Abstract
Purpose
Revegetation of riparian zones is important to improve their soil nitrogen (N) dynamics and to preserve their microbial compositions. However, the success of revegetation projects currently depends on weed control to reduce non-target vegetation competing over nutrients and to ensure the target plant species growth and survival. Different weed control methods affect soil microbial composition and N cycling. However, the long-term effects of herbicides on soil nitrogen (N) pools and microbial community composition remain uncertain even after cessation of the herbicide application.
Materials and methods
This study compared the impacts of different herbicides (Roundup®, BioWeed™, Slasher®, and acetic acid) with mulch on soil N dynamics and microbial community structure 3 years after vegetation establishment (herbicides applied repeatedly in the first 2 years after which no herbicides were applied in the third final year).
Results and discussion
Soil microbial biomass carbon (MBC) was significantly higher in mulch compared with Roundup®, BioWeed™, Slasher®, and acetic acid at month 26 at the Kandanga site and month 10 at the Pinbarren site. Soil MBC remained significantly higher in mulch compared with Roundup® and BioWeed™, 12 months after the cessation of herbicide application at the Pinbarren site. Soil MBC in the Roundup® and BioWeed™ groups was also lower than the acceptable threshold (160 mg kg−1) at month 34 at the Pinbarren site. Soil NO3−-N was significantly higher in the mulch than the Roundup® at months 22 and 34 after revegetation at the Pinbarren site which could be partly explained by the decreased abundance of the denitrifying bacteria (Candidatus solibacter and C. koribacter). Additionally, both soil bacterial and fungal communities at the Pinbarren site and only fungal community at the Kandanga site were different in the mulch group compared with all other herbicides. The differences persisted 12 months after the cessation of herbicide application at the Pinbarren site.
Conclusion
Our study suggested that the application of mulch to assist with riparian revegetation would be beneficial for soil microbial functionality. The use of herbicides may have long-lasting effects on soil microbial biomass and diversity and therefore herbicides should be used with caution as part of an integrated land management plan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Deforestation is a major factor that contributes to land degradation and soil loss within riparian zones with consequences for biodiversity loss, water quality deterioration, and soil nutrient limitations, particularly depletion of soil nitrogen (N) (Ye et al. 2012; Hale et al. 2018). Revegetation of riparian zones is a best management practice for reversing soil degradation, increasing soil N, and reducing sediment and nutrient delivery to waterways (Chen et al. 2012; Li et al. 2012; Olley et al. 2015). However, competitive biological and biochemical interactions between target restorer plants and weeds have the potential to hinder riparian revegetation projects (Bottrill et al. 2020). This may lead to revegetation failure, which can be costly (Schirmer and Field 2014). Therefore, it is essential to control the competing weed species to increase resource availability, ensure plant survival, and optimise the growth of the target revegetation plants (Campoe et al. 2014).
Nitrogen is a growth-limiting nutrient and plays an important role in plant productivity (Atkinson et al. 2010; Bai et al. 2012a, b). Mineralisation of organic N produces inorganic N, mainly as ammonium (NH4+-N) and nitrate (NO3−-N), which are the plant-available forms of N. A recent meta-analysis showed that revegetation increases soil N pools within riparian zones (Omidvar et al. 2021). However, N mineralisation depends on many environmental factors including soil types, vegetation types, environmental factors, and land management practices (Broadbent 1984; Groot and Houba 1995; Williams et al. 2007; Ros et al. 2011; Omidvar et al. 2021). Both short- and long-term soil N indicators must be assessed to measure the success of revegetation, including inorganic N concentrations and potentially mineralisable N (PMN) (Bai et al. 2014). Optimal land management practices need to sustain both short- and long-term N availability within riparian soils for revegetating plants. PMN is an indicator of biologically active soil N that is responsive to changes in land management practices (Mahal et al. 2019), which has a strong influence on the soil microbial diversity and composition (Wang et al. 2018).
Soil microbial composition (e.g. bacteria and fungi) and biomass are important measurable soil biotic components that are highly responsive to changes in land management practices (Falkowski et al. 2008; Urbanova et al. 2015; Nguyen et al. 2018). Land management practices typically alter the soil environment including soil pH, soil water content, and soil organic matter, which lead to shifts in soil microbial composition and abundance (Jangid et al. 2008; Lauber et al. 2008; Rousk et al. 2010). Herbicides used for weed control, and their residues, can be toxic to soil-borne microbes (Bai and Ogbourne 2016), and repeated applications may eventually alter soil microbial community (Helander et al. 2018).
Soil microbial biomass carbon (MBC) and N (MBN) are used as an early indicator of soil microbial community changes in response to land management practice and/or external environmental factors (Jordan et al. 1995; Trasar-Cepeda et al. 1998; Bending et al. 2004; Romaniuk et al. 2011). Soil MBC and MBN alteration may subsequently influence soil organic carbon (SOC) turnover and soil N availability (Yang et al. 2010). As an alternative to herbicides, an organically derived mulch such as wood chips can be applied to control weeds, which increases soil organic matter (SOM), preserves soil water, and reduces soil temperature fluctuations (Huang et al. 2008; Murungu et al. 2011; Bai et al. 2014). Mulch application may also improve both the physical and chemical properties of the soil (Huang et al. 2008) and increase soil N retention (Bai et al. 2014) due to high organic matter inputs. Although physiochemical changes can increase soil microbial biomass (MB) (Bai et al. 2014), the extent to which mulch or other weed control methods can alter the soil microbial composition, abundance, and biomass is largely unknown.
Chemical application is a globally utilised and relatively cheap weed control option and glyphosate is one of the most commonly used post-emergence, non-selective, and broad-spectrum herbicides (Bai and Ogbourne 2016). Glyphosate is applied on leaves but can exude through the roots within 24 h of application (Laitinen et al. 2007). Literature describing the potential glyphosate toxicity on soil microbial communities and diversity due to the compound’s mode of action remains contradictory. Some studies report that a single application of glyphosate changes soil microbial community structure (Puertolas et al. 2010; Banks et al. 2014). However, in a recent short-term field study, no significant impacts of glyphosate on soil microbial diversity and community structure were observed (Bottrill et al. 2020). Glyphosate may be directly toxic to soil microorganisms due to reduced amino acid biosynthesis via inhibition of the shikimate synthesis pathway (Anza et al. 2016; Nguyen et al. 2016) and initial longer-term studies have indicated some impacts on soil microbial communities (Bai and Ogbourne 2016; Lancaster et al. 2009; de Andrea et al. 2003). The half-life of glyphosate is reported to be within a few days to a few weeks (0.8–151 days) (Bai and Ogbourne 2016). However, the frequency of application may prolong the presence of glyphosate in soil (Primost et al. 2017) and later may have adverse consequences on the microbial community and abundance (Helander et al. 2018). Furthermore, the degradation of glyphosate may be delayed due to different environmental factors and soil properties (Bai and Ogbourne 2016). The impact of glyphosate on soil microbes is likely to be dependent on many factors including dosage, frequency of application, and soil characteristics (Bai et al. 2012a, b; Nguyen et al. 2016). Alternative organic chemical herbicides exist in the commercial retail marketplace and include the registered products BioWeed™, Slasher® (pelargonic acid), and horticultural vinegar (acetic acid). Currently, it is unknown what effects, if any, of these organic preparations have on the soil microbiota. This study aimed to assess and compare impacts of different weed control methods on (a) soil N dynamics, (b) soil microbial biomass C (MBC) and N (MBN), and (c) soil fungal and bacterial community composition in revegetated riparian zones over 2 years following repeated application of glyphosate and organic-based herbicides and 1 year after ceasing herbicide application (in total up to 3 years following revegetation establishment).
2 Materials and methods
2.1 Study sites description
The experimental sites were located in the Mary River basin in South East Queensland, Australia (map provided in Fig. 1a, b). These were the (a) Kandanga site (26° 23′ 14.22″ S, 152° 42′ 9.74″ E) and (b) Pinbarren site (26° 18′ 11.92″ S, 152° 51′ 21.39″ E), approximately 150 km and 170 km, north of Brisbane, respectively. Both the Kandanga and Pinbarren sites were bare land with some coverage of patchy weeds before revegetation. The soil type was loamy clay and brown sandy loam in the Kandanga and Pinbarren sites respectively. Temperature and rainfall data were collected from the nearest weather station to each site, being Gympie (Kandanga) and Traveston (Pinbarren) (Bureau of Meteorology 2020). The rainfall and average temperature at the Kandanga site during the study (August 2016 to August 2018) were approximately 1690.3 mm and 27.8 °C, respectively. Similarly, the rainfall and average temperature at the Pinbarren site during the study (September 2017 to September 2020) were approximately 2031.8 mm and 25.8 °C, respectively (Bureau of Meteorology 2020) (Fig. S1a, b). Kandanga site experienced a prolonged period of low rainfall after establishment, which significantly affected plant survival. Therefore, the site was not maintained or monitored after year 2. Tree survival rate was on average 25.8% by year 2 at the Kandanga and 58% by year 3 at the Pinbarren. Average tree height was 1228.50 mm ranging between 1072.74 and 1489.78 mm in the Kandanga site and 3871.52 mm ranging between 3410.20 and 4285.18 mm in the Pinbarren site by the end of the experiment.
2.2 Experimental design and treatments
Each experimental site was established as a randomised complete block design with five subplot replicates. The Kandanga site was established in 2016 and the Pinbarren site was established in 2017. Each site was flat or very close to flat and was divided into five blocks and then each block was separated into five plots, with 2 m distance between treatments to minimise cross contamination (30 m × 2 m). Each plot included a row of 20 different native subtropical trees, planted in the centre of the 2 m wide rows, 1.5 m apart) (Bottrill et al. 2020). The native plants were selected from the regional ecosystem as per Bottrill et al. (2020). The plant species included in this study are provided in Table S1. A slow-release fertilizer (10 g pelletised Agriform - total 20% N) was placed in all planting holes at the time of planting.
Treatments including chemical and organic-based herbicides and mulch were applied within each plot as a spot application around the base of each plant in a circular area with a radius of 0.5 m from the base of each plant. Treatments included Roundup® (glyphosate) at the rate of 10 mL/L, BioWeed™ (derived from pine oil) at 200 mL/L, Slasher® (pelargonic acid) at 700 mL/L, and acetic acid 90% at 125 mL/L, and wood chip mulch (Table S2). At the Kandanga site, the liquid herbicide treatments were applied by a hand sprayer directly onto the ground to cover weeds at the time of planting (month 0) followed by six further applications of each herbicide at months 4, 7, 10, 12, 17, and 22 following revegetation establishment (Table S3). At the Pinbarren site, two pre-establishment sprays were applied, 1 to 2 months before planting directly onto the ground cover weeds and one at the time of planting (with the exception of Roundup®, which was only applied 2 months prior to planting) followed by six maintenance applications with all of the herbicides at 1, 2, 6, 12, 16, and 20 months following revegetation establishment. Mulch was applied only once at the time of planting at both the Kandanga and the PinbarrenSoil sample collection (Table S3).
Soil samples were collected three times following revegetation at each site; at months 2 (August 2016), 14 (August 2017), and 26 (August 2018) at the Kandanga site and months 10 (September 2018), 22 (September 2019), and 34 (September 2020) at the Pinbarren site (Table S3). All soil samples were collected within 2 to 4 months of herbicide application with the exception of the last sampling at the Pinbarren site (months 34), which occurred 12 months after herbicide application. Three soil cores at 0–10 cm depth, 20 cm from the seedling stems were collected from each plot using an augur (60-mm internal diameter) and were combined to provide one sample per plot. All samples were kept on an ice container during transportation to the laboratory. A sub-sample of approximately 20 g of the soil from each plot was stored at − 20 °C for genomics analyses and the remainder was air-dried and passed through a 2-mm sieve for chemical analysis.
2.3 Soil chemical analyses
Soil pH was measured using deionised water with a 1:5 (soil:water) ratio. Soil microbial biomass carbon (MBC) and soil microbial biomass N (MBN) were determined using chloroform fumigation extraction (Jenkinson and Powlson 1976) and the extracts were then analysed using a Shimadzu total organic carbon (TOC) and total nitrogen (TN) analyser (Shimadzu Corp., Kyoto, Japan) (Chen and Xu 2005; Bai et al. 2012a, b). MBC and MBN were derived from the equations as described in Vance et al. (1987) and Brookes et al. (1985), respectively. The K2SO4 extractions from the NF samples were also used to determine dissolved organic carbon (DOC) and dissolved nitrogen (DN).
To measure potentially mineralisable N (PMN), three sub-samples (8 g) from each air-dried sample were weighed. Two sub-samples were incubated at 30 °C for 7 and 14 days. After incubation, 40 mL of 2 M KCl was added to the samples, and the suspension was shaken with an end-over-end shaker for 60 min, centrifuged for 20 min at 2000 rpm followed by filtration through a Whatman No. 42 filter paper. The third sub-sample of soil (namely day 0) was added to 40 mL of 2 M KCl processed as above, without incubation. The 2 M KCl extraction was used to determine the concentration of mineral-N using a SmartChem 200, Discrete Chemistry Analyser (DCA). PMN was measured as the difference between the mineral-N concentration before and after incubation. The KCl extractions from day 0 (non-incubated) were also used to determine NH4+-N and NO3−-N concentrations using a Shimadzu TOC/TN analyser (Shimadzu Corp., Kyoto, Japan).
2.4 Soil DNA extraction
Soil DNA was extracted from 0.25 g soil samples using a Tiangen Soil DNA Extraction Kit (Tiangen, Beijing, China) according to the manufacturer’s instruction. Extracted DNA was visualised on a 1% agarose gel and quantified with a Qubit 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA). Soil DNA samples were extracted for each treatment from each of the five blocks. The five DNA samples from each treatment were pooled to constitute one composite sample per treatment at each sampling occasion. In total, 10 composite samples from the Kandanga site (collected at months 14 and 26) and 15 composite samples from the Pinbarren site (collected at months 10, 22, and 34) were analysed.
2.5 Next-generation sequencing (NGS) library preparation and Illumina MiSeq sequencing
NGS library preparation and Illumina MiSeq sequencing were conducted by GENEWIZ® (GENEWIZ, Biotechnology Co., Ltd, Suzhou, China) according to standard protocols (Huang et al. 2019). In brief, a total of 30–50 ng of each DNA sample was used to generate amplicons using a MetaVx™ Library Preparation kit (GENEWIZ, Inc., South Plainfield, NJ, USA) (Han et al. 2018). The V3 and V4 hypervariable regions of prokaryotic 16S rDNA were selected and amplified using the forward primer 5′CCTACGGRRBGCASCAGKVRVGAAT-3′ and the reverse primer 5′ GGACTACNVGGGTWTCTAATCC-3′ (You et al. 2016). For the fungal community, 50–100 ng DNA was used to generate amplicons using a panel of primers designed by Genewiz (Huang et al. 2019). Oligonucleotide primers were designed to anneal to the relatively conserved sequences spanning fungal ITS regions (Huang et al. 2019). The hypervariable regions of ITS2 were selected and amplified using the forward primer sequence 5′GTGAATCATCGARTC3′ and the reverse primer was 5′-TCCTCCGCTTATTGAT-3′ (Huang et al. 2019; Cai et al. 2018). At the same time, a linker with index was added to the ends of the PCR product of 16S rDNA amplicons to generate indexed libraries ready for downstream NGS sequencing on the Illumina Miseq platform (Qiao et al. 2018). DNA libraries were validated using an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA), and quantified with a Qubit 3.0 Fluorometer (Applied Biosystems, Carlsbad, CA, USA), and then multiplexed and loaded on an Illumina MiSeq instrument according to the manufacturer’s instructions (Illumina, San Diego, CA, USA) (Cai et al. 2018; Fu et al. 2016). Next-generation sequencing was performed (paired-end, 2 × 300 bp) on the MiSeq instrument (Fu et al. 2016; Cai et al. 2018; Huang et al. 2019).
2.6 Statistical analyses
Two-way analysis of variance (ANOVA) tests was conducted where treatments and sampling times were assumed as the main effects, for soil pH, DOC, DN, NH4+-N, NO3−-N, PMN, MBC, and MBN. Where interaction between treatment and sampling time was found to be significant, a series of one-way ANOVA tests were performed to explore differences among specific factors at each sampling time. Stepwise regression using a linear model was developed to ascertain which soil variables explained Shannon index and soil chemical properties. SPSS Statistics (IBM, version 26) was used for all statistical analyses.
The Quantitative Insights into Microbial Ecology (QIIME) package was used for 16S rRNA and ITS rRNA data analysis whereby any low-quality reads were filtered out, and any sequences that did not fulfil the following criteria were discarded: sequence length < 200 bp, no ambiguous bases, and no mean quality scores ≤ 20 (Han et al. 2018; Huang et al. 2019). The sequences were compared with the reference database (RDP Gold database) using the UCHIME algorithm to detect and remove chimeric sequences (Han et al. 2018). The effective remaining sequences were used in the final analysis. The operational taxonomic units (OTUs) with 97% similarity were grouped using the clustering program VSEARCH (v.1.9.6) against the SILVA119 and UNITE ITS database (https:/unite.ut.ee/) (Han et al. 2018; Huang et al. 2019). The Ribosomal Database Program (RDP) classifier was applied to assign a taxonomic category to all OTUs at a confidence threshold of 0.8 (Han et al. 2018). The bacterial and fungal alpha diversity indices were calculated in QIIME from rarefied samples using the Shannon index for diversity analysis and the ACE and Chao1 indices for richness analysis (Cai et al. 2018; Han et al. 2018; Huang et al. 2019). Non-metric multidimensional scaling (NMDS) was performed to visualise similarities among bacterial and fungal communities using R software (v.3.3.1). Heatmap analysis was performed using MeV (v.4.2) to visualise similarities within and between the bacterial and fungal species under different weed control methods. A bipartite interaction network using the R package was created to visualise the bacterial and fungal communities in the different treatments (Dormann et al. 2009).
3 Results
3.1 Effect of different treatments on soil pH, C, and N dynamics
During the term of the study and across all treatments, soil pH ranged from 4.8 to 6.2 at the Kandanga site and from 5.2 to 6.4 at the Pinbarren site (Table 1). Soil pH did not differ significantly among the treatments at any sampling time at the Kandanga site. However, at the Pinbarren site, soil pH was significantly higher in the mulch treatment group compared with the other herbicide treatment groups at months 10, 22, and 34 after revegetation, with the exception of the acetic acid treatment group at month 34 (Table 1).
At the Kandanga site, at month 14, soil DOC was significantly higher in the mulch treatment group compared with the acetic acid treatment group (Table 1). However, at month 26, soil DOC was significantly higher in the mulch treatment group compared with all other treatments, with the exception of the Slasher® treatment group where soil DOC did not differ from the mulch (Table 1). Soil DOC ranged from 42.1 to 103.7 μg g−1 during the period of study at the Kandanga site (Table 1). At the Pinbarren site, at month 10, soil DOC was significantly higher in the mulch treatment group compared with all other treatments (Table 1). Soil DOC ranged from 77.1 to 169.8 μg g−1 over the study and across all treatments at the Pinbarren site (Table 1).
Soil DN was not significantly different following any treatment at any sampling time at the Kandanga site, which ranged from 54.6 to 158.4 μg g−1 over the study period (Table 1). At the Pinbarren site, soil DN ranged from 61.5 to 177.7 μg g−1 during the study period and was significantly higher in the mulch treatment group compared with all other treatment groups only at month 34 (Table 1).
Neither soil NH4+-N nor NO3−-N concentrations were significantly different among treatments at the Kandanga site regardless of sampling time (Table 2). However, at the Pinbarren site, at month 22, treatment with mulch significantly lowered soil NH4+-N compared with all other treatments (Table 2). Also, NO3−-N was the dominant inorganic N in the mulch treatment group at the Pinbarren site at months 22 and 34, at a level of 78.5 and 72.4 mg kg−1, respectively (Table 2). Furthermore, at the Pinbarren site, at months 22 and 34, soil NO3−-N was significantly higher in the mulch treatment group compared to the Roundup® treatment group (Table 2).
Total N mineralised in days 7 and 14 of incubation (PMN-7 days and PMN-14 days) was not significantly different between the treatment groups regardless of sampling times at the Kandanga site (Table 2). However, at the Pinbarren site, at month 22, soil PMN-7 days and PMN-14 days were significantly higher in the Roundup® treatment group compared with the acetic acid and BioWeed™ treatment groups (Table 2).
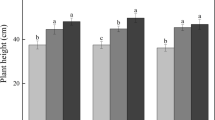
At the Kandanga site, MBC was not significantly different among treatments at months 2 and 14 following revegetation (Fig. 2a). However, by month 26, MBC was significantly higher in the mulch treatment group compared to the Roundup® or organic-based herbicide groups (Fig. 2a). Similarly, at the Pinbarren site, MBC was significantly higher in the mulch treatment group compared to the Roundup® or organic-based herbicide treatment groups at month 10. However, at month 34 at the Pinbarren site, soil MBC was only significantly higher in the mulch treatment group compared with the Roundup® and BioWeed™ treatment groups (Fig. 2b). Soil MBN did not differ significantly among the treatments at all sampling times at both Kandanga and Pinbarren sites (Fig. 2c, d).
Soil microbial biomass C and N (MBC and MBN) for each treatment at the Kandanga site (a, c) at months 2, 14, and 26 and at the Pinbarren site (b, d) at months 10, 22, and 34 following revegetation establishment. Different lower-case letters at each bar indicate significant differences among treatments at P < 0.05. No letters indicate no significant difference among treatments
3.2 Soil bacterial and fungal community composition
A total of 5,345,488 high-quality sequences, in a range of 101,676–297,692 sequences per sample, were detected from the 16S rDNA gene region. These clustered into 6272 OTUs at a 97% sequence similarity threshold. Taxonomic classification indicated these were representative of 28 bacterial phyla, comprising 530 genera and 583 species. Of these, the dominant bacterial phyla were Proteobacteria (38.16%), Actinobacteria (21.42%), and Acidobacteria (19.64%) (Fig. 3). The bacterial Shannon diversity index ranged from 9.53 to 10.16 (Table 3). Non-metric multidimensional scaling (NMDS) ordinations based on the Bray–Curtis similarity matrices showed no segregation of treatments in the community structure of bacteria at the Kandanga site at either sampling time (Fig. 4a). However, clear segregation of the bacterial community structure was evident between the mulch treatment group and the other treatment groups at the Pinbarren site, at months 10, 22, and 34 (Fig. 4b).
NMDS ordinations derived from the Bray–Curtis dissimilarity matrices indicating the changes in the community structure of soil bacteria in different weed control methods at the (a) Kandanga and (b) Pinbarren sites, and soil fungi at the (c) Kandanga and (d) Pinbarren sites. Treatments including mulch (circle symbols), BioWeed™ (square symbol), Slasher® (diamond symbols), Roundup® (right-side-up triangle symbols), acetic acid (upside-down triangle symbols) at the Kandanga site at months 14 (green) and 26 (purple), and the Pinbarren site at months 10 (grey), 22 (brown), and 34 (blue) following revegetation establishment. Treatments from the same sampling time were marked in the same color
A total of 17 fungal phyla, comprising 310 genera and 532 species, were identified from hypervariable regions of ITS2. The three most abundant fungal phyla identified were Ascomycota (40.13%), Basidiomycota (34.25%), and unclassified fungi (11.87%) (Fig. 5). Interestingly, at months 34 at the Pinbarren site, the fungal community shifted in composition from Basidiomycota (41.28%) towards Ascomycota (15.98%) domination (Fig. 5). The fungal Shannon diversity index ranged from 5.34 to 7.49 (Table 3). Similarly, the NMDS ordinations based on the Bray–Curtis similarity matrices showed that there was clear segregation of the fungal community structure between the mulch treatment group and the other treatment groups at both Kandanga and Pinbarren sites at all sampling times (Fig. 4c, d). Soil pH and DN explained 44% and 25% variations of Shannon index bacteria and Shannon index fungi, respectively (Table 4).
At the bacterial species level, C. Solibacter, unclassified Acidothermus, Bacillus, and uncultured bacterium were most abundant at both the Kandanga and Pinbarren sites after all the treatments, regardless of the sampling times (Fig. 6). The bacterial-focussed heatmap indicated that unclassified Bradyrhizobium, Mycobacterium, and Acidibacter bacterial species were more abundant in the mulch-treated samples compared with the other treatments at months 22 and 34 at the Pinbarren site (Fig. 6). Similarly, there was a lower abundance of C. solibacter and C. koribacter in the mulch treatment group compared with the other treatment groups including at both sites and at all sampling times (Fig. 6). The 30 most abundant fungal species detected among all samples were plotted in the heatmap with the most abundant being Mortierella Elongata at the Kandanga site and unclassified Mortierella at the Pinbarren site (Fig. 7).
Heatmap of bacterial distribution of the top 30 abundant species present in the microbial community of samples. The heatmap plot depicted the relative abundance of bacteria in the soil after different treatments at the Kandanga site at months 14 and 26 and the Pinbarren site at months 10, 22, and 34 following revegetation establishment. The relative value for bacterial species is indicated by color intensity
Heatmap of fungal distribution of the top 30 abundant species present in the microbial community of samples. The heatmap plot depicted the relative abundance of fungi in the soil after different treatments at the Kandanga site at months 14 and 26 and at the Pinbarren site at months 10, 22, and 34 following revegetation establishment. The relative value for fungal species is indicated by color intensity
The bacterial co-occurrence patterns and network analysis at the phylum level showed no major differences in the communities among the treatments at either site. However, Proteobacteria showed higher abundance with all treatments at month 26 at the Kandanga site and at month 34 at the Pinbarren site (Fig. S2a, b) compared to the earlier sampling times. The soil fungal phyla Mortierellomycota and unclassified fungi increased in abundance at month 34 in Pinbarren in all treatments (Fig. S3a, b) compared with earlier sampling times.
4 Discussion
Soil microbial biomass carbon (MBC) was significantly lower in soil samples after treatment with Roundup® and BioWeed™ compared with mulch, 12 months after treatments had ceased (month 34 at Pinbarren). In general, an attainable soil MBC limit for normal functioning of soil ecosystems in forest soil is 520 mg kg−1 and soils with MBC as low as 160 mg kg−1 have soil constraints (Gonzalez-Quiñones et al. 2011). None of the soils treated with mulch and herbicides groups had a MBC level lower than 160 mg kg−1 up to 2 years in this study. However, 12 months after cessation of herbicide application at Pinbarren (month 34), soil MBC was lower than 160 mg kg−1 in the Bioweed™ and Roundup® treatment groups, and therefore MBC could be a soil constraining factor for plant growth at this site in the longer term. The relatively high soil MBC in the mulch group was likely the result of increased SOM inputs and enhanced soil moisture retention in the soil layer (Li et al. 2004; Grigg et al. 2006; Bai et al. 2014; Kader et al. 2016). No effect of herbicide was found on soil MBN.
Inorganic N concentrations were responsive to the treatments in the Pinbarren site, where soil NO3−-N was significantly higher in the mulch treatment group compared to the Roundup® treatment group at months 22 and 34, but not in comparison to the organic-based herbicides. Decreased denitrifying bacteria is one of the driving factors to increase soil NO3−-N. Both C. solibacter and C. koribacter are denitrifying bacteria involved in N cycling and are associated with the reduction of nitrate, nitrite, and possibly nitric oxide (Ward et al. 2009). We observed a lower abundance of C. solibacter and C. koribacter in the mulch treatment group compared with the other treatment groups at the Pinbarren site, which could partly explain the higher NO3−-N through decreased denitrification in the mulch treatment group compared with the Roundup® treatment group. However, there were no differences in soil NO3−-N between the mulch treatment group and the other organic-based herbicides despite the fact that C. solibacter and C. koribacter were still lower in the mulch treatment than those other organic-based herbicides. Differences in soil NO3−-N were only observed between the mulch and Roundup® treatment groups, which may suggest that glyphosate may have affected soil nitrification and denitrification differently from the other organic-based herbicides.
Interestingly, segregation of bacterial and fungal communities in soil treated with herbicide compared with mulch was still observable 12 months after ceasing herbicide application at the Pinbarren site. Bacteria and fungi are sensitive to changes in soil physical and chemical properties and different environmental conditions such as substrate availabilities, soil moisture content, climate conditions, and management practices (Dong et al. 2017; Zhao et al. 2018). Soil pH and moisture content changes can also influence soil microbial composition (Schimel et al. 1999; Bååth and Anderson 2003). We observed a higher pH in the soil of the mulch treatment group compared to the herbicide treatment groups at months 10, 22, and 34 at the Pinbarren site, except for acetic acid at month 34. It is likely that both bacterial and fungal diversity segregation between the mulch treatment group and the herbicide treatment groups was partly driven by differences in pH. Therefore, our study highlights the importance of soil pH as one of the driving factors in the bacterial and fungal communities and that the application of mulch can catalyse these changes.
We also observed changes in the abundance of bacteria at the species level in response to the treatments. For example, we found that an unclassified Bradyrhizobium species was less abundant in the Roundup® treatment group, compared with the mulch treatment group and the organic-based herbicide treatment groups at month 34, 1 year after ceasing application of the herbicides. Bacteria of the genus Bradyrhizobium (Proteobacteria) are biologically important in soil due to their role in N fixation (Yao et al. 2014; Wongdee et al. 2018; Praeg et al. 2020). The lower abundance of Bradyrhizobium in the Roundup® treatment group indicated that glyphosate had a negative influence on the relative abundance of Bradyrhizobium species. The toxicity of glyphosate on the abundance of some strains of Bradyrhizobium species has also been previously reported (Zablotowicz and Reddy 2004; dos Santos et al. 2005; Malty et al. 2006), which may negatively impact the nodulation process by Bradyrhizobium, thus affecting soil N cycling. The lower abundance of Bradyrhizobium (Proteobacteria) species was also observed in the organic-based herbicide treatment groups at month 22 at the Pinbarren site, but not at month 34. Our study suggested that although the acute effect of herbicides on soil bacterial communities is relatively low, glyphosate may have negative effects on the abundance of some bacterial species such as Bradyrhizobium even 12 months after cessation of herbicide application, which may have long-term implications for soil N cycling.
Interestingly, at the Pinbarren site, fungal community composition shifted at month 34, which was not associated with treatments. For example, Ascomycota was initially the dominant fungal phyla followed by Basidiomycota at months 10 and 22 at the Pinbarren site. However, Basidiomycota became the most abundant phylum at month 34. Both Ascomycota and Basidiomycota metabolise organic substrates (Hanson et al. 2008) and are sensitive to changes in soil physical and chemical properties such as soil pH, moisture, temperature, and soil nutrient content (Zhao et al. 2018). However, our data do not suggest that this change over time was treatment-dependent because it occurred across all treatments. Microbial shifts can also result from changes in environmental factors such as vegetation cover and revegetation age (Guo et al. 2018). It has been previously reported that there is a distinct shift in fungal communities from Ascomycota in stands of young trees to Basidiomycota in stands of older trees due to changes in litter quality and quantity (Zumsteg et al. 2012; Zhang et al. 2018a) and changes in vegetation cover (Zhang et al. 2018b). Furthermore, in our study, the abundance of Mortierellomycota had increased at month 34 at the Pinbarren site compared with months 10 and 22. Mortierellomycota decomposes plant litter and degrades aromatic hydrocarbons (Osono 2005; Ellegaard-Jensen et al. 2013), and increased abundance of Mortierellomycota is used as an indicator of healthy soil (Zhang et al. 2020). In this study, we did not assess the leaf litter quality, but it is likely that the characteristics of the litter changed as the vegetation matured (Zhang et al. 2018a, b), and may to some extent explain the alteration in the abundance of some communities in our study. We did not observe any major differences in the soil microbial communities among the herbicide treatment groups.
Our study indicated that overall soil bacterial and fungal composition may not be significantly affected by the application of herbicides in the short term. However, 12 months after cessation of treatment, soil MBC levels were lower than the acceptable threshold of 160 mg kg−1 in the Roundup® and BioWeed™ treatment groups and were significantly lower than in the mulch treatment group. Furthermore, the abundance of a small number of microbial species was lower than in the Roundup® treatment group compared with the mulch treatment group, 12 months after cessation of herbicide application. Our data is therefore suggestive that the application of herbicides may have long-term implications on soil microbes and soil health.
5 Conclusion
This study examined the effects of different weed control methods on soil N cycling and microbial communities in two revegetated riparian zones. No major alteration of soil inorganic N was found among the different herbicides. However, we observed an effect of glyphosate on soil nitrification and denitrification because soil NO3−-N was lower in soil following application of Roundup® compared with soil treated with mulch at months 22 and 34 at the Pinbarren site. We did not observe any significant differences in the microbial communities or structure in the soil after the application of Roundup®, BioWeed™, Slasher®, and acetic acid. However, the abundance of a small number of microbial species was lower in the Roundup® treatment group compared with the mulch treatment group,12 months after cessation of herbicide application. Most significantly, we observed that the level of soil MBC after application of Roundup® and BioWeed™ was lower than acceptable thresholds (160 mg kg−1) at month 34 at the Pinbarren site. Furthermore, we observed segregation in soil bacterial and fungal communities between all herbicides and mulch, which persisted 1 year after cessation of herbicide application. This was only observed at one field site, however, which suggested that microbial responses to mulch and herbicides were site-specific. Our study suggested that the application of mulch to assist with riparian revegetation would be beneficial for soil microbial functionality and soil health and that the use of herbicides may have long-lasting effects on soil microbial biomass and microbial communities and should be used with caution as part of an integrated land management plan.
Availability of data
Data available on request from the authors
References
Anza M, Epelde L, Artetxe U, Becerril JM, Garbisu C (2016) Control of Cortaderia selloana with a glyphosate-based herbicide led to a short-term stimulation of soil fungal communities. Environ Monit Assess 188:631. https://doi.org/10.1007/s10661-016-5649-9
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18. https://doi.org/10.1007/s11104-010-0464-5
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Bai SH, Ogbourne SM (2016) Glyphosate: environmental contamination, toxicity and potential risks to human health via food contamination Environ Sci Pollut Res 23:18988–19001. https://doi.org/10.1007/s11356-016-7425-3
Bai SH, Blumfield TJ, Reverchon F (2014) The impact of mulch type on soil organic carbon and nitrogen pools in a sloping site. Biol Fertility Soils 50:37–44. https://doi.org/10.1007/s00374-013-0829-z
Bai SH, Blumfield TJ, Xu Z, Chen C, Wild CH (2012a) Effects of pre-planting site management on soil organic matter and microbial community functional diversity in subtropical Australia. Appl Soil Ecol 62:31–36. https://doi.org/10.1016/j.apsoil.2012.07.006
Bai SH, Blumfield TJ, Xu Z, Chen C, Wild C (2012b) Soil organic matter dynamics and nitrogen availability in response to site preparation and management during revegetation in tropical Central Queensland. Australia J Soils Sediments 12:386–395. https://doi.org/10.1007/s11368-011-0466-9
Banks ML, Kennedy AC, Kremer RJ, Eivazi F (2014) Soil microbial community response to surfactants and herbicides in two soils. Appl Soil Ecol 74:12–20. https://doi.org/10.1016/j.apsoil.2013.08.018
Bending GD, Turner MK, Rayns F, Marx MC, Wood M (2004) Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol Biochem 36:1785–1792. https://doi.org/10.1016/j.soilbio.2004.04.035
Bottrill D, Ogbourne SM, Citerne N, Smith T, Farrar MB, Hu HW, Omidvar N, Wang J, Burton J, Kaamper W, Bai SH (2020) Short-term application of mulch, roundup and organic herbicides did not affect soil microbial biomass or bacterial and fungal diversity. Chemosphere 244:125436. https://doi.org/10.1016/j.chemosphere.2019.125436
Broadbent FE (1984) Plant use of soil nitrogen. Nitrogen in Crop Production. 171–182. https://doi.org/10.2134/1990.nitrogenincropproduction.c11
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842. https://doi.org/10.1016/0038-0717(85)90144-0
Bureau of Meteorology (BOM) (2020) Climate Statistics for Australian Locations. Australian Government, Bureau of Meteorology. Bureau of Meteorology. Viewed: 04/01/2020. Available at: Climate statistics for Australian locations. Commonwealth of Australia 2020
Cai H, Zhang T, Zhang Q, Luo J, Cai C, Mao J (2018) Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol 73:319–326. https://doi.org/10.1016/j.fm.2018.02.002
Campoe OC, Iannelli C, Stape JL, Cook RL, Mendes JCT, Vivian R (2014) Atlantic forest tree species responses to silvicultural practices in a degraded pasture restoration plantation: from leaf physiology to survival and initial growth. For Ecol Manage 313:233–242. https://doi.org/10.1016/j.foreco.2013.11.016
Chen CR, Xu ZH (2005) Soil carbon and nitrogen pools and microbial properties in a 6-year-old slash pine plantation of subtropical Australia: impacts of harvest residue management. For Ecol Manage 206:237–247. https://doi.org/10.1016/j.foreco.2004.11.005
Chen H, Zhang W, Wang K, Hou Y (2012) Soil organic carbon and total nitrogen as affected by land use types in karst and non-karst areas of northwest Guangxi, China. J Sci Food Agric 92:1086–1093. https://doi.org/10.1002/jsfa.4591
de Andrea MM, Peres TB, Luchini LC, Basarin S, Papini S, Matallo MB (2003) Influence of repeated applications of glyphosate on its persistence and soil bioactivity. Pesqui Agropecu Bras 38:1329–1335. https://doi.org/10.1590/S0100-204X2003001100012
Dong W, Si P, Liu E, Yan C, Zhang Z, Zhang Y (2017) Influence of film mulching on soil microbial community in a rainfed region of northeastern China. Sci Rep 7:8468. https://doi.org/10.1038/s41598-017-08575-w
Dormann CF, Fründ J, Blüthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J 2:7–24. https://doi.org/10.2174/1874213000902010007
dos Santos JB, Ferreira EA, Kasuya MCM, da Silva AA, de Oliveira PS (2005) Tolerance of Bradyrhizobium strains to glyphosate formulations. Crop Prot 24:543–547. https://doi.org/10.1016/j.cropro.2004.10.007
Ellegaard-Jensen L, Aamand J, Kragelund BB, Johnsen AH, Rosendahl S (2013) Strains of the soil fungus Mortierella show different degradation potentials for the phenylurea herbicide diuron. Biodegradation 24:765–774. https://doi.org/10.1007/s10532-013-9624-7
Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320:1034–1039. https://doi.org/10.1126/science.1153213
Fu SF, Wang F, Shi XS, Guo RB (2016) Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem Eng J 287:523–528. https://doi.org/10.1016/j.cej.2015.11.070
Gonzalez-Quiñones V, Stockdale EA, Banning NC, Hoyle FC, Sawada Y, Wherrett AD, Jones DL, Murphy DV (2011) Soil microbial biomass—interpretation and consideration for soil monitoring. Soil Res 49:287–304. https://doi.org/10.1071/SR10203
Grigg AH, Sheridan GJ, Pearce AB, Mulligan DR (2006) The effect of organic mulch amendments on the physical and chemical properties and revegetation success of a saline-sodic mine spoil from central Queensland, Australia. Aust J Soil Res 44:97–105. https://doi.org/10.1071/SR05047
Groot JJR, Houba VJG (1995) A comparison of different indices for nitrogen mineralization. Biol Ferti Soils 19:1–9. https://doi.org/10.1007/BF00336338
Guo Y, Chen X, Wu Y, Zhang L, Cheng J, Wei G, Lin Y (2018) Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci Total Environ 635:598–606. https://doi.org/10.1016/j.scitotenv.2018.04.171
Hale R, Reich P, Daniel T, Lake PS, Cavagnaro TR (2018) Assessing changes in structural vegetation and soil properties following riparian restoration. Agric Ecosyst Environ 252:22–29. https://doi.org/10.1016/j.agee.2017.09.036
Han Z, Deng M, Yuan A, Wang J, Li H, Ma J (2018) Vertical variation of a black soil’s properties in response to freeze-thaw cycles and its links to shift of microbial community structure. Sci Total Environ 625:106–113. https://doi.org/10.1016/j.scitotenv.2017.12.209
Hanson CA, Allison SD, Bradford MA, Wallenstein MD, Treseder KK (2008) Fungal taxa target different carbon sources in forest soil. Ecosystems 11:1157–1167. https://doi.org/10.1007/s10021-008-9186-4
Helander M, Saloniemi I, Omacini M, Druille M, Salminen JP, Saikkonen K (2018) Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci Total Environ 642:285–291. https://doi.org/10.1016/j.scitotenv.2018.05.377
Huang Y, Pan H, Wang Q, Ge Y, Liu W, Christie P (2019) Enrichment of the soil microbial community in the bioremediation of a petroleum-contaminated soil amended with rice straw or sawdust. Chemosphere 224:265–271. https://doi.org/10.1016/j.chemosphere.2019.02.148
Huang Z, Xu Z, Chen C (2008) Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl Soil Ecol 40:229–239. https://doi.org/10.1016/j.apsoil.2008.04.009
Jangid K, Williams MA, Franzluebbers AJ, Sanderlin JS, Reeves JH, Jenkins MB, Endale DM, Coleman DC, Whitman WB (2008) Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol Biochem 40:2843–2853. https://doi.org/10.1016/j.soilbio.2008.07.030
Jenkinson DS, Powlson DS (1976) The effects of biocidal treatments on metabolism in soil V: a method for measuring soil biomass. Soil Biol Biochem 8:209–213. https://doi.org/10.1016/0038-0717(76)90005-5
Jordan D, Kremer RJ, Bergfield WA, Kim KY, Cacnio VN (1995) Evaluation of microbial methods as potential indicators of soil quality in historical agricultural fields. Biol Fertil Soils 19:297–302. https://doi.org/10.1007/BF00336098
Kader MA, Senge M, Mojid MA, Ito K (2016) Recent advances in mulching materials and methods for modifying soil environment. Soil till Res 168:155–166. https://doi.org/10.1016/j.still.2017.01.001
Laitinen P, Rämö S, Siimes K (2007) Glyphosate translocation from plants to soil–does this constitute a significant proportion of residues in soil? Plant Soil 300:51–60. https://doi.org/10.1007/s11104-007-9387-1
Lancaster SH, Hollister EB, Senseman SA, Gentry TJ (2009) Effects of repeated glyphosate applications on soil microbial community composition and the mineralization of glyphosate. Pest Manage Sci 66:59–64. https://doi.org/10.1002/ps.1831
Lauber CL, Strickland MS, Bradford MA, Fierer N (2008) The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol Biochem 40:2407–2415. https://doi.org/10.1016/j.soilbio.2008.05.021
Li D, Niu S, Luo Y (2012) Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: a meta-analysis. New Phytol 195:172–181. https://doi.org/10.1111/j.1469-8137.2012.04150.x
Li F, Song Q, Jjemba PK, Shi Y (2004) Dynamics of soil microbial biomass C and soil fertility in cropland mulched with plastic film in a semiarid agro-ecosystem. Soil Biol Biochem 36:1893–1902. https://doi.org/10.1016/j.soilbio.2004.04.040
Mahal NK, Castellano MJ, Miguez FE (2019) Potentially mineralizable nitrogen: a soil health indicator. Crops Soils 52:8–10. https://doi.org/10.2134/cs2019.52.0406
Malty JDS, Siqueira JO, Moreira FMDS (2006) Effects of glyphosate on soybean symbiotic microorganisms, in culture media and in greenhouse. Pesqui Agropecu Bras 41:285–291. https://doi.org/10.1590/S0100-204X2006000200013
Murungu FS, Chiduza C, Muchaonyerwa P, Mnkeni PNS (2011) Mulch effects on soil moisture and nitrogen, weed growth and irrigated maize productivity in a warm-temperate climate of South Africa. Soil till Res 112:58–65. https://doi.org/10.1016/j.still.2010.11.005
Nguyen DB, Rose MT, Rose TJ, Morris SG, Van Zwieten L (2016) Impact of glyphosate on soil microbial biomass and respiration: a meta-analysis. Soil Biol Biochem 92:50–57. https://doi.org/10.1016/j.soilbio.2015.09.014
Nguyen TT, Wallace HM, Xu CY, Zwieten LV, Weng ZH, Xu Z, Che R, Tahmasbian I, Hu HW, Bai SH (2018) The effects of short term, long term and reapplication of biochar on soil bacteria. Sci Total Environ 636:142–151. https://doi.org/10.1016/j.scitotenv.2018.04.278
Omidvar N, Xu Z, Nguyen TT, Salehin B, Ogbourne S, Ford R, Bai SH (2021) A global meta-analysis shows soil nitrogen pool increases after revegetation of riparian zones. J Soils Sediments 21:665–677. https://doi.org/10.1007/s11368-020-02864-0
Olley J, Burton J, Hermoso V, Smolders K, McMahon J, Thomson B, Watkinson A (2015) Remnant riparian vegetation, sediment and nutrient loads, and river rehabilitation in subtropical Australia. Hydrop Process 29:2290–2300. https://doi.org/10.1002/hyp.10369
Osono T (2005) Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97:589–597. https://doi.org/10.1080/15572536.2006.11832789
Praeg N, Seeber J, Leitinger G, Tasser E, Newesely C, Tappeiner U, Illmer P (2020) The role of land management and elevation in shaping soil microbial communities: insights from the Central European Alps. Soil Biol Biochem 150:107951. https://doi.org/10.1016/j.soilbio.2020.107951
Primost JE, Marino DJ, Aparicio VC, Costa JL, Carriquiriborde P (2017) Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem. Argentina Enviro Pollut 229:771–779. https://doi.org/10.1016/j.envpol.2017.06.006
Puértolas L, Damasio J, Barata C, Soares AM, Prat N (2010) Evaluation of side-effects of glyphosate mediated control of giant reed (Arundo donax) on the structure and function of a nearby Mediterranean river ecosystem. Environ Res 110:556–564. https://doi.org/10.1016/j.envres.2010.05.004
Qiao H, Zhang L, Shi H, Song Y, Bian C (2018) Astragalus affects fecal microbial composition of young hens as determined by 16S rRNA sequencing. AMB Express 8:70. https://doi.org/10.1186/s13568-018-0600-9
Romaniuk R, Giuffré L, Costantini A, Nannipieri P (2011) Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol Indic 11:1345–1353. https://doi.org/10.1016/j.ecolind.2011.02.008
Ros GH, Temminghoff EJ, Hoffland E (2011) Nitrogen mineralization: a review and meta-analysis of the predictive value of soil tests. Eur J Soil Sci 62:162–173. https://doi.org/10.1111/j.1365-2389.2010.01318.x
Rousk J, Brookes PC, Bååth E (2010) Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol Biochem 42:926–934. https://doi.org/10.1016/j.soilbio.2010.02.009
Schimel JP, Gulledge JM, Clein-Curley JS, Lindstrom JE, Braddock JF (1999) Moisture effects on microbial activity and community structure in decomposing birch litter in the Alaskan taiga. Soil Biol Biochem 31:831–838. https://doi.org/10.1016/S0038-0717(98)00182-5
Schirmer,J, Field J (2014) The cost of revegetation: final report. ANU forestry & greening Australia project. Available at: https://www.researchgate.net/publication/240630054. Accessed 29 Sep 2017
Trasar-Cepeda C, Leiros C, Gil-Sotres F, Seoane S (1998) Towards a biochemical quality index for soils: an expression relating several biological and biochemical properties. Biol Fertil Soils 26:100–106. https://doi.org/10.1007/s003740050350
Urbanová M, Šnajdr J, Baldrian P (2015) Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol Biochem 84:53–64. https://doi.org/10.1016/j.soilbio.2015.02.011
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Wang J, Fu X, Sainju UM, Zhao F (2018) Soil carbon fractions in response to straw mulching in the Loess Plateau of China. Biol Fertil Soils 54:423–436. https://doi.org/10.1007/s00374-018-1271-z
Ward NL, Challacombe JF, Janssen PH, Henrissat B, Coutinho PM, Wu M, Xie G, Haft DH, Sait M, Badger J, Barabote RD (2009) Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microbiol 75:2046–2056. https://doi.org/10.1128/AEM.02294-08
Williams JD, Crozier CR, White JG, Sripada RP, Crouse DA (2007) Illinois soil nitrogen test predicts southeastern U.S. corn economic optimum nitrogen rates. Soil Sci Soc Am J 71:735–744. https://doi.org/10.2136/sssaj2006.0135
Wongdee J, Boonkerd N, Teaumroong N, Tittabutr P, Giraud E (2018) Regulation of nitrogen fixation in Bradyrhizobium sp. strain DOA9 involves two distinct NifA regulatory proteins that are functionally redundant during symbiosis but not during free-living growth. Front Microbiol 9:1644. https://doi.org/10.3389/fmicb.2018.01644
Yang K, Zhu J, Zhang M, Yan Q, Sun OJ (2010) Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol 3:175–182. https://doi.org/10.1093/jpe/rtq022
Yao Y, Wang R, Lu JK, Sui XH, Wang ET, Chen WX (2014) Genetic diversity and evolution of Bradyrhizobium populations nodulating Erythrophleum fordii, an evergreen tree indigenous to the southern subtropical region of China. Appl Environ Microbiol 80:6184–6194. https://doi.org/10.1128/AEM.01595-14
Ye C, Cheng X, Zhang Y, Wang Z, Zhang Q (2012) Soil nitrogen dynamics following short-term revegetation in the water level fluctuation zone of the Three Gorges Reservoir, China. Ecol Eng 38:37–44. https://doi.org/10.1016/j.ecoleng.2011.10.005
You J, Wu G, Ren F, Chang Q, Yu B, Xue Y, Mu B (2016) Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl Microbiol Biotechnol 100:1469–1478. https://doi.org/10.1007/s00253-015-7073-4
Zablotowicz RM, Reddy KN (2004) Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: a minireview. J Environ Qual 33:825–831. https://doi.org/10.2134/jeq2004.0825
Zhang G, Zhang P, Cao Y (2018a) Ecosystem carbon and nitrogen storage following farmland afforestation with black locust (Robinia pseudoacacia) on the Loess Plateau, China. J Res 29:761–771. https://doi.org/10.1007/s11676-017-0479-3
Zhang K, Cheng X, Shu X, Liu Y, Zhang Q (2018b) Linking soil bacterial and fungal communities to vegetation succession following agricultural abandonment. Plant Soil 431:19–36. https://doi.org/10.1007/s11104-018-3743-1
Zhang S, Wang Y, Sun L, Qiu C, Ding Y, Gu H, Wang L, Wang Z, Ding Z (2020) Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol 20:1–13. https://doi.org/10.1186/s12866-020-01794-8
Zhao FZ, Ren CJ, Zhang L, Han XH, Yang GH, Wang J (2018) Changes in soil microbial community are linked to soil carbon fractions after afforestation. Eur J Soil Sci 69:370–379. https://doi.org/10.1111/ejss.12525
Zumsteg A, Luster J, Göransson H, Smittenberg RH, Brunner I, Bernasconi SM, Zeyer J, Frey B (2012) Bacterial, archaeal and fungal succession in the forefield of a receding glacier. Microbial Ecol 63:552–564. https://doi.org/10.1007/s00248-011-9991-8
Acknowledgements
We acknowledge the assistance of the University of the Sunshine Coast, Griffith University, the Chemistry Centre, and Soil, Catchment and Riverine Processes Group, Department of Environment and Science. We also acknowledge the Mary River Catchment Coordinating Committee (MRCCC) and Australian Government Clean Energy Future package project for funding the project. We appreciate the support received from the landholders, J and C Smith, for providing access to the project site, Noosa District Landcare group for the application of treatments in this project and BOS Rural for advice on choosing organic herbicides. We are also grateful to Ms. Roya Esmaeilani and Dr. Trong Tran for their kind assistance with the fieldwork.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Yan He
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omidvar, N., Ogbourne, S.M., Xu, Z. et al. Effects of herbicides and mulch on the soil carbon, nitrogen, and microbial composition of two revegetated riparian zones over 3 years. J Soils Sediments 23, 2766–2782 (2023). https://doi.org/10.1007/s11368-023-03530-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-023-03530-x