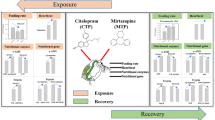
Abstract
The antidepressant effect of zinc on mammals has been documented in recent decades, and the concentration of the antidepressant fluoxetine (FLX) in aquatic environments has been rising constantly. The aim of the present study is to evaluate the combined toxicity of a serotonin reuptake inhibitor (FLX) and Zn2+ on a non-target aquatic model organism Daphnia magna. Animals were exposed to single and binary combinations of FLX (20.5 and 41 µg/L for subchronic and 41 and 82 µg/L for acute exposures) and Zn2+ (40 µg/L for subchronic and 80 µg/L for acute exposures). In vivo experiments were done for 7 days subchronic and 48 h acute exposure, while subcellular supernatants of whole Daphnia lysate (WDL) were directly treated with the same concentrations used in the acute experiments. Morphological characteristics, Ca2+-ATPase, antioxidant enzyme activities, and lipid peroxidation were examined. There was antioxidant system suppression and Ca2+-ATPase inhibition despite the diverse response patterns due to duration, concentration, and toxicant type. After acute exposure, biomarkers showed a diminishing trend compared to subchronic exposure. According to integrated biomarker response index (IBR) analysis, in vivo Zn2+ exposure was reasonably effective on the health of D. magna, whereas exposure of WDL to Zn2+ had a lesser impact. FLX toxicity increased in a concentration-dependent manner, reversed by the combined exposure. We concluded that potential pro-oxidative and adverse Ca2+-ATPase effects of FLX and Zn2+ in D. magna may also have harmful impact on ecosystem levels. Pharmaceutical exposure (FLX) should be considered along with their potential to interact with other toxicants in aquatic biota.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Daphnia magna is an important component of aquatic biota which is a critical link between primary producers and higher-level consumers in freshwater ecosystems (Cox et al. 2018). This species is frequently found in these environments and is frequently employed as a model organism in toxicological research (OECD 2004); as a result, its possible reactions to a wide variety of pollutants are well-known (Campos et al. 2012b; Ding et al. 2017). Moreover, D. magna is the most studied model organism to determine the aquatic toxicity of pharmaceuticals (Bergmann et al. 2011). Hence, the rationale behind choosing this species for investigating the combined effects of aquatic toxicants, such as specific serotonin reuptake inhibitors (SSRIs), may be justified in the absence of an exhaustive exploration of detailed mechanistic pathophysiological pathways. Concurrently, certain physiological mechanistic details at the gene and/or protein level for this species have already been elucidated. For example, the presence of serotonin and functionally related proteins in daphnid species has been demonstrated by different studies (Ehrenström and Berglind 1988; McCoole et al. 2012). Therefore, any substance that modifies serotonin activity can subtly change these species’ regular endocrine and metabolic processes.
The daily dose of antidepressants taken per 1000 people across all OECD member nations showed a steady rise in usage (OECD Statistics 2021). One of the top 10 most given antidepressant medications is fluoxetine (FLX), an SSRI (Barakat et al. 2018). By binding to serotonin transporter proteins (SERT), FLX prevents the reuptake of serotonin at the presynaptic opening (Roman et al. 2003). FLX has been identified often in aquatic environments across the globe (Vasskog et al. 2008; Metcalfe et al. 2010; Mezzelani et al. 2018), in concentrations that can reach up to 0.6 µg/L (Hughes et al. 2013). In addition, higher FLX and its metabolite norFLX have been documented recently in wastewater treatment plant influents (3.5 and 10.4 µg/L, respectively) (Mole and Brooks 2019). FLX has accumulation potential in D. magna (Ding et al. 2017). It was underlined that FLX can easily enter the body with the nutrient flow and be taken up via the thin wall of the carapace during respiratory gas exchange in this species because of its thin exoskeleton and high surface area/volume ratio.
Numerous biological endpoints including growth and reproduction, oxidative stress response, and transcriptomic and metabolomic responses have been analyzed in studies investigating the effects of FLX in daphnid species (Flaherty and Dodson 2005; Campos et al. 2012a, 2016; Jordão et al. 2016; Ding et al. 2017; Stremmel et al. 2023). According to these studies, it is understood that FLX has a harmful potential on daphnid populations. After 7 days of exposure, superoxide dismutase (SOD) activity was reduced by 0.5 µg/L and increased by 5 µg/L FLX, although lipid peroxidation levels showed an opposite trend in D. magna (Ding et al. 2017). No alterations were observed in glutathione S-transferase (GST) activity or lipid peroxidation levels in D. magna following a 72-h exposure to 1 and 5 mg/L of FLX (Varano 2014). We previously demonstrated that FLX at environmental concentration (0.091 µg/L) has no prooxidant potential in D. magna after 21 days of chronic exposure (Över et al. 2020). However, glutathione peroxidase (GPX) activity and lipid peroxidation levels increased at 10 × and 100 × of this concentration after 4 and 21 days of exposure. Exposure to FLX at concentrations of 40 and 80 µg/L has been associated with various documented biological consequences (Campos et al. 2012a, 2016). According to Stremmel et al. (2023), FLX concentrations in aquatic environments will eventually reach lethal concentrations (up to 800 µg/L) in the worst-case scenario for future projections; therefore, we selected higher concentrations than current environmental levels for this study.
The antidepressant-like effect of Zn in mammals has been recognized for several decades. This element is a natural component of aquatic habitats. Human activity can cause the concentration of Zn to increase to dangerous levels (Trevisan et al. 2014). The possibility of coexistence of Zn with some pharmaceuticals like antidepressant FLX in aquatic environments is increasing with the increased usage in human treatments. Moreover, combined therapeutic treatment with Zn and antidepressant drugs like FLX in humans was proposed by many authors because of synergistic effects (Cunha et al. 2008; Refaey et al. 2015), while concentration-dependent antagonistic effects were presented (García-Colunga et al. 2005) as explained below. Therefore, the assessment of combined toxicity of these substances may be indicative of aquatic ecosystem health, as Zn has the potential to amplify the effects of low environmental concentrations of antidepressants.
Zinc, an essential micronutrient, plays multifaceted roles in physiological pathways. (Muyssen and Janssen 2002; Oteiza 2012). However, its excess can induce neurotoxicity in various animal groups such as crustaceans and mammals (Szewczyk 2013; Kukavica et al. 2023), while deficiency in mammals elevates the risk of neurological disorders like depression (Szewczyk 2013). In mammals, Zn can affect a range of receptors or transporters at pre- and post-synaptic sides (Doboszewska et al. 2017). For example, it exhibits a concentration-dependent biphasic effect on agonist binding to the serotonin receptor 5-HT1a (Satała et al. 2016). The antidepressant-like effect of zinc is impeded by serotonin synthesis inhibition or specific receptor antagonism (Szewczyk et al. 2009). Structural disparities in serotonin receptors between vertebrates and arthropods (and other invertebrates) suggest functional variations (Tierney 2001). D. pulex have been found to express 5-HT1a receptors, which are structural and functional similarity with Drosophila 5-HT1a receptors (McCoole et al. 2012). It has been suggested that Zn may interact with these receptors to influence the physiological processes related to serotonin in these species.
The stress brought about by toxicant exposure triggers many biological reaction mechanisms and simultaneous analysis of numerous biomarkers can provide valuable insights into the stress and toxicity mechanism of a particular toxicant, offering a comprehensive understanding of their effects on biological systems (Van der Oost et al. 2003). These biomarkers play a crucial role in various stages of the risk assessment process, including effect, exposure, and hazard assessment, as well as risk characterization or classification, and monitoring the environmental quality of aquatic ecosystems (Iturburu et al. 2018). Determining the susceptibility of an organism to a specific substance through biomarkers poses challenges without a statistical approach to evaluate the global trend of toxicity. According to Devin et al. (2014), the integrated biomarker response index (IBR) approach yields a graphical synthesis of the various responses and a numerical value that simultaneously incorporates all the biomarker responses. The IBR method is an effective tool for visualization of the pollutant impact and simplifying the interpretation of relationships between multiple biomarkers of different pathways and exposure levels (Devin et al. 2014), especially when biomarkers within the same metabolic pathway show no correlation across different exposure scenarios (Potet 2017). Additionally, it aids in evaluating the sensitivity of an organism, tissue, or cell to a toxicant or its concentrations by considering the responses of various biomarkers (Kim et al. 2014). Oxidative stress-related processes garner significant attention due to their sensitivity and prevalence in numerous pathophysiological situations (Van der Oost et al. 2003). The most studied parameters to comprehend the prooxidative nature of toxicants are SOD, catalase (CAT), antioxidant tripeptide glutathione (GSH), and its related enzymes GPX and GST, as well as lipid peroxidation levels (measured as thiobarbituric acid reactive substances [TBARS]). In D. magna, ATPase enzymes were successfully used as a sensitive biomarker of metal and pesticide exposures in earlier studies because they are crucial for ionic equilibrium (Mahmut et al. 2022; Sevgiler and Atli 2022). For example, there is an active Ca2+ cycle in daphnid species because of loss of 90% of their calcium with their carapace during moulting period, and Ca2+-ATPase is important for active transport of calcium into the body (Cox et al. 2018).
Fluoxetine and Zn co-administration can affect the antioxidant response in mammals. Separate Zn treatment had no effect on the expression of the genes involved in the antioxidant system, such as nuclear factor-erythroid 2-related factor 2 (Nrf-2) and heme oxygenase-1 (HO-1). However, FLX exhibited an antioxidant-response inducing effect, leading to enhanced expression of these genes. The metallothionein gene’s expression rose in the presence of these substances, and the greatest rise was found in the FLX and Zn combined treatment group (Omar and Tash 2017). These results increase the possibility that FLX and Zn may also have an impact on the antioxidant system in non-target species like D. magna. Using biomarkers of stress, we attempted to explain the combined toxicity of two distinct chemicals, which might be connected in their therapeutic/toxic mechanism, in a model non-target aquatic organism. To identify potential toxicity, morphometric characteristics, Ca2+-ATPase, and oxidative stress-related biomarkers were analyzed; and the IBR approach was used to identify the global trend in biological responses to these compounds and their combinations in D. magna.
Materials and methods
Animal husbandry
Animals were supplied by the Carolina Biological Supply Company, located in Burlington, North Carolina, and were cultured in our facility for almost 7 years prior to the experiments. Seventy-two D. magna samples were divided into two cassettes, each containing 3000 mL of hard water per the American Society for Testing and Materials International's guidelines (ASTM 2002). These animals were less than 24-h old and the third offspring of their mothers, who were the fifth-generation nestlings of one mother. The water’s physicochemical properties were pH 7.8–8.0, hardness 160–180 mg/L as CaCO3, and alkalinity 110–120 mg/L as CaCO3 (ASTM 2002). The culture was maintained under 12.15 µmol m−2 s−1 light with 16 h: 8 h light: dark photoperiod at 21 °C (Cleresci et al. 1999). The mixture of trout chow, yeast, dried alfalfa (Cleresci et al. 1999), and Roti-Rich™ were used for feeding. Animals were fed every other day at a rate of 1.5 mL/L trout chow-yeast-alfalfa mixture and 200 µL/L Roti-Rich™. After a week, the water was replaced with brand-new ASTM hard water. The first generation was removed and not used in toxicity tests. Prior to the toxicity tests, a typical 24-h EC50 sensitivity test was carried out using the second offspring and K2Cr2O7. The calculated EC50 fell within the interval recommended by the OECD (Organization for Economic Co-operation and Development, (OECD 2004) as 1.108 mg/L (C.I. 1.066–1.140 mg/L). The third offspring were used in subchronic toxicity experiments. For the acute and whole Daphnia lysate (WDL) treatment experiments, 860, less than 24-h old neonates of the fourth offspring were divided equally into three different cassettes containing 12 L ASTM hard water. The cassettes were gently aerated with filtered ambient air. As noted, the animals were fed. After 1 week, the water was replaced with brand-new ASTM hard water. When these animals were 10 days old, acute toxicity experiments were started or they collected for WDL treatment when they were 14 days old.
Toxicity experiments
Less than 24-h or 10 days old, third or fourth offspring animals were divided into six groups for semi-static subchronic (i.e., 7-d) or acute (i.e., 48-h) toxicity experiments, respectively. For each group, five glass beakers with 800 mL of ASTM hard water served as replicates. There were 21 or 20 animals placed into each beaker for subchronic or acute tests, respectively. Stock FLX solution was prepared with fluoxetine hydrochloride, which was a pharmaceutical secondary standard grade (CAS No: 56296–78-7, Supelco). Zn solution was prepared with ZnCl2 (CAS No: 7646–85-7, Sigma-Aldrich). The 48-h EC50 values of FLX and Zn for D. magna were 820 µg/L and 800 µg/L, respectively (Diamantino et al. 2001; Brooks et al. 2003). These values were considered as 1 toxic unit (TU). The sublethal 0.025 and 0.05 TU values of FLX were selected for subchronic (corresponding to 20.5 and 41 µg/L, respectively), while 0.05 and 0.1 TU values for acute and WDL experiments (corresponding to 41 and 82 µg/L, respectively). Zn concentrations were 0.05 and 0.1 TU values corresponding to 40 µg/L and 80 µg/L for subchronic or acute and WDL toxicity experiments, respectively.
The water used in the subchronic experiments was entirely replaced with fresh medium every other day, and 1.5 mL/L of a trout chow-yeast-alfalfa mixture and two drops of Roti-Rich™ were added for animal feeding. Only at the beginning of acute studies where experimental animals fed. The experimental water was totally replaced after 24-h and animals were not fed during this time. Throughout the experimentation, no animal perished.
The physicochemical properties of the experimental water for subchronic experiments were as follows: pH 7.97 ± 0.02, the temperature 21.58 ± 0.78 °C, dissolved oxygen 9.86 ± 0.09 mg/L, and conductivity 544.00 ± 4.55 µS/cm. For acute tests, the corresponding values were pH 7.99 ± 0.02, the temperature 21.53 ± 0.21 °C, dissolved oxygen 9.94 ± 0.16 mg/L, and conductivity 546.67 ± 9.87 µS/cm. Twenty animals from each beaker were collected at the conclusion of the exposure durations, pooled as one replication; the extra water was blotted out with tissue paper, the animals were weighed, and they were kept at – 80 °C until biochemical analyses. The pooled animals underwent homogenization (1:10 w/v) for 2 min on ice using a stainless steel homogenizer in a chilled homogenisation buffer (250 mM sucrose, 20 mM Tris, 1 mM EDTA, pH 7.8). Homogenates were centrifuged at 13,000 × g for 20 min at 4 °C. In the supernatant, total protein concentrations and enzyme activities with TBARS and GSH levels were assessed spectrophotometrically.
The direct effects of FLX and Zn2+ on the selected biomarkers by reducing the organismal and/or cellular borders, 13,000 × g supernatants of D. magna were divided into the groups mentioned in acute experiments. After this point, this experimental setting was named as “WDL.” A total of 36 14-day-old animals per group were aggregated in microtubes, subjected to homogenization, and centrifuged to assess the WDL effect. Six microtubes were prepared for this purpose. To compare the outcomes of acute in vivo and WDL effects, acute exposure concentrations were employed. Each toxicant was promptly mixed with the acquired supernatant, and a 30-min incubation period was allowed at room temperature. For a minimum of triplicate biochemical examination, the processed supernatant was utilized.
Morphometric characteristics analysis
After subchronic experiments, one animal from each beaker was randomly selected and collected in tubes containing 50% ethanol and kept at 4 °C until morphometric analysis. Concave microscope slides containing lactic acid solutions were used. Animals were captured on camera using an Olympus BX53 binocular microscope that has a Canon EOS1200D camera attached. The caudal spine was excluded from this measurement, which assessed the length of the carapace between the anterior head and the base of the spine (Zhu et al. 2010). This morphometric analysis was conducted as an additional toxicity parameter. Maximum carapace width was measured laterally from the entire width of carapace ends. Micam v3.0 imaging software (http://www.science4all.nl/?Microscopy_and_Photography) was used for scaling. An analytical balance (HR250AZ, AND Company Ltd., Tokyo, Japan) was used to measure the combined weight of the pooled animals that were gathered for biochemical examination. The average weight of one animal was subsequently expressed as mg of wet weight.
Biochemical analysis
Ca2+-ATPase activity was measured in an incubation medium (pH 7.7) comprising 40 mM Tris–HCl, 4 mM MgCl2, 1 mM CaCl2, and 1 mM ethylene glycol‐bis (2‐aminoethyl ether)‐,N,N′,N′‐tetra acetic acid (EGTA). Then, 8 μL of supernatant was added to incubation medium, reaching a total volume of 250 µL, and preincubated for 5 min at 37 °C. The reaction was initiated by adding 25 μL Na2ATP (3 mM) and incubating for 30 min. To stop the reaction, 125 μL of ice‐cold distilled water was added. Inorganic phosphate (Pi) was measured as described by Atkinson et al. (1973). Appropriate blanks were included with each assay to correct the non-enzymatic hydrolysis of ATP. KH2PO4 (25–250 μM) was used as the Pi standard and the spectrophotometric analysis was performed at 390 nm. The Ca2+-ATPase activity was quantified as the absorbance difference between the presence and absence of CaCl2, expressed as µmol Pi/mg protein/h.
SOD activity was measured by the indirect method, involving the inhibition of cytochrome c reduction at 550 nm for 1 min (McCord and Fridovich 1969). The reaction buffer, in a final volume of 1 mL, comprised 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 10 µM cytochrome c, 0.05 mM hypoxanthine, and 10 µL of the supernatant. The reaction was started by adding 1.88 mU/mL xanthine oxidase (XOD), with the XOD blank was used to adjust the XOD concentration. SOD activity was expressed as unit/mg protein, defined as the amount of enzyme that causes 50% inhibition of cytochrome c reduction.
CAT activity was measured according to the method of Lartillot et al. (1988). Twenty μL of supernatant, containing 0.2 mg protein/mL, were mixed with 2.5 mL of 25 mM H2O2 in a 75 mM phosphate buffer at pH 7.0. The CAT activity was calculated as μmol H2O2 decomposed/mg protein/min, using a specific absorption coefficient of 0.0392 cm2 μmol−1 H2O2, at 240 nm.
GPX activity was measured in a 1 mL reaction buffer consisting of 100 mM potassium phosphate buffer (pH 7.0), 2 mM GSH, 0.12 mM NADPH, 2 U glutathione reductase (GR), 10 µL supernatant, and 3 mM cumene hydroperoxide (Livingstone et al. 1992). The activity was expressed as µmol/mg protein/min estimating the decrease of NADPH per minute at 340 nm, with a specific absorption coefficient is 6.22 M−1 cm−1 NADPH at 340 nm.
GST activity was evaluated by an increase of absorbance per minute at 340 nm resulting from the conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) (Habig et al. 1974). The specific absorption coefficient of the conjugate S-(2,4-dinitrophenyl)-glutathione is 9.6 mM−1 cm−1 at 340 nm. In a 1-mL reaction buffer containing 100 mM potassium phosphate buffer (pH 7.5), 1 mM GSH, 1 mM CDNB, and 10 µL supernatant, GST activity was expressed as µmol/mg protein/min.
GSH levels were analyzed by measuring the absorbance increase at 412 nm per minute and expressed as micromoles of GSH equivalents per milligrams of protein (Griffith 1980). A standard curve was prepared with GSH, and the reaction buffer in a final volume of 1 mL contained 100 mM sodium phosphate buffer (pH 7.5), 4.2 mM NADPH, 0.8 mM DTNB, 75 U/mL GR, and 25 µL supernatant.
Lipid peroxidation levels was assessed using the TBARS assay, which measures the lipid peroxidation products that react with thiobarbituric acid (TBA). The TBARS analysis was performed based on the incubation of supernatants and TBA in aerobic conditions at the temperature of 100 °C. TBARS concentrations were determined by measuring the formation of pink-coloured complex at 532 nm (Wills 1966). The calculations were based on an external standard curve generated using 1,1′,3,3′-tetramethoxypropane, and the values were expressed in nanomoles/mg protein.
Total protein content was measured spectrophotometrically at 750 nm using the Lowry method (Lowry et al. 1951), with bovine serum albumin serving as an external standard. The total protein content was expressed as mg per mL supernatant. All assays were conducted in duplicate.
Integrated biomarker response index calculations
The integrated biomarker response index (IBR), as described by Devin et al. (2014), was calculated to combine all results from various biomarkers and comprehend global/general reactions. We used the code presented on the website (https://liec-univ-lorraine.shinyapps.io/calibri/) by Devin et al. (2023). Briefly, the general mean (m) and standard deviation (s) for each biomarker were calculated across all data. Subsequently, a standardization was employed for each circumstance to yield Y, where Y = (X − m)/s, where X is the mean value for the biomarker at a specific group. The Z value was then determined using Z = − Y or Z = Y, signifying inhibition or stimulation, respectively, in the case of a biological effect. The S value was calculated using the formula S = Z +|Min|, where Min represents the smallest value observed across all exposure groups for each biomarker in a specific exposure time. The Si values, representing the standardized values of each biomarker, were plotted on a radar diagram. The total area depicted on the radar diagram is used to calculate the IBR. When seven or eight biomarkers are considered in a k-biomarker study, the area of the triangle formed by two subsequent biomarkers is defined as follows:
The IBR value is then determined as follows:
Due to the neighborhood effect, all conceivable circular permutations of k biomarkers were taken into consideration when calculating IBR values for subchronic, acute, or WDL exposure regimes. For all exposure regimes, the same set of parameters were used in the IBR calculations except for the animal weight in subchronic duration. This adjustment was necessitated by the 8-biomarker limitation in the code.
Statistical analysis
Statistical analysis was performed in IBM SPSS Statistics 23 statistical software. The Kolmogorov–Smirnov normality test was applied before further analysis. One-way ANOVA was applied for normally distributed data before Levene’s homogeneity of variance test. All the data were categorised as homogeneous subsets, and then Duncan’s post hoc multiple range tests were used. Mann–Whitney U test was applied after the Kruskal–Wallis analysis for non-normal distributed data. All the results were given as mean ± standard error (N = 5). Spearman rho correlation was also applied to compare the studied parameters’ correlations (P < 0.05). Paired two-sample t-test was used to compare the IBR values in a specific exposure regime.
Results
Table 1 shows the subchronic effect of FLX, Zn, or their mixtures on the morphometric traits of D. magna. The maximum carapace width and length remained unaffected. Subchronic Zn0.05TU exposure decreased the caudal spine length in comparison to the control and FLX0.025TU + Zn0.05TU by 15% and 14%, respectively (P < 0.05). All exposed daphnids were impacted by Zn exposure in comparison to the control group, leading to weight decreases of 6.5%, 11% (P < 0.05), and 14% (P < 0.05) in the groups exposed to Zn0.05TU, FLX0.025TU + Zn0.05TU, and FLX0.05TU + Zn0.05TU, respectively.
Subchronic FLX0.025TU exposure caused a 36% decrease in Ca2+-ATPase activity compared to the control (P < 0.05). However, there was an increased activity of 30–63% upon FLX0.05TU + Zn0.05TU exposure compared to the other groups (P < 0.05), excluding the control (P > 0.05). Ca2+-ATPase activity enhanced in the range of 39–161% following acute FLX0.05TU exposure compared to the other groups (P < 0.05), while Zn significantly declined the activity to 44–62% in comparison with all groups apart from the FLX0.1TU group (P < 0.05). WDL exposure significantly reduced the Ca2+-ATPase activity in the percent range of 42–72 in all groups in contrast to the control (P < 0.05). On the other hand, Ca2+-ATPase activity exhibited a declining value after Zn (84–112%) and Zn + FLX exposures (51–112%) compared to the single FLX effect (P < 0.05) (Table 2).
SOD activity increased by 24% in the FLX0.05TU group and by 27–28% in the FLX0.05TU + Zn0.05TU group, contrasting with the FLX0.025TU and Zn0.05TU groups, respectively after subchronic exposure (P < 0.05). However, its activity decreased in Zn0.1TU and FLX0.05TU + Zn0.1TU groups when compared to the control, with a range of 40–43% (P < 0.05), and compared to single FLX groups, with a range of 48–54% (P < 0.05), upon acute exposure. WDL exposure inhibited SOD activity in all groups ranging from 17 to 34% compared to the control (P < 0.05). Notably, SOD activity in the FLX0.05TU group was higher by 17% and 26% than in the Zn0.1TU and FLX0.05TU + Zn0.1TU groups, respectively (P < 0.05) (Table 3, 4, and 5).
Subchronic FLX0.025TU exposure decreased the CAT activity in contrast to the control, FLX0.05TU, and Zn0.05TU groups in a percent range of 37–38% (P < 0.05). CAT activity increased upon acute FLX0.05TU exposure in a range of 30–41% when compared to all groups (P < 0.05), except for the control (P > 0.05). However, the FLX0.05TU + Zn0.1TU group significantly decreased to 26% and 37% when compared to both the control and FLX0.05TU groups, respectively (P < 0.05). WDL exposure also caused a decline in CAT activity of FLX0.05TU (19%), FLX0.1TU (14%), Zn0.1TU (19%), and FLX0.1TU + Zn0.1TU (21%) groups when compared to the control (P < 0.05). On the other hand, FLX0.05TU + Zn0.1TU exposure led to a higher CAT activity in order of 20%, 20%, and 23% than the groups of FLX0.05TU, Zn0.1TU, and FLX0.1TU + Zn0.1TU (P < 0.05) (Table 3, 4, and 5).
In single FLX-exposed groups, GPX activity reduced by 33–47% when compared to the control, Zn0.05TU, and FLX0.025TU + Zn0.05TU groups after subchronic exposure (P < 0.05). GPX activity was significantly decreased in all exposed groups, ranging from 44 to 100%, in contrast to the control (P < 0.05) while acute FLX0.05TU + Zn0.1TU exposure completely inhibited it (P < 0.05). GPX activity remained unaffected in single FLX groups (P > 0.05), while its activity was totally inhibited (100%) in single Zn and combined groups when compared to the control after WDL exposure (P < 0.05) (Table 3, 4, and 5).
Subchronic Zn0.05TU exposure caused a significant decline to 19–26% in GST activity in contrast to all exposed groups (P < 0.05) except the FLX0.05TU + Zn0.05TU group (P > 0.05). On the other hand, GST activity was higher at 36% and 22% in the FLX0.05TU group compared to both Zn0.05TU and FLX0.05TU + Zn0.05TU groups, respectively (P < 0.05) (Table 3). However, GST activity was not affected by both acute and WDL exposures (P > 0.05) (Table 4 and 5).
A decrease was observed in GSH level in subchronic exposure (P < 0.05) as well excluding the FLX0.025TU group which was higher than all groups at a 49–100% range (P < 0.05). Similarly, following acute exposure, GSH levels declined to 41–100% across all groups in contrast to the control (P < 0.05). Additionally, FLX0.05TU exposed group had higher GSH level than the other exposure groups (P < 0.05). WDL exposure also caused a GSH decrement (82–100%) in groups following single Zn0.1TU and Zn + FLX exposures (P < 0.05), whereas its level increased after FLX0.1TU exposure when compared to others (P < 0.05) (Table 3, 4, and 5).
Subchronic FLX0.05TU + Zn0.05TU exposure increased TBARS levels in comparison with the control (29%), Zn0.05TU (23%), and FLX0.025TU + Zn0.05TU (40%) groups (P < 0.05) (Table 3). The same pattern was observed in acute exposure to FLX0.1TU + Zn0.1TU in the range of 28–38% after when compared to the other groups (P < 0.05) except for the control (P > 0.05) (Table 4). Elevated levels of TBARS (27–46%) were also observed in all exposed groups (P < 0.05) except the FLX0.05TU group in contrast to the control (P > 0.05) after WDL exposure (Table 5). Among the groups, FLX0.05TU + Zn0.1TU exposure caused the highest TBARS levels.
Subchronic exposure caused any significant alteration in protein levels (P > 0.05) (Table 3). However, they were significantly stimulated (14–31%) by all exposed groups following acute exposure when compared to the control (P < 0.05) (Table 4).
All exposure groups and regimes yielded harmful consequences when compared to their respective controls (P < 0.05), as determined by the generalized biomarker response analysis through IBR calculation (Table 6, 7, and 8). The highest IBR value corresponds the most pronounced biological effect, but a decrease in IBR values can also indicate a negative impact of a toxicant in our experimental setting (Dr Simon Devin, personal communication). Therefore, both increases or decreases in IBR values compared to control groups signify harmful pollutant effects. These changes might be related to the contamination level or toxicant concentration.
Following acute or subchronic in vivo exposures, damage from Zn2+ alone or in combination with FLX was enhanced (P < 0.05). On the contrary, the lowest effect was observed after WDL Zn2+ exposure. It is interesting to note that the highest FLX concentrations when combined with Zn2+ showed an improvement after acute in vivo and WDL exposures compared to control, single FLX, and/or Zn2+ groups. The IBR value decreased when FLX concentration was increased after in all exposure regimes (P < 0.05), indicating that the negative impacts produced in individual FLX groups were concentration-dependent.
In subchronic exposure regime, the most detrimental impact was observed in the FLX0.025TU + Zn0.05TU exposure group (Table 6), and CAT, GPX, GSH, TBARS, Ca2+-ATPase, and animal weight appear to be the factors that caused this effect (Fig. 1). In this group, all biomarkers, except GSH, exhibited a declining trend without statistical significance (Tables 1, 2, and 3). In acute exposure regime, Zn2+ demonstrated the greatest harmful consequences (Table 7), with its effects primarily driven by SOD, GPX, TBARS, and Ca2+-ATPase. A statistical analysis revealed that these biomarkers had already declined following acute Zn exposure (Table 4). The FLX0.05TU + Zn0.1TU group experienced decreased IBR values that were comparable to those of the Zn exposure, indicating that this group also experienced the effects of Zn. SOD, CAT, GST, GSH, and TBARS all had an impact on the IBR value in this group (Fig. 2). In WDL exposure regime, the most significant biological alteration is caused by FLX0.1TU exposure (Table 8), influenced by all the parameters analysed (Fig. 3). A statistical analysis of all biomarkers in this group, except for GPX and GST enzyme activities, revealed a trend toward either a decreasing or an increasing pattern (Table 5).
Discussion
In this study, the detrimental effects of both single and combined FLX and Zn were revealed in the non-target organism D. magna. This revelation was made possible through the sensitive responses of the antioxidant system and Ca2+-ATPase biomarkers along with IBR analyses. According to obtained results, it is suggested that the animals face substantial toxicity of Zn2+ and FLX, albeit not reaching lethal levels.
Morphometric characteristics
Single FLX did not exert any influence on morphometric traits at the tested concentrations. However, FLX exacerbated the effects of single Zn2+ on animal weight. Muyssen et al. (2006) proposed that Zn-mediated inhibition of Ca2+ absorption led to restricted body Ca2+ content, subsequently causing a decline in food intake and, consequently, diminished energy reserves, which ultimately inhibited body growth. Reduced food intake may also be a cause of weight decrease. It was reported that a nonselective beta-blocker and serotonin antagonist propranolol reduced the feeding rate in mussel, Mytilus galloprovincialis (Solé et al. 2010). D. magna with a serotonin biosynthesis enzyme mutation displays lower growth rate (Rivetti et al. 2018). Therefore, decreased weight after combined FLX and Zn2+ exposure is thought to be caused by an imbalance in Ca2+ and serotonin levels; however, further mechanistic research should be conducted. Caudal spine length in FLX and Zn combination groups was attenuated compared to the single Zn2+ effect. In daphnids, this trait can be affected by chemical toxicity; for instance, lesser sulfoxaflor concentrations increased caudal spine length, while higher concentrations decreased it (Sevgiler and Atli 2022).
Ca2+-ATPase activity
According to our results, FLX increased Ca2+-ATPase activity except in the lowest concentration tested in subchronic exposure and WDL exposures. Majeed et al. (2015) suggested that FLX increased cytosolic Ca2+ levels through the ER stores in the crayfish, Procambarus clarkii. Different mammalian cell types experienced an increase in the cytosolic Ca2+ content because of ER Ca2+ release driven on via FLX exposure. The mechanism may involve Ca2+ leakage from the translocon, which is located on the ER membrane. Because of Ca2+ release from the ER driven by the exposure to FLX, the mitochondrial membrane depolarizes and consequently oxygen consumption and ATP production will be reduced. Lower ATP levels then result in lower SERCA activity, and cytosolic Ca2+ levels rise (Charles et al. 2017). In our prior investigation, FLX0.012TU and FLX0.0015TU exposures in D. magna resulted in mitochondrial membrane depolarization after 96 h and 21 days (Över et al. 2020). Since Ca2+-ATPase balances cytosolic Ca2+ levels, induction of Ca2+-ATPase activity following FLX exposure is inevitable. In light of our previous study, the in vivo and WDL inhibition of Ca2+-ATPase activity should be concentration-dependent because of the unaltered activity after 48-h Zn0.05TU exposure in D. magna (Sevgiler and Atli 2022). In the present study, this concentration also has no impact following subchronic exposure contrasting the inhibition after in vivo and WDL Zn0.1TU. Analyzing in vivo studies reveals a consistent trend of decreased Ca2+-ATPase activity in acute and chronic exposures to various metals and pesticides across different aquatic organisms (Rogers and Wood 2004; Atli and Canli 2007; Mahmut et al. 2022; Sevgiler and Atli 2022). It was concluded that Zn2+ may have inhibited Ca2+-ATPase activity by competing with Ca2+ for the Ca-selective channel, which caused an ion disruption (Heath 1995). Zn2+ has an inhibitory effect on the NMDA receptor complex in mammals, and this effect is attributed to its antidepressant function (Nowak 2015). The NMDA receptor complex in D. pulex is crucial for intracellular Ca2+ regulation and plays a pivotal role in the production of male offspring (Toyota et al. 2015). Zn2+ and FLX has complex relations on serotonin and nicotinic acetylcholine receptors (nAChRs), and the inhibitory potential of FLX on nAChRs is enhanced by Zn2+ co-exposure in mammals (for further details, see Nowak (2015). FLX ameliorated the inhibitory potential of Zn2+ on Ca2+-ATPase activity in D. magna after in vivo exposures in the current study. While complex interactions between Zn2+ and FLX on different receptor types, which affect the intracellular Ca2+ regulation, were found in mammals, the interaction mechanism of FLX and Zn2+ on cellular ion regulation in crustaceans should be a subject for further research. After the in vitro exposure to Zn2+, inhibition of Ca2+-ATPase activity was found in the rainbow trout gill (Hogstrand et al. 1996) and tilapia muscle (Atli and Canli 2013), which are in accordance with our data. It has been concluded that decreased heart rate in D. magna may through an inhibition of plasma Ca2+ and Na+ channels (if they exist) by SSRIs including FLX (Halliwushka 2016). However, we hypothesized that the suppression of Ca2+-ATPase activity following single or combination FLX exposure in the present investigation was due to the absence of complicated compensatory mechanisms in WDL experiments. This may also be partially a result of the toxicants' direct effects on the enzyme molecule and/or isolated structure in the absence of the whole-body physiological and biochemical processes that allow the organism to adapt to the toxicant effect (Atli and Canli 2013). To the best of our knowledge from the literature, this study offers a first and important source of data regarding the effect of single and binary exposure to FLX and metals on Ca2+-ATPase activity.
Oxidative stress response
Variable response patterns were observed due to exposure duration (acute or subchronic) and treatment condition (in vivo or WDL), single and binary exposure, concentration levels, and toxicant type differences such as reverse effect or non-responsive situations for some cases.
It is suggested that the SOD and CAT inhibitory effects of all toxicants are duration specific. Inhibited SOD activity was also noticed in D. magna in our prior study following acute in vivo and WDL Zn0.05TU exposure (Sevgiler and Atli 2022). Exaggerated Zn2+ levels can inhibit certain glycolytic enzymes and components of the electron transport chain, leading to the production of ROS (Lee 2018). In turn, more H2O2 can reduce SOD activity (Ma et al. 2017). Inhibitory effects of FLX on SOD activity have been documented in the literature in a variety of aquatic invertebrates, including mussels, clams, and daphnids (Gonzalez-Rey and Bebianno 2013; Chen et al. 2015; Ding et al. 2017; Magni et al. 2017). In mammals, FLX induced mitochondrial electron leakage and affected the mitochondrial redox parameters to induce ROS formation (de Oliveira 2016). Oxidative modification can cause the loss of function due to their protein nature of antioxidant enzymes, and their gene expression may be induced as a compensation mechanism. SSRIs have been shown to increase the synthesis of antioxidant enzymes. For example, 500 ng/L FLX exposure increased SOD, CAT, and GPX mRNA expression levels in zebrafish larvae while GST expression did not change (Parolini et al. 2019). The decrease in CAT activity should mostly be attributed to the reduced SOD activity brought on by substrate limitation. Mitochondrial effects of FLX was also reported in D. magna, as evidenced by the loss of mitochondrial membrane polarization (MMP) and higher TBARS levels that signal the ROS production in our previous study (Över et al. 2020). Although the precise mechanism of ROS formation in FLX-exposed non-target aquatic invertebrates is unknown, it may be connected to uncompensated cytosolic Ca2+ levels and loss of MMP, while Ca2+-ATPase, at least in acute exposure, did not support this hypothesis. Declined SOD and CAT activities could be attributed to the loss of ability to compensate for the effect of the enhanced amount of ROS. Our data demonstrated a positive correlation (r2 = 0.96, P < 0.05) between the loss in GSH and SOD activity following subchronic Zn0.05TU exposure. Due to acute Zn combination groups consuming all of their GSH, such a correlation could not be assessed. In our previous research, the sulfoxaflor and Zn0.05TU effect on D. magna antioxidant system also indicated the correlations of GSH with SOD and CAT upon acute and subchronic durations. This could highlight the crucial function that GSH levels play in the antioxidant system's response. The GSH function may also be important after subchronic FLX0.025TU exposure. In this group, SOD activity remained unaffected while CAT activity decreased and GSH levels increased.
Besides, increases in GSH levels after subchronic FLX0.025TU and WDL FLX0.1TU could be associated with increased tolerance to cope with oxidative stress. Byeon et al. (2020) reported GSH alterations in the marine rotifer Brachionus koreanus in response to FLX and sertraline, two antidepressants that caused both augmentation and reduction, respectively. Following acute (4-h) and chronic (42 days) 200 µg/L FLX exposure, the GSH-GSSG system in the liver of fish Pseudorasbora parva was likewise identified as being most impacted among all antioxidants studied (Chen et al. 2018). GSH reduction could indicate its consumption in order to decrease the cytotoxic effects and may be brought on by the loss of adaptive mechanisms. It is therefore regarded as a sensitive indicator of oxidative damage in aquatic organisms (Atli et al. 2020).
Following exposure to FLX0.012TU and FLX0.0015TU for 96-h and 21 days in D. magna in vivo, the GPX activity was increased (Över et al. 2020). Similarly, Orozco-Hernández et al. (2022) found that 96-h exposure increased lipid peroxidation levels, and SOD, CAT, and GPX activities in Danio rerio embryos at concentrations between 15 and 40 ng/L. Therefore, while evaluating the effects of FLX alone on GPX activity, it could be required to take the concentration-dependence into account. However, the antioxidant system has a complicated structure, and before drawing meaningful conclusions, various elements that are impractical to examine all at once must be considered. For instance, GPX and CAT activities compete in their substrate, which is H2O2, and GPX activity depends on its substrate, which is GSH. Nevertheless, a negative correlation (r2 = − 1.00, P < 0.05) was found between CAT and GPX activity after acute FLX0.1TU exposure. Our earlier investigation found that D. magna had increased GPX and decreased SOD activities upon subchronic Zn0.05TU exposure (Sevgiler and Atli 2022), while GPX activity was found to be unaffected in the current research. Therefore, we propose that differentiation in the outcomes is typical and that compensation mechanisms divaricate to counteract ROS effects. In both acute and WDL tests, exposure to Zn0.1TU reduced GPX activity, whereas combined exposure to FLX had no impact on the decreased activity after Zn2+ exposure. A positive correlation in acute Zn0.1TU group was accompanied by decreased SOD and GPX activity (r2 = 1.00, P < 0.05) in the present study. After acute or WDL exposures, decreased SOD and GPX activities in the Zn2+ alone or Zn + FLX combination groups may have indicated ROS-inducing potentials of FLX and Zn, which were also associated by greater lipid peroxidation levels in these groups.
In contrast to the other antioxidant system parameters analysed, GST activity was found to be less susceptible to in vivo and WDL FLX and Zn2+ exposures. However, subchronic exposures to Zn0.05TU and FLX0.05TU + Zn0.05TU caused a reduction in its activity. This decrease may be linked to GSH loss, which is a sign of consumption. According to Chen et al. (2018), GST activity was induced in the gills of the fish P. parva after 4-h of exposure to 200 µg/L FLX, as opposed to being inhibited in the liver and gills after 42 days of exposure. This decline was consistent with the antioxidant capacity being exceeded during long-term FLX exposure, which was also linked to an increase in the levels of lipid peroxidation in these tissues (Chen et al. 2018). A possible connection between the increased TBARS levels and the decline in GST activity in D. magna following subchronic exposure to Zn0.05TU and FLX0.05TU + Zn0.05TU is plausible in the current investigation. Similar to our findings, the GST activity in common goby Pomatoschistus microps was unaffected by short-term (96-h) FLX concentrations of 0.1, 0.5, 10, and 100 µg/L. It was concluded that these FLX concentrations did not generate overt oxidative stress in P. microps, which was also in association with lack of lipid peroxidation changes. Therefore, GST should not be considered a reliable biomarker for FLX’s oxidative effects.
Increased ROS levels after subchronic FLX0.05TU + Zn0.05TU exposure, which drastically decreased the GSH levels in D. magna, may have contributed to the rise in TBARS levels. WDL experiments also revealed a decline in GSH levels along with suppressed SOD activity, particularly in the Zn2+ alone or combination groups. Actually, FLX and its ultimate target serotonin operate as in silico hydroxyl and peroxyl radical scavengers (Muraro et al. 2019). This suggests that FLX’s antioxidant capability may be important pharmacological mechanism in its mode of action (Novío et al. 2011). In contrast, FLX has led to adverse effects such as nose contraction and paralysis in the nematode Caenorhabditis elegans under in vivo conditions and at high concentrations, independently of serotonin (Ranganathan et al. 2001). Thus, serotonin accumulation may not be an important mediator of the prooxidative effects of FLX. High ROS levels in the liver of crucian carp Carassius auratus can be inferred with high lipid peroxidation levels and insufficient antioxidant capacity to defend against the oxidative stress caused by FLX at its environmental or higher concentrations (Ding et al. 2016). It was stated that loss in SOD activity was likely due to the elevation in SOD-consuming superoxide anion radicals produced by FLX effect; therefore, the elevated TBARS levels become apparent (Gonzalez-Rey and Bebianno 2013; Chen et al. 2015; Ding et al. 2016). The current data also provided support for FLX-induced lipid peroxidation in aquatic invertebrates, demonstrated by exposure to 5 and 50 µg/L FLX for 30 days in adult Asian clam Corbicula fluminea (Chen et al. 2015), and 5.4 µg/L for 14 days in juvenile oysters Crassostrea gigas (Di Poi et al. 2016). Despite the unchanged TBARS content in M. galloprovincialis after 7 days of 75 ng/L FLX exposure, a significant increase was recorded after 15 days of exposure (Gonzalez-Rey and Bebianno 2013). Consistent with these observations, FLX at lower concentrations increased lipid peroxidation levels over longer durations. Our earlier findings showed that 96-h and 21 days of FLX0.012TU and FLX0.0015TU exposures, respectively, increased the levels of lipid peroxidation in D. magna (Över et al. 2020). This highlights the importance of accounting for the exposure duration when evaluating the impact of FLX, either alone or in conjunction with Zn, on lipid peroxidation levels.
The current investigation discovered complex response patterns in individual biomarkers following exposure to various combinations of FLX, Zn, or their mixtures during WDL and subchronic or acute in vivo exposures. The multi-biomarker technique can be used to assess the effects of a single toxicant or toxicant combinations on numerous biological systems while incorporating the overall trend of “effects on biomarkers.” In this regard, IBR analysis leads us to the conclusion that Zn2+ at the tested concentrations was fairly effective on D. magna in subchronic and acute in vivo experiments; nevertheless, WDL Zn2+ exposure had a lower impact compared to these exposure durations. This result suggests that Zn needs intact biological response mechanism to reveal its toxicity. Due to the lowest IBR values, the observed effect of Zn2+ on D. magna is congruent with its harmonized classification codes (H400, H410) even at low environmental concentrations (0.05TU and 0.1TU for subchronic and acute periods, respectively). The H400 and H410 indicates that the chemical is extremely harmful to aquatic life in both short- and long-term exposures under the GHS (Global Harmonized System of Classification and Labelling of Chemicals, United Nations) classification. The harmonized classification codes H400 and H410 are also assigned to FLX. Based on current data and previous researches in invertebrates (De Castro-Català et al. 2017; Över et al. 2020), as well as findings from aquatic vertebrates such as zebrafish (Orozco-Hernández et al. 2023), FLX poses an extreme hazard to aquatic organisms even at very low environmental concentrations following both short- and long-term exposures. In fact, FLX0.025TU (the study’s lowest concentration) had the greatest harmful impact on overall health status of D. magna over the course of seven days, both alone and in combination with Zn2+ according to IBR analysis. Environmental specialists should pay particular attention to the low FLX concentrations because these levels are the most likely to be detected in various aquatic systems. The counter impact of Zn2+ and FLX on serotonin absorption mechanisms, as was reported in mammalian studies (García-Colunga et al. 2005), can be linked to the observed improvements in IBR values following acute in vivo and WDL exposures. The diverse directions of FLX and Zn2+ in the IBR values of the single and combined groups after the subchronic period may also serve as evidence for the different impacts of Zn2+ and FLX. The involvement of different response pathways, which warrants further investigation, may also contribute to the contradictory directions observed in IBR results after FLX exposure. At higher concentrations (0.22–0.44 mM), FLX induced effects in a nematode Caenorhabditis elegans that are independent of SERT and serotonin, such as nose contraction and paralysis (Ranganathan et al. 2001). This suggests that FLX may influence various response pathways depending on its concentration, rather than solely acting on serotonergic pathways as was already mentioned previously. Even though FLX is considered safe and useful for a variety of problems in humans, it should be seriously considered because both its single and combined effects resulted in significant changes in biomarker levels leading to failures in the antioxidant system and Ca2+-ATPase functions, two crucial processes for daphnid species.
In conclusion, the environmental risks associated with FLX and Zn2+, two substances that are similar in their ability to act as an antidepressant in humans, overlap. The provided data has shown the potential pro-oxidative and anti-Ca2+-ATPase effects of FLX and Zn2+ on D. magna, impacting both Ca2+-ATPase activity and the antioxidant system across all exposure durations and patterns. IBR analysis revealed that Zn2+ was reasonably effective on the health of D. magna, particularly following in vivo exposures, and FLX toxicity showed a concentration-dependent increase, but was reversed by the combined exposure. The observed adverse effects in Daphnia may also have harmful impact on ecosystem levels due to ecological significance of non-target D. magna, holds environmental value for higher trophic levels due to its ecological significance. Pharmaceutical exposure, such as FLX, should therefore be considered even at low environmental concentrations (ng to µg/L). Therefore, it is crucial to assess their toxicity and interactions with other aquatic toxins, as these substances may disrupt vital biological systems in aquatic organisms.
Data availability
Not applicable.
References
ASTM (2002) Standard guide for conducting acute toxicity tests on test materials with fishes, macroinvertebrates, and amphibians. ASTM International, West Conshohocken, PA. https://doi.org/10.1520/E0729-96R02
Atkinson A, Gatenby AD, Lowe AG (1973) The determination of inorganic orthophosphate in biological systems. Biochim Biophys Acta BBA - Gen Subj 320:195–204. https://doi.org/10.1016/0304-4165(73)90178-5
Atli G, Canli M (2007) Enzymatic responses to metal exposures in a freshwater fish Oreochromis niloticus. Comp Biochem Physiol Part C 145:282–287. https://doi.org/10.1016/j.cbpc.2006.12.012
Atli G, Canli M (2013) Metals (Ag+, Cd2+, Cr6+) affect ATPase activity in the gill, kidney, and muscle of freshwater fish Oreochromis niloticus following acute and chronic exposures. Environ Toxicol 28:707–717. https://doi.org/10.1002/tox.20766
Atli G, Guasch H, Rubio-Gracia F et al (2020) Antioxidant system status in threatened native fish Barbus meridionalis from the Osor River (Iberian Peninsula): I. Characterization and II. In vitro Zn assays. Environ Toxicol Pharmacol 79:103428. https://doi.org/10.1016/j.etap.2020.103428
Barakat A, Hamdy MM, Elbadr MM (2018) Uses of fluoxetine in nociceptive pain management: a literature overview. Eur J Pharmacol 829:12–25. https://doi.org/10.1016/j.ejphar.2018.03.042
Bergmann A, Fohrmann R, Weber F-A (2011) Zusammenstellung von Monitoringdaten zu Umweltkonzentrationen von Arzneimitteln. Umweltbundesamt, Dessau-Roßlau, Forschungskennzahl 360 14 013 UBA-FB 001525. http://www.uba.de/uba-info-medien/4188.html. Accessed 20 Mar 2024
Brooks BW, Turner PK, Stanley JK et al (2003) Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere 52:135–142. https://doi.org/10.1016/S0045-6535(03)00103-6
Byeon E, Park JC, Hagiwara A et al (2020) Two antidepressants fluoxetine and sertraline cause growth retardation and oxidative stress in the marine rotifer Brachionus koreanus. Aquat Toxicol 218:105337. https://doi.org/10.1016/j.aquatox.2019.105337
Campos B, Piña B, Barata CC (2012a) Mechanisms of action of selective serotonin reuptake inhibitors in Daphnia magna. Environ Sci Technol 46:2943–2950. https://doi.org/10.1021/es203157f
Campos B, Piña B, Fernández-Sanjuán M et al (2012b) Enhanced offspring production in Daphnia magna clones exposed to serotonin reuptake inhibitors and 4-nonylphenol. Stage- and food-dependent effects. Aquat Toxicol 109:100–110. https://doi.org/10.1016/j.aquatox.2011.12.003
Campos B, Rivetti C, Kress T et al (2016) Depressing antidepressant: fluoxetine affects serotonin neurons causing adverse reproductive responses in Daphnia magna. Environ Sci Technol 50:6000–6007. https://doi.org/10.1021/acs.est.6b00826
Charles E, Hammadi M, Kischel P et al (2017) The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget 8:3181–3196. https://doi.org/10.18632/oncotarget.13689
Chen H, Zha J, Yuan L, Wang Z (2015) Effects of fluoxetine on behavior, antioxidant enzyme systems, and multixenobiotic resistance in the Asian clam Corbicula fluminea. Chemosphere 119:856–862. https://doi.org/10.1016/j.chemosphere.2014.08.062
Chen H, Zeng X, Mu L et al (2018) Effects of acute and chronic exposures of fluoxetine on the Chinese fish, topmouth gudgeon Pseudorasbora parva. Ecotoxicol Environ Saf 160:104–113. https://doi.org/10.1016/j.ecoenv.2018.04.061
Cleresci LS, Greenberg AE, Eaton AD (eds) (1999) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC, p 1325
Cox AR, Arnott SE, Riessen HP (2018) Nonlinear effects of aqueous calcium concentration on antipredator response in Daphnia. Hydrobiologia 820:79–89. https://doi.org/10.1007/s10750-018-3640-x
Cunha MP, Machado DG, Bettio LEB et al (2008) Interaction of zinc with antidepressants in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry 32:1913–1920. https://doi.org/10.1016/j.pnpbp.2008.09.006
De Castro-Català N, Muñoz I, Riera JL, Ford AT (2017) Evidence of low dose effects of the antidepressant fluoxetine and the fungicide prochloraz on the behavior of the keystone freshwater invertebrate Gammarus pulex. Environ Pollut 231:406–414. https://doi.org/10.1016/j.envpol.2017.07.088
de Oliveira MR (2016) Fluoxetine and the mitochondria: a review of the toxicological aspects. Toxicol Lett 258:185–191. https://doi.org/10.1016/j.toxlet.2016.07.001
Devin S, Burgeot T, Giambérini L et al (2014) The integrated biomarker response revisited: optimization to avoid misuse. Environ Sci Pollut Res Int 21:2448–2454. https://doi.org/10.1007/s11356-013-2169-9
Devin S, Arnould P-Y, Minguez L et al (2023) Correction to: CalIBRi: a web interface to calculate integrated biomarker index. Environ Sci Pollut Res 30:67912–67913. https://doi.org/10.1007/s11356-023-27447-7
Di Poi C, Evariste L, Séguin A et al (2016) Sub-chronic exposure to fluoxetine in juvenile oysters (Crassostrea gigas): uptake and biological effects. Environ Sci Pollut Res Int 23:5002–5018. https://doi.org/10.1007/s11356-014-3702-1
Diamantino TC, Almeida E, Soares AM, Guilhermino L (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna Straus. Chemosphere 45:553–560. https://doi.org/10.1016/s0045-6535(01)00029-7
Ding J, Lu G, Li Y (2016) Interactive effects of selected pharmaceutical mixtures on bioaccumulation and biochemical status in crucian carp (Carassius auratus). Chemosphere 148:21–31. https://doi.org/10.1016/j.chemosphere.2016.01.017
Ding J, Zou H, Liu Q et al (2017) Bioconcentration of the antidepressant fluoxetine and its effects on the physiological and biochemical status in Daphnia magna. Ecotoxicol Environ Saf 142:102–109. https://doi.org/10.1016/j.ecoenv.2017.03.042
Doboszewska U, Wlaź P, Nowak G et al (2017) Zinc in the monoaminergic theory of depression: its relationship to neural plasticity. Neural Plast 2017:3682752. https://doi.org/10.1155/2017/3682752
Ehrenström F, Berglind R (1988) Determination of biogenic amines in the water flea, Daphnia magna (Cladocera, Crustacea) and their diurnal variations using ion-pair reversed phase hplc with electrochemical detection. Comp Biochem Physiol Part C Comp Pharmacol 90:123–132. https://doi.org/10.1016/0742-8413(88)90108-9
Flaherty CM, Dodson SI (2005) Effects of pharmaceuticals on Daphnia survival, growth, and reproduction. Chemosphere 61:200–207. https://doi.org/10.1016/j.chemosphere.2005.02.016
García-Colunga J, Reyes-Haro D, Godoy-García IU, Miledi R (2005) Zinc modulation of serotonin uptake in the adult rat corpus callosum: modulation of serotonin uptake by zinc. J Neurosci Res 80:145–149. https://doi.org/10.1002/jnr.20421
Gonzalez-Rey M, Bebianno MJ (2013) Does selective serotonin reuptake inhibitor (SSRI) fluoxetine affects mussel Mytilus galloprovincialis? Environ Pollut 173:200–209. https://doi.org/10.1016/j.envpol.2012.10.018
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212. https://doi.org/10.1016/0003-2697(80)90139-6
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwushka K (2016) The sub-lethal and transgenerational effects of fluoxetine and sertraline on Daphnia magna. MSc Thesis, University of Guelph, p 105. https://atrium.lib.uoguelph.ca/items/c9f16a7e-5b87-4c22-b874-e19695ae8f26. Accessed 20 Mar 2024
Heath AG (1995) Water pollution and fish physiology, 2nd edn. Lewis Publishers, Boca Raton
Hogstrand C, Verbost PM, Bonga SE, Wood CM (1996) Mechanisms of zinc uptake in gills of freshwater rainbow trout: interplay with calcium transport. Am J Physiol 270:R1141-1147. https://doi.org/10.1152/ajpregu.1996.270.5.R1141
Hughes SR, Kay P, Brown LE (2013) Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol 47:661–677. https://doi.org/10.1021/es3030148
Iturburu FG, Bertrand L, Mendieta JR et al (2018) An integrated biomarker response study explains more than the sum of the parts: oxidative stress in the fish Australoheros facetus exposed to imidacloprid. Ecol Indic 93:351–357. https://doi.org/10.1016/j.ecolind.2018.05.019
Jordão R, Garreta E, Campos B et al (2016) Compounds altering fat storage in Daphnia magna. Sci Total Environ 545–546:127–136. https://doi.org/10.1016/j.scitotenv.2015.12.097
Kim W-K, Lee S-K, Park J-W et al (2014) Integration of multi-level biomarker responses to cadmium and benzo[k]fluoranthene in the pale chub (Zacco platypus). Ecotoxicol Environ Saf 110:121–128. https://doi.org/10.1016/j.ecoenv.2014.08.025
Kukavica B, Davidović-Plavšić B, Savić A et al (2023) Oxidative stress and neurotoxicity of cadmium and zinc on Artemia franciscana. Biol Trace Elem Res 201:2636–2649. https://doi.org/10.1007/s12011-022-03352-x
Lartillot S, Kedziora P, Athias A (1988) Purification and characterization of a new fungal catalase. Prep Biochem 18:241–246. https://doi.org/10.1080/00327488808062526
Lee SR (2018) Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev 2018:e9156285. https://doi.org/10.1155/2018/9156285
Livingstone DR, Lips F, Martinez PG, Pipe RK (1992) Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar Biol 112:265–276. https://doi.org/10.1007/BF00702471
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma X, Deng D, Chen W (2017) Inhibitors and activators of SOD, GSH‐Px, and CAT. In: enzyme inhibitors and activators. Senturk M (ed.) InTechOpen, London, pp 207–224. https://doi.org/10.5772/65936
Magni S, Parolini M, Della Torre C et al (2017) Multi-biomarker investigation to assess toxicity induced by two antidepressants on Dreissena polymorpha. Sci Total Environ 578:452–459. https://doi.org/10.1016/j.scitotenv.2016.10.208
Mahmut K, Demiray GA, Sevgiler Y (2022) Oxidative and osmoregulatory effects of imidacloprid, cadmium, and their combinations on Daphnia magna. Environ Toxicol Pharmacol 95:103963. https://doi.org/10.1016/j.etap.2022.103963
Majeed ZR, Ritter K, Robinson J et al (2015) New insights into the acute actions from a high dosage of fluoxetine on neuronal and cardiac function: Drosophila, crayfish and rodent models. Comp Biochem Physiol Part C 176–177:52–61. https://doi.org/10.1016/j.cbpc.2015.07.010
McCoole MD, Atkinson NJ, Graham DI et al (2012) Genomic analyses of aminergic signaling systems (dopamine, octopamine and serotonin) in Daphnia pulex. Comp Biochem Physiol - D 7:35–58. https://doi.org/10.1016/j.cbd.2011.10.005
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Metcalfe CD, Chu S, Judt C et al (2010) Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ Toxicol Chem 29:79–89. https://doi.org/10.1002/etc.27
Mezzelani M, Gorbi S, Regoli F (2018) Pharmaceuticals in the aquatic environments: evidence of emerged threat and future challenges for marine organisms. Mar Environ Res 140:41–60. https://doi.org/10.1016/j.marenvres.2018.05.001
Mole RA, Brooks BW (2019) Global scanning of selective serotonin reuptake inhibitors: occurrence, wastewater treatment and hazards in aquatic systems. Environ Pollut 250:1019–1031. https://doi.org/10.1016/j.envpol.2019.04.118
Muraro C, Dalla Tiezza M, Pavan C et al (2019) Major depressive disorder and oxidative stress: in silico investigation of fluoxetine activity against ROS. Appl Sci 9:3631. https://doi.org/10.3390/app9173631
Muyssen BTA, Janssen CR (2002) Accumulation and regulation of zinc in Daphnia magna: links with homeostasis and toxicity. Arch Environ Contam Toxicol 43:492–496. https://doi.org/10.1007/s00244-002-1245-9
Muyssen BTA, De Schamphelaere KAC, Janssen CR (2006) Mechanisms of chronic waterborne Zn toxicity in Daphnia magna. Aquat Toxicol 77:393–401. https://doi.org/10.1016/j.aquatox.2006.01.006
Novío S, Núñez MJ, Amigo G, Freire-Garabal M (2011) Effects of fluoxetine on the oxidative status of peripheral blood leucocytes of restraint-stressed mice. Basic Clin Pharmacol Toxicol 109:365–371. https://doi.org/10.1111/j.1742-7843.2011.00736.x
Nowak G (2015) Zinc, future mono/adjunctive therapy for depression: mechanisms of antidepressant action. Pharmacol Rep 67:659–662. https://doi.org/10.1016/j.pharep.2015.01.015
OECD (2004) OECD Guidelines for the testing of chemicals, section 2: effects on biotic systems. OECD Library. https://doi.org/10.1787/20745761
OECD Statistics (2021) Pharmaceutical market: pharmaceutical consumption. Organisation for economic co-operation and development. https://www.oecd-ilibrary.org/sites/5689c05c-en/index.html?itemId=%2Fcontent%2Fcomponent%2F5689c05c-en. Accessed 20 Mar 2024
Omar NN, Tash RF (2017) Fluoxetine coupled with zinc in a chronic mild stress model of depression: providing a reservoir for optimum zinc signaling and neuronal remodeling. Pharmacol Biochem Behav 160:30–38. https://doi.org/10.1016/j.pbb.2017.08.003
Orozco-Hernández JM, Gómez-Oliván LM, Elizalde-Velázquez GA et al (2022) Effects of oxidative stress induced by environmental relevant concentrations of fluoxetine on the embryonic development on Danio rerio. Sci Total Environ 807:151048. https://doi.org/10.1016/j.scitotenv.2021.151048
Orozco-Hernández JM, Elizalde-Velázquez GA, Gómez-Oliván LM et al (2023) Acute exposure to fluoxetine leads to oxidative stress and hematological disorder in Danio rerio adults. Sci Total Environ 905:167391. https://doi.org/10.1016/j.scitotenv.2023.167391
Oteiza PI (2012) Zinc and the modulation of redox homeostasis. Free Radic Biol Med 53:1748–1759. https://doi.org/10.1016/j.freeradbiomed.2012.08.568
Över SB, Guven C, Taskin E, Sevgiler Y (2020) Oxidative and apoptotic effects of fluoxetine and its metabolite norfluoxetine in Daphnia magna. Arch Ind Hyg Toxicol 71:211–222. https://doi.org/10.2478/aiht-2020-71-3473
Parolini M, Ghilardi A, De Felice B, Del Giacco L (2019) Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ Sci Pollut Res 26:34943–34952. https://doi.org/10.1007/s11356-019-06619-4
Potet M (2017) De l’acclimatation à l’adaptation: mécanismes évolutifs, conséquences populationnelles et implication en biosurveillance. Ph.D. Thesis, Université de Lorraine, p 338. https://hal.univ-lorraine.fr/tel-01908679. Accessed 20 Mar 2024
Ranganathan R, Sawin ER, Trent C, Horvitz HR (2001) Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci 21:5871–5884. https://doi.org/10.1523/JNEUROSCI.21-16-05871.2001
Refaey HE, Amri HSA, Ashour AE, Ahmed AF (2015) Administration of zinc with paroxetine improved the forced swim test behavioral pattern of treated mice in acute and sub-acute study. J Behav Brain Sci 05:213. https://doi.org/10.4236/jbbs.2015.57022
Rivetti C, Campos B, Piña B et al (2018) Tryptophan hydroxylase (TRH) loss of function mutations induce growth and behavioral defects in Daphnia magna. Sci Rep 8:1518. https://doi.org/10.1038/s41598-018-19778-0
Rogers JT, Wood CM (2004) Characterization of branchial lead-calcium interaction in the freshwater rainbow trout Oncorhynchus mykiss. J Exp Biol 207:813–825. https://doi.org/10.1242/jeb.00826
Roman DL, Walline CC, Rodriguez GJ, Barker EL (2003) Interactions of antidepressants with the serotonin transporter: a contemporary molecular analysis. Eur J Pharmacol 479:53–63. https://doi.org/10.1016/j.ejphar.2003.08.056
Satała G, Duszyńska B, Stachowicz K et al (2016) Concentration-dependent dual mode of Zn action at serotonin 5-HT1A receptors: in vitro and in vivo studies. Mol Neurobiol 53:6869–6881. https://doi.org/10.1007/s12035-015-9586-3
Sevgiler Y, Atli G (2022) Sulfoxaflor, Zn2+ and their combinations disrupt the antioxidant and osmoregulatory (Ca2+-ATPase) system in Daphnia magna. J Trace Elem Med Biol 73:127035. https://doi.org/10.1016/j.jtemb.2022.127035
Solé M, Shaw JP, Frickers PE et al (2010) Effects on feeding rate and biomarker responses of marine mussels experimentally exposed to propranolol and acetaminophen. Anal Bioanal Chem 396:649–656. https://doi.org/10.1007/s00216-009-3182-1
Stremmel H, Weiss L, Parra G et al (2023) Ecotoxicological assessment of the effects of fluoxetine on Daphnia magna based on acute toxicity, multigenerational reproduction effects, and attraction-repellence responses. Chemosphere 312:137028. https://doi.org/10.1016/j.chemosphere.2022.137028
Szewczyk B (2013) Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 5:33. https://doi.org/10.3389/fnagi.2013.00033
Szewczyk B, Poleszak E, Wlaź P et al (2009) The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry 33:323–329. https://doi.org/10.1016/j.pnpbp.2008.12.011
Tierney AJ (2001) Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol A Mol Integr Physiol 128:791–804. https://doi.org/10.1016/s1095-6433(00)00320-2
Toyota K, Miyakawa H, Yamaguchi K et al (2015) NMDA receptor activation upstream of methyl farnesoate signaling for short day-induced male offspring production in the water flea, Daphnia pulex. BMC Genom 16:186. https://doi.org/10.1186/s12864-015-1392-9
Trevisan R, Flesch S, Mattos JJ et al (2014) Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp Biochem Physiol Part C Toxicol Pharmacol 159:22–30. https://doi.org/10.1016/j.cbpc.2013.09.007
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Varano V (2014) Use of bioassays and biomarkers in Daphnia magna to assess the effect of pharmaceutical residuals in freshwater ecosystems. Ph.D. Thesis, Alma Mater Studiorum - Università di Bologna, p 109. https://amsdottorato.unibo.it/6372/#. Accessed 20 Mar 2024
Vasskog T, Anderssen T, Pedersen-Bjergaard S et al (2008) Occurrence of selective serotonin reuptake inhibitors in sewage and receiving waters at Spitsbergen and in Norway. J Chromatogr A 1185:194–205. https://doi.org/10.1016/j.chroma.2008.01.063
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676. https://doi.org/10.1042/bj0990667
Zhu X, Chang Y, Chen Y (2010) Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78:209–215. https://doi.org/10.1016/j.chemosphere.2009.11.013
Acknowledgements
We would like to express our gratitude to Dr Serdar Sönmez from Adiyaman University for his outstanding work in microscopic analyses. We would like to thank Dr Muhsin Aydın for the English editing of the manuscript. We also would like to thank Dr Simon Devin from the Université de Lorraine for his insightful remarks on the IBR analysis.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The Research Fund of Çukurova University (FBA-2021–14145) provided funding for this study.
Author information
Authors and Affiliations
Contributions
GA: data curation, formal analysis, writing–original draft, validation, visualization, conceptualization, methodology, and investigation. YS: conceptualization, methodology, supervision, software, validation, investigation, data curation, visualization, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
The use of animals in this investigation did not require ethical approval. The study did not involve any human or animal participants, which would have required getting permission to participate and/or publish the findings.
Consent to participate
All authors are informed and provided consent for this submission.
Consent for publication
All authors have approved the manuscript and declare that this is an original contribution and none of the material in this paper is under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• FLX and Zn supressed the antioxidant system status in D. magna.
• Ca2+-ATPase was altered by FLX and Zn as a sign of ionic equilibrium failure.
• Biomarkers were more responsive to a decreasing trend after in vivo acute exposure.
• Zn2+ was reasonably effective on D. magna after in vivo experiments (IBR data).
• FLX toxicity increased in a concentration-dependent manner upon single exposure.
• Combined FLX and Zn2+ has opposing trend at their toxicity compared to single exposure (IBR data).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Atli, G., Sevgiler, Y. Binary effects of fluoxetine and zinc on the biomarker responses of the non-target model organism Daphnia magna. Environ Sci Pollut Res 31, 27988–28006 (2024). https://doi.org/10.1007/s11356-024-32846-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32846-5