leiocalyx suggest that it is more likely to acquire N from air, which is beneficial for soil recovery due to its higher BNF capacity.
4.2 Soil profile mineral N dynamics
N processes in the topsoil involve mineralization, nitrification, and ammonia volatilization, while in the deeper soil, processes include NO3−-N leaching, denitrification, and microbial and plant assimilation (Choi et al. 2017; Liu et al. 2021). Therefore, the significantly higher NH4+-N concentration in 0–5 cm soil come from plant litter accumulation and topsoil mineralization (Mlambo et al. 2007; Hobbie and Högberg 2012). In 10–20 cm soil, significantly higher mineral N (NH4+-N and NO3−-N) concentrations and their δ15N values were observed. These results are consistent with previous studies, with enriched 15N accumulating in the deep soil (Hobbie and Högberg 2012; Zeng and Han 2020). Soil profile N transfer and fractionation processes, including ammonia volatilization, nitrate leaching, and denitrification, result in the depletion of N-depleted mineral N in the deep soil (Hobbie and Ouimette 2009; Hobbie and Högberg 2012; Gurmesa et al. 2022). Moreover, plant uptake and microbial fixation can also increase δ15N in deep soil, especially the transfer of N-depleted N into the plant mediated by mycorrhizal fungi (Hobbie and Ouimette 2009).
4.3 Soil mineral N and regression with SMC under biochar application
N turnover can be controlled to some extent by biochar application (Reverchon et al. 2014). Biochar is capable of adsorbing soil mineral N, thereby reducing the N-leaching process (Dempster et al. 2012; Sika and Hardie 2014; Sun et al. 2017). Observations indicated that the lowest soil NH4+-N and NO3−-N concentration were under the 10 t ha−1 biochar treatment, especially significant at the 0–5 cm depth (Tables 3 and 4). While adsorbing NH4+-N, biochar reduced the production of NO3−-N and N2O from nitrification (Liang et al. 2006; Teutscherova et al. 2018). Moreover, biochar directly adsorbs NO3−-N in soil solution, which can prolong its residence time in soil (Mukherjee et al. 2014). Lower δ15N of NO3−-N values indicate that biochar can reduce nitrate N losses, especially with 10 t ha−1 addition rate.
SMC is a key factor regulating nutrient levels in plant and soil, as well as microbial activity (Farooq et al. 2021). Variations in soil moisture conditions promote N mineralization, providing substrates for nitrification and denitrification (Liu et al. 2017; Li et al. 2022b). In the topsoil (0–5 cm), δ15N of NH4+-N decreased with increasing soil moisture, due to enhanced microbial activity producing more 15N-depleted products. Under the biochar treatment, a higher application rate resulted in a faster decline of δ15N of NH4+-N. On the one hand, it benefits from the porous physical structure, which increases soil porosity highlighting the improved soil retention capacity (Zhang et al. 2008; Li et al. 2018). On the other hand, biochar enhances CEC of soil, providing more adsorption sites, thereby reducing NH4+-N leaching (Liang et al. 2006; Sun et al. 2017). Notably, the application of biochar at 10 t ha−1 appeared to be the optimal biochar addition rate to reduce NH4+-N loss and improve N utilization in the soil compared to the control and 5 t ha−1.
4.4 Effects of acacia species on soil mineral N dynamics
The difference in plant N metabolism during growth is closely related to the absorption, utilization, and transformation of N (Nunes-Nesi et al. 2010; Ohyama 2010). Among both species, A. leiocalyx significantly decreased soil NO3−-N concentration and enriched δ15N of NO3−-N, especially at 0–5 and 5–10 cm soil. This is attributed to discrimination and fractionation effects in N metabolic processes of different species (Hobbie and Ouimette 2009; Luo et al. 2013). A. leiocalyx showed a preference for assimilating more N-depleted compounds. The increase in N-enriched nitrification substrates and gas emissions during nitrification lead to soil δ15N of NO3−-N enrichment (Falxa-Raymond et al. 2012; Gauthier et al. 2013).
Legumes supply NH4+-N to the soil through BNF and promote microbial N mineralization (Jensen and Hauggaard-Nielsen 2003; Chu et al. 2004). Compared to A. disparimma, A. leiocalyx exhibited a significant reduction in δ15N of NH4+-N, especially at 0–5 cm, emphasizing more N-depleted NH4+-N input. It is noteworthy that at soil depth of 10–20 cm, A. leiocalyx showed a significantly lower NH4+-N. This suggested that A. leiocalyx was more favorable in reducing the leaching of mineral N as the soil profile deepens than A. disparimma.
4.5 Regression of soil mineral N and SMC in different acacia species
In addition to regulating the BNF rate of legumes, increased precipitation have either positive or negative impacts on controlling soil litter decomposition and N mineralization levels (Di Blasio et al. 2010). Differences in species physiology may result in varying strategies for utilizing soil water and nutrients in different soil depth (Graciano et al. 2005).
In the topsoil (0–5 cm), δ15N of NH4+-N of both acacia species decreased with increasing SMC. Bobbink et al. (2010) and Abdalla et al. (2019) indicated that when soil moisture is abundant, N2 fixation increases and enters the soil through plant root exudates exchange, resulting in a decrease in soil δ15N values. Compared with A. disparimma, fast-growing A. leiocalyx promoted soil N mineralization and BNF by rhizosphere microorganisms, resulting in a decrease in soil δ15N of NH4+-N (Jensen and Hauggaard-Nielsen 2003; Chu et al. 2004).
At the soil depth of 5–10 cm, A. disparimma soil NH4+-N concentration was related positively with SMC. This can be attributed to its lower N demands resulting in NH4+-N from soil N mineralization more responsive to SMC (Verma and Sagar 2020). A. leiocalyx soil NO3−-N concentration increased with SMC. It indicated stronger soil microbial activity under non-waterlogged conditions, promoting nitrification (Bobbink et al. 2010).
Some studies have indicated that many temperate and tropical legumes have reduced BNF under soil moisture deficiency (Reed et al. 2011; Warshan et al. 2016; Rousk and Michelsen 2017). Due to differences in root distribution, A. disparimma caused minimal disturbance in the 10–20 cm soil. While A. leiocalyx had the nonlinear relationships between SMC and NH4+-N concentration, and the dynamics of δ15N of NH4+-N also exhibited a similar trend. Legume growth and BNF are limited at low SMC (Chalk et al. 2010; Salemaa et al. 2019). SMC limited the uptake of NH4+-N by A. leiocalyx causing the NH4+-N concentration to rise with increasing SMC. At this stage, A. leiocalyx preferred to assimilate the lighter isotopic form of NH4+-N, enriching the soil with δ15N of NH4+-N. However, once SMC constraints were lifted, greater absorption by A. leiocalyx caused soil NH4+-N concentrations to decrease. At this time, there was higher BNF capacity of A. leiocalyx with more biologically fixed N into soil, which reduced soil δ15N of NH4+-N. These results emphasize that BNF requires a certain level of soil moisture and may not be achieved until meets the basic growth needs of plants.
5 Conclusions
Forest burning management and extreme rainfall have altered soil mineral N dynamics and ecosystem functions in the suburban native forests of subtropical Australia. The long-term biochar application and BNF capacity of legumes can conserve soil mineral N and moisture, thereby reducing N losses from N transformation processes and leaching. This study found that the optimal biochar application rate was 10 t ha−1, which significantly promoted plant growth and limited NO3−-N leaching. We evaluated the BNF of two acacia species and found that they still relied on this process to supply N 4–5 years after prescribed burning. A. leiocalyx, with a higher BNF capacity, surpasses A. disparimma in soil moisture retention and improving mineral N utilisation. The A. leiocalyx demonstrated a stronger potential for restoring soil N availability.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
References
Abdalla M, Hastings A, Cheng K, Yue Q, Chadwick D, Espenberg M, Truu J, Rees RM, Smith P (2019) A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Global Change Biol 25(8):2530–2543
Adjesiwor AT, Islam MA (2016) Rising nitrogen fertilizer prices and projected increase in maize ethanol production: The future of forage production and the potential of legumes in forage production systems. Grassl Sci 62(4):203–212
Ariz I, Cruz C, Moran JF, González-Moro MB, García-Olaverri C, González-Murua C, Martins-Loução MA, Aparicio-Tejo PM (2011) Depletion of the heaviest stable N isotope is associated with NH4+/NH3 toxicity in NH4+-fed plants. BMC Plant Biol 11:1–13
Asadyar L, Xu C-Y, Wallace HM, Xu Z, Reverchon F, Bai SH (2021) Soil-plant nitrogen isotope composition and nitrogen cycling after biochar applications. Environ Sci Pollut Res 28:6684–6690
Bai SH, Reverchon F, Xu C-Y, Xu Z, Blumfield TJ, Zhao H, Van Zwieten L, Wallace HM (2015a) Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol Biochem 90:232–240
Bai SH, Sun F, Xu Z, Blumfield TJ, Chen C, Wild C (2012) Appraisal of 15N enrichment and 15N natural abundance methods for estimating N2 fixation by understorey Acacia leiocalyx and A. disparimma in a native forest of subtropical Australia. J Soils Sediments 12:653–662
Bai SH, Xu Z, Blumfield TJ, Reverchon F (2015b) Human footprints in urban forests: implication of nitrogen deposition for nitrogen and carbon storage. J Soils Sediments 15:1927–1936
Blanco-Canqui H (2017) Biochar and soil physical properties. Soil Sci Soc Am J 81(4):687–711
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20(1):30–59
Boddey RM, Peoples MB, Palmer B, Dart PJ (2000) Use of the 15N natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr Cycl Agroecosyst 57:235–270
Bruckman VJ, Terada T, Uzun BB, Apaydın-Varol E, Liu J (2015) Biochar for climate change mitigation: tracing the in-situ priming effect on a forest site. Energy Procedia 76:381–387
Bureau of Meteorology (2023) Queensland in 2022: record rainfall in the south-east; warmer in the tropics, cooler days across the south. http://www.bom.gov.au/climate/current/annual/qld/archive/2022.summary.shtml. Accessed 25 Jan 2023
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162(2):145–173
Chalk PM, Alves BJ, Boddey RM, Urquiaga S (2010) Integrated effects of abiotic stresses on inoculant performance, legume growth and symbiotic dependence estimated by 15N dilution. Plant Soil 328(1–2):1–16
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Zhao Q, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7(1):1–18
Choi W-J, Kwak J-H, Lim S-S, Park H-J, Chang SX, Lee S-M, Arshad MA, Yun S-I, Kim H-Y (2017) Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric Ecosyst Environ 237:1–15
Chu GX, Shen QR, Cao J (2004) Nitrogen fixation and N transfer from peanut to rice cultivated in aerobic soil in an intercropping system and its effect on soil N fertility. Plant Soil 263:17–27
Collins AL, Burak E, Harris P, Pulley S, Cardenas L, Tang Q (2019) Field scale temporal and spatial variability of δ13C, δ15N, TC and TN soil properties: implications for sediment source tracing. Geoderma 333:108–122
Craine JM, Brookshire E, Cramer MD, Hasselquist NJ, Koba K, Marin-Spiotta E, Wang L (2015) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396:1–26
Craine JM, Elmore AJ, Aidar MP, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992
Cusack DF, Silver W, McDowell WH (2009) Biological nitrogen fixation in two tropical forests: ecosystem-level patterns and effects of nitrogen fertilization. Ecosystems 12:1299–1315
Das SK, Ghosh GK, Avasthe R (2022) Valorizing biomass to engineered biochar and its impact on soil, plant, water, and microbial dynamics: a review. Biomass Convers Biorefin 12(9):4183–4199
Dempster D, Gleeson D, Zi S, Jones D, Murphy D (2012) Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 354(1):311–324
Di Blasio L, Droetto S, Norman J, Bussolino F, Primo L (2010) Protein Kinase D1 Regulates VEGF-A-Induced αvβ3 Integrin Trafficking and Endothelial Cell Migration. Traffic 11(8):1107–1118
Di HJ, Cameron KC (2016) Inhibition of nitrification to mitigate nitrate leaching and nitrous oxide emissions in grazed grassland: a review. J Soils Sediments 16:1401–1420
El Amin YR, Luukkanen O (2006) Adaptive genetic variation in water-use efficiency and gum yield in Acacia senegal provenances grown on clay soil in the Blue Nile region. Sudan Ecol Manag 226(1–3):219–229
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6(3):121–126
Falxa-Raymond N, Patterson AE, Schuster WS, Griffin KL (2012) Oak loss increases foliar nitrogen, δ15N and growth rates of Betula lenta in a northern temperate deciduous forest. Tree Physiol 32(9):1092–1101
Farhangi-Abriz S, Ghassemi-Golezani K, Torabian S (2021a) A short-term study of soil microbial activities and soybean productivity under tillage systems with low soil organic matter. Appl Soil Ecol 168:104122
Farhangi-Abriz S, Ghassemi-Golezani K, Torabian S, Qin R (2022) A meta-analysis to estimate the potential of biochar in improving nitrogen fixation and plant biomass of legumes. Biomass Convers Biorefin 1–11
Farhangi-Abriz S, Torabian S, Qin R, Noulas C, Lu Y, Gao S (2021b) Biochar effects on yield of cereal and legume crops using meta-analysis. Sci Total Environ 775:145869
Farooq TH, Chen X, Shakoor A, Li Y, Wang J, Rashid MHU, Kumar U, Yan W (2021) Unraveling the influence of land-use change on δ13C, δ15N, and soil nutritional status in coniferous, broadleaved, and mixed forests in southern china: a field investigation. Plants 10(8):1499
Franche C, Lindström K, Elmerich C (2008) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321(1–2):35–59
Gauthier PP, Lamothe M, Mahé A, Molero G, Nogués S, Hodges M, Tcherkez G (2013) Metabolic origin of δ15N values in nitrogenous compounds from Brassica napus L. leaves. Plant Cell Environ 36(1):128–137
Graciano C, Guiamét JJ, Goya JF (2005) Impact of nitrogen and phosphorus fertilization on drought responses in Eucalyptus grandis seedlings. For Ecol Manag 212(1–3):40–49
Güereña DT, Lehmann J, Thies JE, Enders A, Karanja N, Neufeldt H (2015) Partitioning the contributions of biochar properties to enhanced biological nitrogen fixation in common bean (Phaseolus vulgaris). Biol Fertil Soils 51:479–491
Gurmesa GA, Hobbie EA, Zhang S, Wang A, Zhu F, Zhu W, Koba K, Yoh M, Wang C, Zhang Q (2022) Natural 15N abundance of ammonium and nitrate in soil profiles: New insights into forest ecosystem nitrogen saturation. Ecosphere 13(3):e3998
Hobbie EA, Högberg P (2012) Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol 196(2):367–382
Hobbie EA, Ouimette AP (2009) Controls of nitrogen isotope patterns in soil profiles. Biogeochemistry 95:355–371
Hosseini Bai S, Sun F, Xu Z, Blumfield TJ (2013) Ecophysiological status of different growth stage of understorey Acacia leiocalyx and Acacia disparrima in an Australian dry sclerophyll forest subjected to prescribed burning. J Soils Sediments 13:1378–1385
Huang Z, Xu Z, Blumfield TJ, Bubb K (2008) Variations in relative stomatal and biochemical limitations to photosynthesis in a young blackbutt (Eucalyptus pilularis) plantation subjected to different weed control regimes. Tree Physiol 28(7):997–1005
Iannetta PP, Young M, Bachinger J, Bergkvist G, Doltra J, Lopez-Bellido RJ, Monti M, Pappa VA, Reckling M, Topp CF (2016) A comparative nitrogen balance and productivity analysis of legume and non-legume supported cropping systems: the potential role of biological nitrogen fixation. Front Plant Sci 7:1700
Jackson BG, Martin P, Nilsson MC, Wardle DA (2011) Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 120(4):570–581
Jensen ES, Hauggaard-Nielsen H (2003) How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 252:177–186
Kasper M, Foldal C, Kitzler B, Haas E, Strauss P, Eder A, Zechmeister-Boltenstern S, Amon B (2019) N 2 O emissions and NO 3− leaching from two contrasting regions in Austria and influence of soil, crops and climate: a modelling approach. Nutr Cycl Agroecosyst 113:95–111
Kiers ET, Rousseau RA, West SA, Denison RF (2003) Host sanctions and the legume–rhizobium mutualism. Nature 425(6953):78–81
Li S, Zhang Y, Yan W, Shangguan Z (2018) Effect of biochar application method on nitrogen leaching and hydraulic conductivity in a silty clay soil. Soil Tillage Res 183:100–108
Li L, Hao Y, Zheng Z, Wang W, Biederman JA, Wang Y, Wen F, Qian R, Xu C, Zhang B (2022a) Heavy rainfall in peak growing season had larger effects on soil nitrogen flux and pool than in the late season in a semiarid grassland. Agric Ecosyst Environ 326:107785
Li Z, Wang S, Nie X, Sun Y, Ran F (2022b) The application and potential non-conservatism of stable isotopes in organic matter source tracing. Sci Total Environ 155946
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70(5):1719–1730
Liu M, Han G, Li X (2021) Using stable nitrogen isotope to indicate soil nitrogen dynamics under agricultural soil erosion in the Mun River basin. Northeast Thailand Ecol Indic 128:107814
Liu W, Li L, Biederman J, Hao Y, Zhang H, Kang X, Cui X, Wang Y, Li M, Xu Z (2017) Repackaging precipitation into fewer, larger storms reduces ecosystem exchanges of CO2 and H2O in a semiarid steppe. Agric for Meteorol 247:356–364
Luo J, Li H, Liu T, Polle A, Peng C, Luo Z-B (2013) Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J Exp Bot 64(14):4207–4224
Ma L, Rao X, Lu P, Bai SH, Xu Z, Chen X, Blumfield T, Xie J (2015) Ecophysiological and foliar nitrogen concentration responses of understorey Acacia spp. and Eucalyptus sp. to prescribed burning. Environ Sci Pollut Res 22:10254–10262
Macil PJ, Ogola JB, Odhiambo JJ (2020) Response of soil pH and nodulation of three chickpea genotypes to biochar and rhizobium inoculation. Commun Soil Sci Plant Anal 51(18):2377–2387
May B, Attiwill P (2003) Nitrogen-fixation by Acacia dealbata and changes in soil properties 5 years after mechanical disturbance or slash-burning following timber harvest. For Ecol Manag 181(3):339–355
Mia S, Dijkstra FA, Singh B (2017) Long-term aging of biochar: a molecular understanding with agricultural and environmental implications. Adv Agron 141:1–51
Mia S, Dijkstra FA, Singh B (2018) Enhanced biological nitrogen fixation and competitive advantage of legumes in mixed pastures diminish with biochar aging. Plant Soil 424:639–651
Mlambo D, Mwenje E, Nyathi P (2007) Effects of tree cover and season on soil nitrogen dynamics and microbial biomass in an African savanna woodland dominated by Colophospermum mopane. J Trop Ecol 23(4):437–448
Mukherjee A, Lal R, Zimmerman A (2014) Effects of biochar and other amendments on the physical properties and greenhouse gas emissions of an artificially degraded soil. Sci Total Environ 487:26–36
Nelson NO, Agudelo SC, Yuan W, Gan J (2011) Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci 176(5):218–226
Nessa A, Bai SH, Wang D, Karim Z, Omidvar N, Zhan J, Xu Z (2021) Soil nitrification and nitrogen mineralization responded non-linearly to the addition of wood biochar produced under different pyrolysis temperatures. J Soils Sediments 21:3813–3824
Nguyen TTN, Xu C-Y, Tahmasbian I, Che R, Xu Z, Zhou X, Wallace HM, Bai SH (2017) Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma 288:79–96
Niinemets Ü, Wright IJ, Evans JR (2009) Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. J Exp Bot 60(8):2433–2449
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3(6):973–996
Ohyama T (2010) Nitrogen as a major essential element of plants. Nitrogen Assim Plants 37:1–17
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512
Regus J, Wendlandt C, Bantay R, Gano-Cohen K, Gleason N, Hollowell A, O’Neill M, Shahin K, Sachs J (2017) Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 414:159–170
Reverchon F, Abdullah KM, Bai SH, Villafán E, Blumfield TJ, Patel B, Xu Z (2020) Biological nitrogen fixation by two Acacia species and associated root-nodule bacteria in a suburban Australian forest subjected to prescribed burning. J Soils Sediments 20:122–132
Reverchon F, Flicker RC, Yang H, Yan G, Xu Z, Chen C, Hosseini Bai S, Zhang D (2014) Changes in δ15N in a soil–plant system under different biochar feedstocks and application rates. Biol Fertil Soils 50:275–283
Reverchon F, Xu Z, Blumfield T, Chen C, Abdullah K (2011) Impact of global climate change and prescribed burning on understorey legumes and associated belowground communities: implications for biogeochemical cycles in forest ecosystems. J Soils Sediments 12:150–160
Reverchon F, Xu Z, Blumfield TJ, Chen C, Abdullah KM (2012) Impact of global climate change and fire on the occurrence and function of understorey legumes in forest ecosystems. J Soils Sediments 12:150–160
Rousk K, Michelsen A (2017) Ecosystem nitrogen fixation throughout the snow-free period in subarctic tundra: effects of willow and birch litter addition and warming. Global Change Biol 23(4):1552–1563
Salemaa M, Lindroos A-J, Merilä P, Mäkipää R, Smolander A (2019) N2 fixation associated with the bryophyte layer is suppressed by low levels of nitrogen deposition in boreal forests. Sci Total Environ 653:995–1004
Schulz H, Dunst G, Glaser B (2013) Positive effects of composted biochar on plant growth and soil fertility. Agron Sustain Dev 33:817–827
Seymour CL, Huyser O (2008) Fire and the demography of camelthorn (Acacia erioloba Meyer) in the southern Kalahari–evidence for a bonfire effect? Afr J Ecol 46(4):594–601
Sika M, Hardie A (2014) Effect of pine wood biochar on ammonium nitrate leaching and availability in a S outh A frican sandy soil. Eur J Soil Sci 65(1):113–119
Stark JM, Hart SC (1996) Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J 60(6):1846–1855
Succarie A, Xu Z, Wang W (2022) The variation and trends of nitrogen cycling and nitrogen isotope composition in tree rings: the potential for fingerprinting climate extremes and bushfires. J Soils Sediments 22(9):2343–2353
Succarie A, Xu Z, Wang W, Liu T, Zhang X, Cao X (2020) Effects of climate change on tree water use efficiency, nitrogen availability and growth in boreal forest of northern China. J Soils Sediments 20:3607–3614
Sun H, Lu H, Chu L, Shao H, Shi W (2017) Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci Total Environ 575:820–825
Sun W, Li Y, Xu Z, Bai Y, Bai SH (2024) Biochar application for enhancing water and nitrogen use efficiency of understory acacia species in a suburban native forest subjected to nitrogen deposition in Southeast Queensland. Plant Soil (published online on 1 April 2024)
Swagathnath G, Rangabhashiyam S, Murugan S, Balasubramanian P (2019) Influence of biochar application on growth of Oryza sativa and its associated soil microbial ecology. Biomass Convers Biorefin 9:341–352
Taresh S, Bai SH, Abdullah KM, Zalucki J, Nessa A, Omidvar N, Wang D, Zhan J, Wang F, Yang J (2021) Long-term impact of prescribed burning on water use efficiency, biological nitrogen fixation, and tree growth of understory acacia species in a suburban forest ecosystem of subtropical Australia. J Soils Sediments 21:3620–3631
Tcherkez G (2010) Natural 15N/14N isotope composition in C3 leaves: are enzymatic isotope effects informative for predicting the 15N-abundance in key metabolites? Funct Plant Biol 38(1):1–12
Teutscherova N, Houška J, Navas M, Masaguer A, Benito M, Vazquez E (2018) Leaching of ammonium and nitrate from Acrisol and Calcisol amended with holm oak biochar: A column study. Geoderma 323:136–145
Thies JE, Rillig MC, Graber ER (2015) Biochar effects on the abundance, activity and diversity of the soil biota. Biochar for Environmental Management: Science, Technology and Implementation 2:327–389
Verma P, Sagar R (2020) Effect of nitrogen (N) deposition on soil-N processes: a holistic approach. Sci Rep 10(1):10470
Voroney P (2019) Chapter 4 - Soils for Horse Pasture Management. In: Sharpe, ed. Horse pasture management 65–79
Wang D, Xu Z, Blumfield TJ, Zalucki J (2020) The potential of using 15N natural abundance in changing ammonium-N and nitrate-N pools for studying in situ soil N transformations. J Soils Sediments 20:1323–1331
Wang T, Arbestain MC, Hedley M, Bishop P (2012) Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org Geochem 51:45–54
Wang X, Chen G, Wang S, Zhang L, Zhang R (2019) Temperature sensitivity of different soil carbon pools under biochar addition. Environ Sci Pollut Res 26:4130–4140
Wang Y, Xu Z, Zheng J, Abdullah KM, Zhou Q (2015) δ15N of soil nitrogen pools and their dynamics under decomposing leaf litters in a suburban native forest subject to repeated prescribed burning in southeast Queensland, Australia. J Soils Sediments 15:1063–1074
Warshan D, Bay G, Nahar N, Wardle DA, Nilsson M-C, Rasmussen U (2016) Seasonal variation in nifH abundance and expression of cyanobacterial communities associated with boreal feather mosses. ISME J 10(9):2198–2208
Whitehead D, Barbour MM, Griffin KL, Turnbull MH, Tissue DT (2011) Effects of leaf age and tree size on stomatal and mesophyll limitations to photosynthesis in mountain beech (Nothofagus solandrii var. cliffortiodes). Tree Physiol 31(9):985–996
Wilson KB, Baldocchi DD, Hanson PJ (2000) Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol 20(9):565–578
Witt GB, English NB, Balanzategui D, Hua Q, Gadd P, Heijnis H, Bird MI (2017) The climate reconstruction potential of Acacia cambagei (gidgee) for semi-arid regions of Australia using stable isotopes and elemental abundances. J Arid Environ 136:19–27
Xiao Y, Wang L, Zhao Z, Che Y (2020) Biochar shifts biomass and element allocation of legume-grass mixtures in Cd-contaminated soils. Environ Sci Pollut Res 27:10835–10845
Xu Z, Saffigna P, Farquhar G, Simpson J, Haines R, Walker S, Osborne D, Guinto D (2000) Carbon isotope discrimination and oxygen isotope composition in clones of the F1 hybrid between slash pine and Caribbean pine in relation to tree growth, water-use efficiency and foliar nutrient concentration. Tree Physiol 20(18):1209–1217
Yang J, Zhan J, Taresh S, Sun W, Li Y, Nessa A, Wu Q, Xu Z (2023) Short-term changes in soil labile carbon and nitrogen pools with biochar application in a suburban native forest in subtropical Australia. J Soils Sediments 23(11):3832–3842
Yang L, Liu N, Ren H, Wang J (2009) Facilitation by two exotic Acacia: Acacia auriculiformis and Acacia mangium as nurse plants in South China. For Ecol Manag 257(8):1786–1793
Yoseph T, Shanko S (2017) Growth, symbiotic and yield response of N-fertilized and Rhizobium inoculated common bean (Phaseolus vulgaris L.). Afr J Plant Sci 11(6):197–202
Yousfi S, Serret MD, Araus JL (2013) Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell Environ 36(6):1214–1227
Zeng J, Han G (2020) Preliminary copper isotope study on particulate matter in Zhujiang River, southwest China: Application for source identification. Ecotoxicol Environ Saf 198:110663
Zhang M, Wang W, Tang L, Heenan M, Xu Z (2018) Effects of nitrification inhibitor and herbicides on nitrification, nitrite and nitrate consumptions and nitrous oxide emission in an Australian sugarcane soil. Biol Fertil Soils 54(6):697–706
Zhang X, Chen S, Sun H, Pei D, Wang Y (2008) Dry matter, harvest index, grain yield and water use efficiency as affected by water supply in winter wheat. Irrig Sci 27:1–10
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by Postgraduate Research Scholarships granted by Griffith University.
Author information
Authors and Affiliations
Contributions
Zhihong Xu designed the experiment; Yinan Li, Weiling Sun and Yifan Bai carried out the sampling and analyses; Yinan Li, Weiling Sun analyzed the data and wrote the manuscript; and Zhihong Xu and Shahla Hosseini Bai improved the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This research does not involve human participants or animals performed by any of the authors.
Competing interests
The authors have no competing interests to declare.
Additional information
Responsible editor: Hailong Wang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Sun, W., Xu, Z. et al. Long-term effects of biochar application on biological nitrogen fixation of acacia species and soil carbon and nitrogen pools in an Australian subtropical native forest. J Soils Sediments 24, 1956–1968 (2024). https://doi.org/10.1007/s11368-024-03816-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-024-03816-8