Abstract
Bulinus truncatus snail is one of the most medically important snails. The goal of this study was to evaluate the molluscicidal effect of saponin on these snails and study how it affects their biological functions. The present results showed that saponin had a molluscicidal activity against adult B. truncatus snails after 24h and 72h with LC50 (57.5 and 27.1 ppm, respectively) and had ovicidal acivity on the snails’ embryos. By studying the effect of the sublethal concentrations (LC10 48.63 ppm or LC25 52.83 ppm) exposure on B. truncatus snails, they resulted in significant decreases in the survivorship, egg-laying, and the reproductive rate compared to untreated snails. Both concentrations caused morphological changes to the snails’ hemocytes, where, after the exposure, granulocytes and hyalinocytes had irregular outer cell membrane and some cell formed pseudopodia. Granulocytes had large number of granules, vacuoles, while hyalinocytes’ nucleus was shrunken. Also, these concentrations resulted in significant increases in sex hormone levels (17β-estradiol and testosterone) in tissue homogenate of B. truncatus snails. It resulted in significant decrease in total antioxidant (TAO) activity, while, significantly increased lipid peroxidase (LPO) level, superoxide dismutase (SOD), nitrogen oxide (NO), and glutathione-S-transferase (GST) as compared to control group. Histopathological and genotoxicological damages occurred in snails’ tissue after exposure to these concentrations. Conclusion, saponin has a molluscicidal effect on B. truncatus snails and might be used for the control of schistosomiasis haematobium. Besides, these snails could be used as invertebrate models to reflect the toxic effects of saponin in the aquatic ecosystem.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomatidae family, which includes the genus Schistosoma, required two hosts, intermediate and final host to complete their life cycle (WHO 2021; Morad et al. 2022). Some of these genera are responsible for cercarial dermatitis (Sodeman, 1989; Loker and Mkoji 2005) and many socioeconomic problems (WHO 2022). It was globally distributed in many parts of Africa, the Middle East, and south America (Ibrahim and Sayed 2021; Ibrahim et al. 2023).
The freshwater snail Bulinus truncatus (Audouin, 1827) (phylum Mollusca, class Gastropoda) is the intermediate host of Schistosoma haematobium and S. bovis (Xing et al. 2021; Hang et al. 2022) in Africa and the Middle East (Konan et al. 2022). Due to the ethics of scientific study, these snails could be employed as models to illustrate the impacts of specific materials in experimental studies to reduce the use of higher vertebrate models (Ibrahim and Sayed 2019; Ibrahim et al. 2021). Schistosomiasis control programs were performed by implementation of a national program that included chemotherapy and/or snail control either naturally or chemically (WHO 2020). The chemical control of these snails caused many deleterious consequences in the non-target organisms’ tissues that could be transferred to the higher food chain organisms like humans (Barathinivas et al. 2022). Besides, they are expensive and not degradable in the ecosystem (WHO 2014; Ibrahim et al., 2022).
Saponins are glucosides that are known to be a type of the plants’ secondary metabolites (Moses et al. 2014). It was extracted from Quillaja saponaria bark and it has been used as molluscicidal agent (Joshi et al. 2008; Adomaitis and Skujiene 2020). Saponins are promising choices for controlling schistosomiasis (Nair and Varalakshmi 2011; Ibrahim and Abdalla 2017; Augusto and de Mello-Silva 2018; Ibrahim and Sayed 2021). A recent study showed that saponin has molluscicidal activity against adult B. alexandrina snails with LC50 70.05 mg/l and reasoned this toxic effect to the amphiphilic nature of saponin that resulted in the cell membrane disturbances (Ibrahim et al. 2023) and also, it has miracidicidal and cercaricidal activities on the free larval stages of S. mansoni. Another study showed that pure saponin is less toxic than the extracted plant parent by three to five times and this was done on the aquatic crustacean Daphnia magna and zebra fish (Danio rerio) embryos (Jiang et al. 2018). It also could be used as insecticidal, antimicrobial, antiviral, anticancer, and anti-inflammatory, antioxidant, and immunomodulatory (Asgharian and Ojani 2017; Trdá et al. 2019; Biswas and Dwivedi 2019). It can be used as bio- surfactants in Daphnia galeata and Bosmina longirostris ecotoxicological testing as it decreased the entrapment of these water fleas in the water surface film (Cui et al. 2017). Saponins could be used cautiously in the aquatic ecosystem because its higher concentration could exceed the water quality limit (Jiang et al. 2018).
Therefore, the aim of the present study is to investigate the toxicological impact of saponin on B. truncatus snails, then studying its impact on different biological, immunotoxicological, endocrine disruption, oxidative parameters alterations, and histopathological alterations of these snails after exposure.
Materials and methods
Saponin
Saponin was purchased from (SERVA Electrophoresis GmbH, D- 69115 Heidelberg- Carl- Benz- Str. 7) as white to beige pure powder extracted from Quillaja saponaria bark with 5% solubility in water. A stock solution was prepared by dissolving the powder in distilled water (on the basis of W/V), which was used to prepare the different serial concentrations.
Snails rearing
Bulinus truncatus snails (5–8 mm) were obtained from the stock reared in Medical Malacology Department, Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt. They were bred in plastic aquaria measured (16 × 23 × 9 cm), each aquaria with 10 snails/L in dechlorinated water (25 ± 1°C), pH: 7± 0.2, 12h/12h photoperiod was controlled and covered with glass plates. Optimization of the water hardness was done by using 30 mg/L calcium carbonate (CaCO3) (Eveland and Haseeb 2011). Blue-green algae (Nostoc muscorum), oven-dried lettuce leaves and Tetramin (ad libitum) were used for snail feeding. For collecting egg masses, small pieces 3×5 of polyethylene sheets were float in the aquaria (Pellegrino and Goncalves, 1965). Water was changed twice a week and dead snails were removed (Eveland and Haseeb 2011). Ethics for collecting and using snails have been done according to NENT (2019).
Bioassay tests
A stock solution of 1000 ppm from saponin was prepared, and dilutions were performed for determining LC10, LC25, LC50, and LC90 against B. truncatus snails. Therefore, those snails were treated with concentrations of 45, 50, 55, 60, 65, and 70 mg/L of saponin for 24h or concentrations of 10, 15, 20, 25, 30 mg/l for 72h. Afterwards, snails were thoroughly washed and transferred to a clean dechlorinated water to recover for 24h. For each concentration, three replicates were used, each of which 10 snails (5–8mm)/L. Mortality of snails was recorded (WHO 1983) and analyzed to obtain the lethal concentration values by probit analysis software (WHO 1965). For control groups, dechlorinated water was allowed to run along with the test samples with tri-replicate manner. About 210 snails were used in this experiment.
Effect of saponin on the survival and the reproductive rates of adult snails
One hundred and eighty B. truncatus snails (6–8mm) were exposed for 24 h/day for 2 weeks to the concentrations LC10 or LC25 of saponin. Three replicates, each of 10 snails/L, were prepared for each concentration, another group considered as control group was maintained in dechlorinated water, the following parameters were weekly recorded: Lx (the survival rate as a proportion of the correct one at time of exposure in weeks (x), fecundity (Mx) (the number of eggs/snail/week), and R0 (the reproductive rate which is the summation of LxMx during the experimental period) (El-Gindy and Radhawy, 1965).
Investigation of ovicidal activity
Egg masses on the sheets were transferred to petri dishes, where they were exposed to the tested concentrations. For each concentration, 100 eggs were used and assays were repeated three times. At the end of exposure (24 h), egg masses were transferred to petri dishes with dechlorinated water and were examined daily under a stereomicroscope up to the 7th day (Ibrahim and Abdalla 2017).
Investigation of the hemocytes morphological alterations after exposure to the sublethal concentration
Hemolymph samples were collected from each group of tested snails that was exposed to LC10, LC25, and control snails group by removing a small portion of the shell and inserting a capillary tube into the heart (Nduku and Harrison 1980). Hemocytes monolayers were prepared by placing 10 μl of hemolymph on a glass slide and were allowed to be adhered the glass surface for 15 min at room temperature. Hemocytes were fixed with absolute methanol for 5 min and then stained with 10% Giemsa stain (Aldrich) for 20 min (Abdul-Salam and Michelson 1980), then examined under microscope.
Assay for the biochemical alterations
Tissue preparation
From each exposed and the control groups, ten snails were crushed between two slides, and their soft tissues were withdrawn, weighed (1g tissue/10 ml phosphate buffer) and then homogenized by a glass Dounce homogenizer. The homogenates were centrifuged at 3000 rpm for 10 min and the supernatants were used.
Investigation of steroid sex hormones (testosterone and 17β-estradiol) after exposure to saponin
The steroid hormones (testosterone, 17β-estradiol) were determined in tissue of snails survived post exposure to LC10 or LC25 of saponin and in the control group. Hormone concentrations (T and E) were assayed for all groups according to the manufacturer instructions of T EIA kit (Enzo Life Science, Michigan, USA, ADI-900-065) and E EIA kit (Cayman Chemical Page 6/18 Company, Michigan, USA, item no. 582251). These kits were used for the quantitative determination of testosterone and estradiol in a colorimetric competitive enzyme immunoassay with results in 3 h.
Investigation of the antioxidant biomarkers (SOD, GST, MDA, and TAC)
Biodiagnostic kits (Biodiagnostic Dokki, Giza, Egypt) were used for the determination of SOD and it relied on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye (Damerval et al. 1986). Also, glutathione S transferase (GST) kit is a colorimetric assay designed for the quantification and detection of glutathione in tissue samples and measured according to (Beutler 1963). In addition, tissue malondialdhyde (lipid peroxide) was done colorimetrically according to Ohkawa et al. (1979) and total antioxidant capacity was estimated by kit (Cat. No. TA 2513) and based on the reaction of antioxidants in the sample with a defined amount of exogenously provide hydrogen peroxide (H2O2). The antioxidants in the sample eliminate a certain amount of the provided hydrogen peroxide and the residual hydrogen peroxide is determined colorimetrically by an enzymatic reaction (Koracevic et al. 2001).
Investigation of the histopathological alterations
The snails were treated with LC10 and LC25 of saponin as mentioned before. Control groups were simultaneously carried out. Three replicates (10 snails/ L for each) were used for both control and the tested groups. Thereafter, the snail’s hermaphrodite gland was dissected out of their shells and was fixed using Bouin's fixative, then embedded in wax blocks, sectioned (5–8μm), and stained with hematoxylin and eosin (Mohamed and Saad 1990). Similarly, sections of control snails’ hermaphrodite glands were prepared.
Molecular changes by comet assay
Snails (8–10 mm) were subjected to LC10 or LC25 of saponin for 24h and then DNA damage was measured by single cell gel assay according to Singh et al. (1988) and Grazeffe et al. (2008).
Statistical analysis
Statistical analyses were run on IBM compatible PC using SPSS for windows statistical package (SPSS, 2006). Lethal concentrations were calculated using Probit analysis software (Finney 1971). The mortality rates of the experimental groups were compared using Pearson’s Chi-square test. Values of the biochemical parameters were expressed as mean± SD. Student’s t-test was applied to locate significant changes between control and treated groups (Murray 1981).
Results
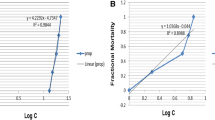
The tested saponin in the current study revealed a toxic molluscicidal activity against the intermediate host of S. haematobium after a 24-h and 72-h exposure with different concentrations. The mortality of B. truncatus snails was positively concentrations dependent. The LC50 value was 57.5 and 27.1 ppm after 24-h and 72-h exposure, respectively. Interestingly, slope value indicated that the lethal concentration probability lines (LCP) of saponin was steep, which indicated that the toxic effect of saponin is concentration dependent (Table 1).
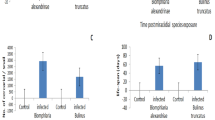
Results revealed that the biological parameters that are tested in B. truncatus snails exposed to either LC10 48.63 ppm or LC25 52.83 ppm of saponin recorded decrease values in the survivorship, egg-laying, and the reproductive rate (R0) of snails compared to untreated snails (Table 2; Fig. 1).
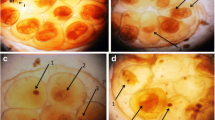
The present results showed that B. truncatus ova exposed to either LC10 48.63 ppm or LC25 52.83 ppm of saponin showed delayed developmental stage of embryos, dead and degenerated dead embryos (Fig. 2).
The current investigation elucidated the presence of two types of large cells (granulocytes and hyalinocytes) and one small type of cells (Fig. 3, A, B, and C). Exposure of B. truncatus snails to either LC10 48.63 ppm or LC25 52.83 ppm of saponin caused morphological changes to these cells. Where, after the exposure, granulocytes had plenty of granules, vacuoles, irregular cell membrane, and forming pseudopodia. The nucleus of some hyalinocytes was shrinked, some had irregular outer cell membrane and some cell formed pseudopodia (Fig. 4, D, E, F, G, H, and I).
B. truncatus ova exposed to LC10 and LC25 concentration of saponin (after 1 week). A Normal healthy embryo with eyes (E) on the head foot (HF) and with shell (SH). B Developmental delayed embryo (DeE) and dead embryo (DE) after exposure to LC10 of saponin; C degenerated dead embryo (DDE), dead embryo (DE), and delayed embryo (DeE) after exposure to LC25 of saponin (50× magnification)
Photomicrographs show B. truncatus hemocytes (×40). (1) Normal hemocytes (A, B, C). (2) After exposure to LC10 48.63 ppm of saponin (D, E, F). (3) After exposure to LC25 52.83 ppm of saponin (G, H, I). CY, Cytoplasm; DM, double membrane; PS, pseudopodia; G, granulocyte; GR, granules; H, hyalinocyte; N, nucleus; S, round small; V, vacuoles
Changes in sex hormone levels (17β-estradiol and testosterone) in tissue homogenate of B. truncatus snails exposed to saponin were occured. After exposure to LC10 48.63 ppm or LC25 52.83 ppm of saponin both the level of 17β-estradiol and testosterone hormones were significantly increased (p< 0.05) compared to control group (Fig. 5).
The present data indicated that exposure of B. truncatus snails to either LC10 48.63 ppm or LC25 52.83 ppm of saponin significantly decreased TAO activity; while, they significantly increased lipideperoxidase (LPO) level, superoxidedismutase (SOD), nitrogen oxide (NO), and glutathione-s-transferees (GST) as compared to control group (Fig. 6).
Examination of the histological sections of the digestive and hermaphrodite glands transverse of snails treated with LC10 and LC25 of saponin (Fig. 7 A, B, and C) showed cleared rupture and swelling of the digestive and secretory cells compared to control snails. Moreover, the hermaphrodite gland transverse sections of snails treated with the same concentrations of saponin (Fig. 7 D, E, and F) showed varying degrees of degenerations, atrophy and rupture of different cell types, ova and sperms in the glands’ acini. Furthermore, the most prominent damages were clear for several vacuolated ova, degeneration and scattered sperms after exposure to LC25 of saponin when compared to the control group.
A Light photomicrograph of the digestive gland transverse section of normal Bulinus truncatus snails (hematoxylin and eosin stain ×40) with normal digestive cells (dc),normal secretory cells (sc), lumen (l), and epithelial layer (e). B Snails exposed to LC10 of saponin and C treated with LC25 of saponin showing: ruptured digestive cells (rdc), shranking, swelling, vacuole (V) formation and degenerated digestive cells (ddc). D Light photomicrograph of transverse section in the hermaphrodite gland of Bulinus truncatus (control), showing: Ov, mature ova; Sp, developed sperms; Ct, connective tissue, spermatocyte (red arrow) and oocytes (green arrow); E treated LC10 and F treated LC25 of saponin: showing degeneration ova (red arrow), scattered degenerated sperm (green arrow) and vacuolated ova (yellow arrow)
The results of the alkaline comet assay showed that the percent tailed comet and the olive tail moment (OTM) of snails exposed to the sublethal concentrations of saponin were increased significantly (p< 0.05) than the control snails (Fig. 8).
Discussion
World Health Organization has tested thousands of agent compounds for the eradication of snails and reported that molluscicides of plant origin were more promising as they could not pollute the environment and were biodegradable (WHO 1990). The present results indicated that saponin induced toxic effect to B. truncatus snails a study that agrees with the data of (Zheng et al. 2021) which recorded the toxicity effects of novel triterpene saponins against B. glabrata snails. This could be due to that saponin has specific ability to perforate the cell membranes (Lorent et al. 2014). Also, the molluscicidal activity of Saponins might be due to their detergent effects on the soft body of these snails (Bahgat et al. 2018).
Results showed that after exposure of B. truncatus snails to either LC10 or LC25 of saponin, the survivorship, egg-laying, and the reproductive rate were significantly decreased compared to untreated snails. These alterations might be due to that B. truncatus snails had no operculum and so, their soft bodies were continuously in contact with the tested saponins during the experiment (He et al. 2017). Similar observations were noticed on the freshwater snail Lanistes lybicus tissues after exposure to saponins in a dose-dependent way (Akinpelu 2012). Moreover, the survival rate of Biomphalaria pfeifferi, and Bulinus globosus snails were decreased after exposure to Cucurbita maxima seed extracts concentration (Mtemeli et al. 2021). Molluscicides could induce death by disrupting the physiological processes that could explain the high mortality rates and long periods of ceasing egg-laying during the recovery period (McCullough et al. 1980). Also, these reductions in Mx and R0 of B. truncatus post-saponin exposure might be due to the harmful effects on the regulation of the oviposition. In addition to the interruption of their physiological activities, confirmed by the deteriorations on the levels of the antioxidant enzymes in the snails’ tissues. This mirrored on the developmental stages of the embryos, where the present results showed that B. truncatus ova exposed to either LC10 or LC25 of saponin showed delayed, dead, and degenerated dead embryos. These alterations were caused because of the great surface area for contact that allowing for the penetration of saponin (Li et al. 2014). Araújo et al. (2018) reasoned the embryotoxicity of saponin to its absorption through the cellular membrane causing changes in its permeability (Li et al. 2009).
Snails immune system consists of cellular and humoral components (Le Clec’h et al. 2016) (Baroudi et al. 2020). Hemocytes have three morphological shapes (Larson et al. 2014; Fried 2016), undifferentiated small, hyalinocytes, and granulocytes (Ibrahim et al. 2018). The immunological responses in B. alexandrina snails have been used as biomarkers of exposure to environmental stressors (Ibrahim and Sayed 2020). The current investigation elucidated the presence of two types of large cells (granulocytes and hyalinocytes) and one small type of cells. Exposure of B. truncatus snails to either LC10 or LC25 of saponin resulted in deformation of cell shape where, both granulocytes and hyalinocytes had irregular cell membrane, vacuoles, and forming pseudopodia, while granulocytes had plenty of granules. The nucleus of some hyalinocyte was shrinked. The pseudopodia formation by cells is an immunological action of these cells to get rid of the foreign materials (Guria 2018; Ibrahim et al. 2022a). Similarly, Morad et al. (2022) reported after exposure to LC10 (31.82 mg/l) of selenium nanoparticles, the granulocytes had many granules and pseudopodia and the hyalinocytes had incomplete cell division with irregular cell membrane and forming pseudopodia. In a good accordance with this study, Ibrahim et al. (2019) elucidated that granulocytes had many granules and pseudopodia, while hyalinocytes had a shrunken nucleus after exposure of B. alexandrina to pendimethalin, butralin, or glyphosate isopropyl ammonium herbicides.
Testosterone and 17β-estradiol hormone required in the gonadal development and might be used as a biomarker for molluscicides toxicity (Omran and Salama 2016). The present results showed that both the level of 17β-estradiol and testosterone hormones were significantly increased after the exposure of B. truncatus snails to LC10 or LC25 of saponin compared to control group. Swan et al. (2000) reported that saponin could interfere with the endocrine functions leading to the increase in sex hormones levels.
These findings were in a good accordance with Morad et al. (2022) who reported elevated levels of both testosterone and 17β-estradiol after exposure of B. alexandrina snails to the sublethal concentration with SeNPs Myco-Synthesis. These alterations were due to the androgenic stimulatory action of saponin which resulted in the increase in sex hormones (Dasofunjo et al. 2020).
Antioxidant enzymes were used as a biomarker for stressors in both marine and freshwater organisms (Tellez-Bañuelos et al. 2009). The aquatic stressors might result in reproductive, oxidative stress parameters and teratogenic alterations in the aquatic organisms (Pašková et al. 2011). The alterations in SOD, CAT, GST, GR, and MDA levels could be useful biomarkers of aquatic system pollution (Khalil et al. 2017). The present data indicated that exposure of B. truncatus snails to either LC10 or LC25 of saponin significantly decreased TAO activity, while, increased LPO level, SOD, NO, and GST as compared to control group. The reduction in total antioxidant level in tissues of B. truncatus snails exposed to saponin due to the severe oxidative stress and reactive oxygen production (ROS) which could lead to suppression of this enzyme and alterations in other parameters (Xu et al. 2009). The oxidative stress might be the main cause of delaying the sexual maturation and puberty onset (Pizzino et al. 2017). Also, the alterations in LPO level, SOD, NO, and GST levels might due to the effect of saponin on membrane fluidity (Halliwell and Gutteridge 2015) and might cause damages to DNA, lipids, and proteins (Di Giulio and Meyer 2008). The antioxidant enzymes played an important role in the elimination of ROS and modulation of the living organisms’ response to the oxidative stressors (Pizzino et al. 2017). Nitric oxide (NO) is one of the innate immune defense mechanisms which lead to kill the invading pathogens (Ray et al. 2013; Ibrahim and Hussein 2022). Wang et al. (2018) reported increase level of NO of B. straminea snail after exposure to pyridyl phenyl urea derivatives.
Examination of the histological sections of the digestive glands of snails treated with LC10 and LC25 of saponin showed scattered vacuole, rupture and swelling of the digestive and secretory cells compared to control snails. Moreover, the hermaphrodite gland sections of snails treated with the same concentrations of saponin showed degenerations, atrophy and rupture of different cell types, ova and sperms in the glands’ acini. These could be due to the genital organs suffered from the saponin treatments which could resulted to decrease their oviposition for some weeks and these may explain why that the reproduction rate (R0) of exposed snails was reduced. In these regards, Abdel-Tawab et al. (2022) reported great damages in both hermaphrodite and digestive glands represented mainly as degeneration rupture and vacuolation of the digestive, secretory cells, sperms, and ova after exposure to cerium oxide nanoparticle synthesized with Moringa oleifera seeds at concentration 314.5 mg/l. In 2019, Ibrahim and Bakry (2019) stated that chlorophyllin extracted from deep-frozen leaves of M. oleifera plant exerted deleterious effects in the digestive gland of B. alexandrina snails treated with LC25 of water soluble chlorophyllin, represented by deformation of secretory cells, disintegration of the digestive cells, and rupturing the connective tissue between the gland tubules. Moreover, Dokmak et al. (2021) reported that destruction of the hermaphrodite and digestive glands cells of B. truncatus snails treated with cupper chlorophyllin and magnesium chlorophyllin and reasoned these damages to the harmful effects exerted by such agents during the photosensitization process.
DNA damages might be used as biomarkers of aquatic pollution in snails (Abdel-Tawab et al. 2022). Alkaline comet assay is a simple, fast, and reliable technique that was used to detect DNA single-strand breaks (Ibrahim et al. 2018). The present investigation showed that both the % of tailed cells and the olive tail moment (OTM) of snails exposed to the sublethal concentrations of saponin were increased compared with the control snails. Similarly, Ibrahim et al. (2023) stated that exposure of B. alexandrina snails to LC10 or LC25 concentrations of saponin caused genotoxic effects and downregulated the metabolic cycles for both genes (cytochrome oxidase subunit I (COI) and NADH dehydrogenase subunit 1 (ND1). These effects might be due to the oxidation of DNA bases resulting in strand breaks (Caixeta et al. 2020) after the exposure to DNA damaging materials compared to the control group (Ye et al. 2012).
Conclusion
Therefore, saponins could be used as a molluscicide against the intermediate host of schistosomiasis and hence decrease the spread of this disease. Also, B. truncatus snails could be used as bio-indicators to reflect the effect of saponin on the biological system of organisms.
Data availability
Data are available on request from the authors
References
Abdel-Tawab H, Ibrahim AM, Hussein T, Mohamed F (2022) Mechanism of action and toxicological evaluation of engineered layered double hydroxide nanomaterials in Biomphalaria alexandrina snails. Environ Sci Pollut Res Int 29. https://doi.org/10.1007/S11356-021-16332-W
Abdul-Salam JM, Michelson EH (1980) Biomphalaria glabrata amoebocytes: effect of Schistosoma mansoni infection on in vitro phagocytosis. J Invertebr Pathol 35:241–248
Adomaitis M, Skujiene G (2020) Lethal doses of saponins from Quillaja saponaria for invasive slug Arion vulgaris and non-target organism Enchytraeus albidus (Olygochaeta: Enchytraeidae). Insects 11:1–11
Akinpelu A (2012) Effect of stem - bark of Erythrophleum suaveolens (Guill. Perri.) saponin on fresh water snail (Lanistes lybicus) tissues. African J Environ Sci Technol 6:446–451. https://doi.org/10.5897/ajest12.007
Araújo HDA, Silva LRS, Siqueira WN et al (2018) Toxicity of usnic acid from Cladonia substellata (Lichen) to embryos and adults of Biomphalaria glabrata. Acta Trop 179:39–43. https://doi.org/10.1016/j.actatropica.2017.11.007
Asgharian A, Ojani S (2017) In vitro antioxidant activity and phytochemical screening of flowers and leaves of Hypericum perforatum L. ethanolic extracts from Tonekabon-Iran. J Phytochem Biochem 1:1–5
Audouin (1827) Bulinus truncatus. In: Molluscabase. https://www.molluscabase.org/aphia.php?p=taxdetails&id=716338. Accessed 29 May 2023
Augusto R, de Mello-Silva C (2018) Phytochemical molluscicides and schistosomiasis: what we know and what we still need to learn. Vet Sci 5:94. https://doi.org/10.3390/vetsci5040094
Bahgat DM, Mossalem HS, Al-Sayed E et al (2018) Influence of saponin fraction from Albizia anthelmintica on Biomphalaria alexandrina snail; the intermediate host of Schistosoma mansoni in Egypt. Egypt J Aquat. Biol Fish 22:231–240
Barathinivas A, Ramya S, Neethirajan K et al (2022) Ecotoxicological effects of pesticides on hematological parameters and oxidative enzymes in freshwater catfish, Mystus keletius. Sustain 14:9529. https://doi.org/10.3390/su14159529
Baroudi F, Al Alam J, Fajloun Z, Millet M (2020) Snail as sentinel organism for monitoring the environmental pollution; a review. Ecol Indic 113:106240
Beutler E (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61:882–888
Biswas T, Dwivedi UN (2019) Plant triterpenoid saponins: biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 256:1463–1486
Caixeta MB, Araújo PS, Gonçalves BB et al (2020) Toxicity of engineered nanomaterials to aquatic and land snails: a scientometric and systematic review. Chemosphere 127654
Cui R, Il KJ, An YJ (2017) A novel method for preventing surface film entrapment of water fleas and its application for toxicity testing with heavy metals. Environ Sci Pollut Res 24:4210–4219. https://doi.org/10.1007/S11356-016-8091-1/TABLES/3
Damerval C, De Vienne D, Zivy M, Thiellement H (1986) Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7:52–54. https://doi.org/10.1002/elps.1150070108
Dasofunjo K, Asuk AA, Nku CI (2020) Evaluating the effect of ethanol leaf extract of Gongronema latifolium on some reproductive hormones of male Wistar rats. GSC Biol Pharm Sci 12(03):166–173. https://doi.org/10.30574/gscbps.2020.12.3.0297
Di Giulio R, Meyer J (2008) Reactive oxygen species and oxidative stress. In: CR C Press Taylor Fr. Gr https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Di++Giulio.++RT%2C++Meyer%2C++JN.+%282008%29++Reactive++oxygen++species+and+oxidative++stress.+In%3A++Di++Giulio++RT%2C++Hinton+DE+%28eds%29%2C+The+toxicology+of+fishes%2C+CR+C+Press+Taylor+%26+Franc. Accessed 16 Jan 2023
Dokmak HAAS, El-Emam MA, Mossalem HS et al (2021) Impact of the photosensitizers copper and magnesium chlorophyllin on biological and biochemical parameters of Bulinus truncatus snail. Egypt J Aquat Biol Fish 25:525–540. https://doi.org/10.21608/EJABF.2021.146539
El-Gindy MS, Radhawy IA, others (1965) Effect of low concentrations of sodium pentachlorophenate on the fecundity and egg viability of Bulinus truncatus from Central Iraq. Bull Endem Dis (Baghdad) 7:44–54
Eveland LK, Haseeb MA (2011) Laboratory rearing of Biomphalaria glabrata snails and maintenance of larval schistosomes in vivo and in vitro. In: Biomphalaria snails and larval trematodes. Springer, New York, New York, NY, pp 33–55
Finney DJ (1971) Probit analysis, 3rd edn. Combrige University Press
Fried B (2016) An update on hemocytes in Biomphalaria snails. J Hematol Oncol Res 2:20–26. https://doi.org/10.14302/issn.2372-6601.jhor-14-401
Grazeffe VS, de Freitas TL, de Sa PA et al (2008) Establishment of the comet assay in the freshwater snail Biomphalaria glabrata (Say, 1818). Mutat Res Toxicol Environ Mutagen 654:58–63
Guria S (2018) Lead (Pb) induced paraptosis like cell death in hemocytes of Lamellidens sp: a preliminary study. World Sci News 94:163–172
Halliwell B, Gutteridge J (2015) Free radicals in biology and medicine
Hang DR, Feng Y, Zhang JF et al (2022) Studies on the ecology of Bulinus globosus snails: evidence against burrowing into the soil during the dry season. Front Environ Sci 10:1–10. https://doi.org/10.3389/fenvs.2022.925065
He P, Wang W, Sanogo B et al (2017) Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasit Vectors 10. https://doi.org/10.1186/s13071-017-2313-3
Ibrahim AM, Abdalla AM (2017) Impact of Moringa oleifera seed aqueous extract on some biological, biochemical, and histological aspects of Biomphalaria alexandrina snails. Environ Sci Pollut Res 24:28072–28078. https://doi.org/10.1007/s11356-017-0397-0
Ibrahim AM, Ahmed AK, Bakry FA et al (2019) Toxicological impact of butralin, glyphosate-isopropylammonium and pendimethalin herbicides on physiological parameters of Biomphalaria alexandrina snails. Molluscan Res 39:224–233. https://doi.org/10.1080/13235818.2019.1592296
Ibrahim AM, Ahmed AK, Bakry FA, Abdel-Ghaffar F (2018) Hematological, physiological and genotoxicological effects of Match 5% EC insecticide on Biomphalaria alexandrina snails. Ecotoxicol Environ Saf 147. https://doi.org/10.1016/j.ecoenv.2017.09.059
Ibrahim AM, Ahmed AK, Hammam OA, Abdel-Ghaffar F (2022a) Immunotoxical, neurotoxical, histopathological and immunohistopathological alterations of Nerium oleander and Tecoma stans methanolic extract on Biomphalaria alexandrina snails. Acta Trop 230:106405. https://doi.org/10.1016/j.actatropica.2022.106405
Ibrahim AM, Bakry FA (2019) Assessment of the molluscicidal impact of extracted chlorophyllin on some biochemical parameters in the nervous tissue and histological changes in Biomphalaria alexandrina and Lymnaea natalensis snails. Invertebr Neurosci 19. https://doi.org/10.1007/s10158-019-0230-1
Ibrahim AM, El-karim RMG, Ali RE, Nasr SM (2023) Toxicological effects of Saponin on the free larval stages of Schistosoma mansoni, infection rate , some biochemical and molecular parameters of Biomphalaria alexandrina snails. Pestic Biochem Physiol 191:105357. https://doi.org/10.1016/j.pestbp.2023.105357
Ibrahim AM, Hussein AAA (2022) Toxicological impact of organophosphorus Chlorpyrifos 48%EC pesticide on hemocytes, biochemical disruption, and molecular changes in Biomphalaria alexandrina snails. Pestic Biochem Physiol 186:105154. https://doi.org/10.1016/j.pestbp.2022.105154
Ibrahim AM, Mohamed F, Al-Quraishy S et al (2021) Green synthesis of cerium oxide / Moringa oleifera seed extract nano-composite and its molluscicidsal activities against Biomophalaria alexanderina. J King Saud Univ - Sci 33:101368. https://doi.org/10.1016/J.JKSUS.2021.101368
Ibrahim AM, Morad MY, Hamdi SAH (2022) Fol MF (2022b) Biocontrol potential of chitosan extracted from Procambarus clarkii (Crustacea: Cambaridae) against Eobania vermiculata snails (Muller 1774) in Egypt. Egypt J Biol Pest Control 321(32):1–10. https://doi.org/10.1186/S41938-022-00521-X
Ibrahim AM, Sayed DA (2019) Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) snails. Environ Sci Pollut Res 26:7960–7968. https://doi.org/10.1007/s11356-019-04251-w
Ibrahim AM, Sayed SSM (2021) Assessment of the molluscicidal activity of the methanolic seed extracts of Ziziphus spina-christi and Carica papaya on immunological and molecular aspects of Biomphalaria alexandrina snails. Aquac Res 52:2014–2024. https://doi.org/10.1111/ARE.15050
Ibrahim AM, Sayed SSM (2020) Assessment of the molluscicidal activity of the methanolic seed extracts of Ziziphus spina-christi and Carica papaya on immunological and molecular aspects of Biomphalaria alexandrina snails. Aquac Res 52:2014–2024. https://doi.org/10.1111/are.15050
Jiang X, Cao Y, von Gersdorff Jørgensen L et al (2018) Where does the toxicity come from in saponin extract? Chemosphere 204:243–250. https://doi.org/10.1016/j.chemosphere.2018.04.044
Joshi RC, San Martín R, Saez-Navarrete C et al (2008) Efficacy of quinoa (Chenopodium quinoa) saponins against golden apple snail (Pomacea canaliculata) in the Philippines under laboratory conditions. Crop Prot 3–5:553–557. https://doi.org/10.1016/J.CROPRO.2007.08.010
Khalil MT, Gad NS, Ahmed NAM, Moustafa SS (2017) Antioxidant defense system alternations in fish as a bio-indicator of environmental pollution. Egypt J Aquat Biol Fish 21:11–28. https://doi.org/10.21608/ejabf.2017.3536
Konan CK, Tian-Bi YNT, Diakité NR et al (2022) Spatial variation of life-history traits in Bulinus truncatus, the intermediate host of schistosomes, in the context of field application of niclosamide in Côte d’Ivoire. BMC Zool 7:1–14. https://doi.org/10.1186/S40850-021-00104-7/FIGURES/7
Koracevic D, Koracevic G, Djordjevic V et al (2001) Papers Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361. https://doi.org/10.1136/jcp.54.5.356
Larson MK, Bender RC, Bayne CJ (2014) Resistance of Biomphalaria glabrata 13-16-R1 snails to Schistosoma mansoni PR1 is a function of haemocyte abundance and constitutive levels of specific transcripts in haemocytes. Int J Parasitol 44:343–353
Le Clec’h W, TJC A, Chevalier FD (2016) Characterization of hemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosoma mansoni infection. Parasit Vectors 9:32. https://doi.org/10.1186/s13071-016-1319-6
Li XY, Dong XY, Bai X et al (2014) The embryonic and postembryonic developmental toxicity of imidazolium-based ionic liquids on Physa acuta. Environ Toxicol 29:697–704. https://doi.org/10.1002/TOX.21797
Li XY, Zhou J, Yu M et al (2009) Toxic effects of 1-methyl-3-octylimidazolium bromide on the early embryonic development of the frog Rana nigromaculata. Ecotoxicol Environ Saf 72:552–556. https://doi.org/10.1016/j.ecoenv.2007.11.002
Loker ES, Mkoji GM (2005) Schistosomes and their snail hosts BT - schistosomiasis. In: Secor WE, Colley DG (eds) Springer. US, Boston, MA, pp 1–11
Lorent JH, Quetin-Leclercq J, Mingeot-Leclercq MP (2014) The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org Biomol Chem 12:8803–8822. https://doi.org/10.1039/C4OB01652A
McCullough FS, Gayral P, Duncan J, Christie JD (1980) Molluscicides in schistosomiasis control. Bull World Health Organ 58:681–689. https://doi.org/10.1016/0048-3575(76)90014-6
Mohamed SH, Saad AA (1990) Histological studies on the hermaphrodite gland of Lymnaea caillaudi and Biomphalaria alexandrina upon infection with certain larval trematodes. Egypt J Histol 13:47–53
Morad MY, El-Sayed H, Elhenawy AA et al (2022) Myco-Synthesized molluscicidal and larvicidal selenium nanoparticles: a new strategy to control Biomphalaria alexandrina snails and larvae of Schistosoma mansoni with an in silico study on induced oxidative stress. J Fungi 8:262. https://doi.org/10.3390/JOF8030262
Moses T, Papadopoulou KK, Osbourn A (2014) Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol 49:439–462
Mtemeli FL, Walter I, Tinago T, Shoko R (2021) An assessment of the molluscicidal potential of Cucurbita maxima seed extracts on Biomphalaria pfeifferi and Bulinus globosus snails. All Life 14:244–255. https://doi.org/10.1080/26895293.2021.1901788
Murray R (1981) Speigel: statistical estimation theory. Schaum’s Outl Ser Theory Probl Stat SI Units. First Ed McGraw Hill B Company, Singapore, p 156
Nair S, Varalakshmi KN (2011) Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J Nat Pharm 2
Nduku WK, Harrison AD (1980) Cationic responses of organs and haemolymph of Biomphalaria pfeifferi (Krauss), Biomphalaria glabrata (Say) and Helisoma trivolvis (Say)(Gastropoda: Planorbirdae) to cationic alterations of the medium. Hydrobiologia 68:119–138
NENT (2019) Ethical guidelines for the use of animals in research | Forskningsetikk. In: Natl. Comm. Res. Ethics Sci. Technol https://www.forskningsetikk.no/en/guidelines/science-and-technology/ethical-guidelines-for-the-use-of-animals-in-research/. Accessed 15 Apr 2023
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omran NE, Salama WM (2016) The endocrine disruptor effect of the herbicides atrazine and glyphosate on Biomphalaria alexandrina snails. Toxicol Ind Health 32:656–665
Pašková V, Hilscherová K, Bláha L (2011) Teratogenicity and embryotoxicity in aquatic organisms after pesticide exposure and the role of oxidative stress. In: Reviews of environmental contamination and toxicology, vol 211. Springer, pp 25–61
Pellegrino J, Goncalves M, others (1965) A simple method for collecting egg clutches of Biomphalaria glabrata (Australorbis glabratus) and for rearing newly hatched snails. J Parasitol 51:
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017:8416763
Ray M, Bhunia NS, Bhunia AS, Ray S (2013) A comparative analyses of morphological variations, phagocytosis and generation of cytotoxic agents in flow cytometrically isolated hemocytes of Indian molluscs. Fish Shellfish Immunol 34:244–253. https://doi.org/10.1016/j.fsi.2012.11.006
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Sodeman WA (1989) The biology of schistosomes from genes to latrines. Am J Trop Med Hyg 41:251–251. https://doi.org/10.4269/ajtmh.1989.41.2.tm0410020251a
SPSS (2006) Statistical programme for social sciences. SPSS for Windows. Release 2006, SPSS Inc.
Swan S, Elkin E, Fenster L (2000) The question of declining sperm density revisited: an analysis of 101 studies. Environ Health Perspect 1934:961–966
Tellez-Bañuelos MC, Santerre A, Casas-Solis J et al (2009) Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol 27:105–111
Trdá L, Janda M, Macková D et al (2019) Dual mode of the saponin aescin in plant protection: antifungal agent and plant defense elicitor. Front Plant Sci 10:1–14. https://doi.org/10.3389/fpls.2019.01448
Wang W, Mao Q, Yao J et al (2018) Discovery of the pyridylphenylureas as novel molluscicides against the invasive snail Biomphalaria straminea, intermediate host of Schistosoma mansoni. Parasit Vectors 11. https://doi.org/10.1186/S13071-018-2868-7
WHO (2021) Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 13 Sep 2021
WHO (2022) Schistosomiasis. In: World Heal. Organ. Fact sheet. Schistosomiasis https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 18 Mar 2022
WHO (2020) Schistosomiasis. https://www.who.int/newsroom/fact-sheets/detail/schistosomiasis
WHO (2014) Schistosomiasis. Fact sheet N°115
WHO (1983) Reports of the scientific working group on plant molluscicides. Bul WHO 61:927–929 https://doi.org/http://apps.who.int/iris/handle/10665/60086
WHO (1965) Molluscicide screening and evaluation. Bull WHO 33:567–581
WHO (1990) World health organization. Vector Biology and control 791:1–60
Xing Y-T, Dai J-R, Yang K et al (2021) Bulinus snails control by China-made niclosamide in Zanzibar: experiences and lessons. In: Sino-African cooperation for schistosomiasis control in Zanzibar. Springer, Cham, pp 147–159
Xu W, Li Y, Wu Q et al (2009) Effects of phenanthrene on hepatic enzymatic activities in tilapia (Oreochromis niloticus female × O. aureus male). J Environ Sci (China) 21:854–857. https://doi.org/10.1016/S1001-0742(08)62352-9
Ye J, Wu H, Wu Y et al (2012) High molecular weight hyaluronan decreases oxidative DNA damage induced by EDTA in human corneal epithelial cells. Eye 26:1012–1020
Zheng L, Deng L, Zhong Y et al (2021) Molluscicides against the snail-intermediate host of Schistosoma: a review. Parasitol Res 120:3355–3393. https://doi.org/10.1007/S00436-021-07288-4
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Amina M. Ibrahim and Hebat-Allah A. Dokmak. The first draft of the manuscript was written by Amina M. Ibrahim, Ali A. Al-Fanharawi, and Hebat-Allah A. Dokmak, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Research data policy
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.M., Al-Fanharawi, A.A. & Dokmak, HA.A. Ovicidal, immunotoxic and endocrine disrupting effects of saponin on Bulinus truncatus snails with special emphasize on the oxidative stress parameters, genotoxicological, and histopathological alterations. Environ Sci Pollut Res 30, 78641–78652 (2023). https://doi.org/10.1007/s11356-023-27668-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27668-w