Abstract
Background
Trematode infections of the genus Schistosoma can induce physiological and behavioral changes in intermediate snail hosts. This is because the parasite consumes essential resources necessary for the host's survival, prompting hosts to adapt their behavior to maintain some level of fitness before parasite-induced mortality occurs.
Methods
In this study, the reproductive and biochemical parameters of Biomphalaria alexandrina and Bulinus truncatus were examined during the cercareal shedding stage of infection with Schistosoma mansoni and Schistosoma haematobium, respectively, compared with controls.
Results
The study revealed an infection rate of 34.7% for S. mansoni and 30.4% for S. haematobium. In B. alexandrina infected with S. mansoni, a survival rate of 65.2% was recorded, along with a mean prepatent period of 30.3 ± 1.41 days, a mean shedding duration of 14.2 ± 0.16 days, and a mean lifespan of 44.1 ± 0.24 days. Meanwhile, in B. truncatus infected with S. haematobium, a survival rate of 56.4% was observed, with a mean prepatent period of 44.3 ± 1.41 days, a mean shedding duration of 22.6 ± 2.7 days, and a mean lifespan of 66.9 ± 1.6 days. Feeding increased in both infected species of snails, while the net reproductive rate (Ro) of the infected snails decreased. Total antioxidant (TAO) and lipid peroxidation activity increased in the two infected snail species during shedding, while Glutathione-S-transferase levels decreased. Lipid peroxidase activity and nitrogen oxide levels significantly decreased in infected B. alexandrina and increased in infected Bulinus. Steroid hormone levels were elevated in infected Biomphalaria, whereas they were reduced in infected Bulinus. Comet assay parameters showed an increase in the two infected genera after infection compared to control snails, indicating genotoxic damage and histopathological damage was observed.
Conclusions
These findings demonstrate that infection with larva species diverse biochemical, hormonal, genotoxic, and histopathological changes in the tissues responsible for fecundity and reproduction in B. alexandrina and B. truncates comparing with controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a chronic parasitic disease caused by trematodes of the genus Schistosoma. It is considered the second most devastating disease worldwide in terms of morbidity and mortality [1, 2]. This disease is prevalent in tropical and subtropical areas, affecting approximately 240 million people globally, with about 700 million people at risk, particularly in poor communities with inadequate sanitation facilities [3,4,5,6]. Schistosoma mansoni and S. haematobium are the two parasites that cause the most widespread forms of intestinal and urogenital schistosomiasis [7]. In our laboratory, we use the infection of B. alexandrina with S. mansoni and B. truncatus with S. haematobium to study the impact of host-parasite infections on physiological and behavioral changes, including reduced fecundity and increased feeding behavior in the two intermediate host species. When B. alexandrina becomes infected with S. mansoni and B. truncatus becomes infected with S. haematobium, the development of the hermaphroditic reproductive system of the two snail species is severely retarded [8,9,10], resulting in the production of almost no eggs. Numerous studies have reported behavioral alterations in hosts, such as changes in feeding and crawling behavior, caused by parasitic infection, and have interpreted these changes as induced adaptations by parasites to facilitate transfer to the next-stage hosts [11,12,13,14]. Increased feeding with infection has been interpreted as compensating for nutrient deprivation caused by parasites or as a modification of the host's growth rate (gigantism) [15, 16]. The comet assay has several advantages over other DNA damage methods, such as sister chromatid exchange, alkali elution, and micronucleus assay, due to its high sensitivity and the ability to determine DNA strand breaks in individual cells [17,18,19]. Gastropod snails have been reported to be intermediate hosts of certain larval digeneans [20, 21]. These snails harbor various developmental stages, such as sporocysts, rediae, and cercariae. During their multiplication and growth, they obtain nutrients from infected tissues, such as the digestive gland and gonads, leading not only to diverse histopathological changes in the snails but also to physiological disturbances [19, 21,22,23,24].
The aim of this study was to expand and update the existing knowledge regarding behavioral alterations in hosts, specifically focusing on feeding and fecundity, caused by parasitic species. The B. alexandrina-S. mansoni and B. truncatus-S. haematobium models were utilized for comparison with uninfected species (controls). In addition, biochemical, histopathological, and genotoxic parameters were measured in the tissue homogenate of both infected and uninfected snails to facilitate the comparison.
Material and Methods
Species Snails with Infections
Juvenile specimens of both B. alexandrina (shell diameter 3–5 mm) and B. truncatus (shell diameter 3–5 mm) were obtained from the stock reared in the Medical Malacology Department at Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt. The snails were originally collected from field populations in Giza Governorate and were used for all experiments. The snail species were bred under standard conditions according to the methodology described by [25].
To induce infections, triplicate groups of 10 B. alexandrina snails were individually exposed to 5–8 freshly hatched S. mansoni miracidia, and triplicate groups of 10 B. truncatus snails were individually exposed to 8–15 freshly hatched S. haematobium miracidia for 3 h at 25 °C in 2 ml vials containing dechlorinated tap water, following the protocol outlined by [10]. Miracidia of S. mansoni and S. haematobium were obtained from the Schistosome Biological Supply Center (SBSC) at Theodor Bilharz Research Institute in Egypt. Triplicate groups of 10 control snails were individually placed in 2 ml vials without exposure to miracidia. Both infected and control snails were housed in plastic aquaria (10 snails per container, with a size of 16 × 23 × 9 cm) filled with dechlorinated water. The infected snails were allowed to develop for 4 weeks after infection with B. alexandrina and 8 weeks after infection with B. truncatus.
The infection rate was calculated 4 weeks after infection in B. alexandrina and 8 weeks after infection in B. truncatus, following the method described by [26]: Infection rate = (number of infected snails/total number of snails examined) × 100. The survival rate at shedding was also calculated for both snail species according to Frank (1963) using the following equation: survival rate = \(\frac{{\text{Number of survived species snails}}}{{\text{total number of exposed miracidia species snails}}} \times 100\). Furthermore, the mean total number of cercariae, mean duration of shedding, mean prepatent period and mean lifespan were calculated for each species with positive infections, following the approach by [26].
In experiments involving quantitative cercarial counting, the standard exposure time was extended to 45–60 min. The water containing cercariae from the test tubes was carefully transferred into Petri dishes lined with graduated paper. To immobilize and stain the cercariae, a few drops of Lugol’s solution (7.5 g KI + 5 g I2 in 100 ml distilled water) were added, facilitating rapid visualization. The cercariae were then counted under a dissecting microscope [27].
Snails Feeding
Approximately 120 snails of the same size (3–5 mm) from each species were used in the infected and control groups. They were housed in a plastic container (16 × 23 × 9 cm) and provided with 50 circles of washed clean fresh lettuce leaves measuring 4 mm2. The snails were starved for one day before the experiment, and then the food was given [28]. The consumption of the lettuce circles was counted and recorded daily, and the number of surviving snails in both species was noted [29]. Triplicate groups were performed for each species and compared side by side with the control groups.
Biological Parameters
To investigate the fecundity of the two snail species, Styrofoam sheets measuring 5 × 5 cm with a thickness of 0.5 cm were used as substrates for egg deposition. These sheets were placed on the water surface of plastic containers. Weekly collection of egg masses was carried out for a period of four to eight consecutive weeks. The egg-laying capacity was quantified as (Mx), which represents the total number of eggs laid in a given week divided by the initial number of living snails (eggs/snail/week) [30]. The survivorship of the snails (Lx) and the total number of eggs laid per snail (Mx) were recorded on a weekly basis for each aquarium. The net reproductive rate (R0) of the snails throughout the experimental period was calculated using the following parameters: Survivorship (Lx), which represents the proportion of snails that survived at any given week relative to the initial population (1.0 = 100% survival rate), and Fecundity (Mx), which refers to the average number of eggs laid per snail per week. The net reproductive (R0) at any given period was determined using the formula (R0 = ΣLxMx).
Species Snail Tissue Homogenates and Biochemical Estimations
To investigate changes in biochemical parameters TAO, LPO, SOD, NO, and GST in two infected species of snails, three replicates of 10 snails per liter were prepared for two infected species at the cercarial shedding stage, as well as two control groups of the tested species. Snails with an average shell diameter of 7–8 mm were carefully crushed between two glass slides, and their shells were removed. Tissue weighing 0.1 g from each species was then homogenized in 1 ml of phosphate buffer (pH 7.1), followed by centrifugation at 4000 rpm for 15 min. The resulting supernatant was collected in Eppendorf tubes and stored at − 20 °C for further analysis.
For the biochemical analyses, Biodiagnostic kits (Biodiagnostic Dokki, Giza, Egypt) were employed to determine the levels of SOD and GST [31, 32]. Tissue malondialdehyde (lipid peroxide) was assessed according to the method described by [33]. Nitric oxide (NO) concentration was determined using a colorimetric NO kit (Biodiagnostic Company, Dokki, Giza, Egypt; Cat. No. GR 2511), based on the approach outlined by [34]. Additionally, the total antioxidant capacity was estimated using a kit (Cat. No. TA 2513) following the methodology established by [35].
Steroid Sex Hormones (Testosterone and 17β-Estradiol)
The study aimed to assess the levels of steroid hormones, specifically testosterone and 17β-estradiol, in the tissues of two snail species: one infected with the trematode species and another serving as an uninfected control group. The hormone levels were measured using the T EIA kit from Enzo Life Science (Michigan, USA, ADI-900-065) and the E EIA kit from Cayman Chemical Company (Michigan, USA, item no. 582251) according to the instructions provided by the manufacturers [36].
Genotoxicity by Comet Assay
A study was conducted to compare DNA damage in snails infected with trematodes at the shedding stage as well as a control species group, following the methods described by [37, 38].
Histopathological Alterations
The experiment included simultaneous positive infections of B. alexandrina with S. mansoni and B. truncatus with S. haematobium, along with their respective control groups. Each group consisted of three replicates with 10 snails per liter. To examine the snails' tissues, the digestive and hermaphrodite glands were dissected from their shells, fixed using Bouin’s fixative, and embedded in wax blocks. Sections of 5–8 µm thickness were prepared and stained with haematoxylin and eosin, following the protocol by [39]. Similar preparations were made for the control snails' digestive and hermaphrodite glands.
Statistical Analysis
The values of biological and biochemical parameters were expressed as mean ± SD (standard deviation). Statistical analysis was performed using the student's "t" test to determine significant changes between the control and infected groups, following the method by [40]. The limit for statistical significance was set at p < 0.05, corresponding to a confidence level of 95%.
Results
Snail’s Infection Rate
The infection rate in B. alexandrina with S. mansoni was recorded as 34.7% (Fig. 1A), while B. truncatus with S. haematobium had a recorded rate of 30.4%.
Impact of S. mansoni with B. alexandrina and S. haematobium with B. truncatus on infection rate (A), pre-patent period and duration of shedding (B), total cercarial production (C), life span post miracidia species exposure (D) and Snail’s survival rate 1st cercarial shedding stage (E) comparing with uninfected snails.
Prepatent Period and Duration of Cercarial Shedding in Snails
The pre-patent period varied from 28 to 32 days (mean: 30.3 ± 1.41) post-infection for B. alexandrina, and it was recorded as 43–50 days (mean: 44.3 ± 1.41) post-infection for B. truncatus (Fig. 1B) at 25 °C. The duration of shedding ranged from 11 to 29 days (mean: 14.2 ± 0.16) in B. alexandrina and from 14 to 36 days (mean: 24.6 ± 2.6) in B. truncatus.
Mean Total Number of Cercariae Per Snail
The mean number of cercariae per snail (Fig. 1C) in B. alexandrina was recorded as 2915 ± 74.394 (p < 0.05), and it was recorded as 1637.3 ± 307.5) (p < 0.05) in positive B. truncatus. Cercariae production typically increased after the first week of patency but often decreased significantly towards the end of the snails'.
Snail’s Mean Life Span
The mean lifespan was recorded as 44.1 ± 0.24 days in B. alexandrina (Fig. 1D) and 66.9 ± 1.6 days in B. truncatus.
Snail’s Survival Rate at First Shedding
The survival rate of B. alexandrina exposed to Schistosoma mansoni at the first cercarial shedding was 65.2%, while the survival rate of B. truncatus exposed to S. haematobium was 56.4%, compared to the survival rate in the respective control groups (Fig. 1E).
Impact of Schistosoma mansoni with Biomphlaria alexandrina and S haematobium with Bulinus truncatus on Feeding, Fecundity and Reproductive Rate
During the prepatent period, the number of feeding B. alexandrina snails on green circles of fresh lettuce leaves exceeded that of their uninfected counterparts, indicating that the infected snails were more voracious feeders (Fig. 2A). The same pattern was observed in B. truncatus infected with S. haematobium (Fig. 2B). Additionally, the fecundity of B. alexandrina showed a pattern of ceasing egg-laying for 4 weeks during the prepatent period (Fig. 2C), which was also observed in B. truncatus after being exposed to miracidia (Fig. 2D). The net reproductive rate (Ro) in infected B. alexandrina and B. truncatus was significantly reduced to 47.7% and 84.6% of its value in the respective control groups (Fig. 2E).
Impact of S. mansoni with B. alexandrina and S. haematobium with B. truncatus on feeding (A, B); on fecundity (C, D) and reproductive rate (E) in two infected snails, Survivorship (Lx): This represents the proportion of snails that survived at any given week relative to the initial population (1.0 = 100% survival rate). Fecundity (Mx): this refers to the average number of eggs laid per snail per week. The net reproductive rate (R0) at any given period was determined using the formula (R0 = ΣLxMx) comparing with uninfected snails
Impact of Infection with Schistosoma mansoni in Biomphlaria alexandrina and Infection with S haematobium in Bulinus truncatus on Oxidative Stress Parameters at 1st Cercarial Shedding Stage
TAO activity showed a significantly higher value (p < 0.05) in the homogenized tissue of B. alexandrina compared to the uninfected group. Similarly, infected B. truncatus snails exhibited a significantly higher TAO activity (Table 1, Fig. 3A). These findings suggest that the infections were stressful for the snails, triggering an increase in their antioxidant defense mechanism.
Lipid peroxidation (LPO) activity displayed contrasting results between the two snail species. In infected B. alexandrina snails, LPO activity was significantly reduced relative to the uninfected group. Conversely, in infected B. truncatus snails, LPO activity increased significantly compared to the control group (Fig. 3B). These observations indicate that S. mansoni infection in B. alexandrina may have a protective effect against lipid peroxidation, while S. haematobium infection in B. truncatus may induce oxidative damage. Furthermore, a significant elevation in the levels of nitric oxide (NO) was observed in the tissue homogenate of infected B. truncatus snails whereas infected B. alexandrina snails exhibited a significant reduction in NO compared to the uninfected group (Fig. 3C).
Superoxide dismutase (SOD) levels were higher in infected B. alexandrina and B. truncatus snails compared to uninfected snails in both species (Table 2, Fig. 3D). This indicates an up regulation of the SOD antioxidant enzyme as a response to the infections in both snail species.
In terms of glutathione-s-transferase (GST) activity, the highest value was measured in infected B. truncatus snails, while infected B. alexandrina snails exhibited a reduction in GST activity compared to uninfected B. alexandrina snails (Fig. 3E). These differences in the antioxidant system response may be attributed to variations in laboratory-infected snail species or the longer prepatent period in B. truncatus compared to B. alexandrina, regardless of the parasite species.
Impact of S. mansoni with B. alexandrina and S. haematobium with B. truncatus on 17β-Esteradiol and Testosterone Hormones in Tissues at 1st Cercarial Shedding Stage
In infected B. alexandrina snails, there were significant increases in the concentrations of 17β-estradiol and testosterone in homogenized tissues post-infection (Table 2, Fig. 4A). On the other hand, infected B. truncatus snails exhibited a notable reduction in the concentrations of 17β-estradiol and testosterone hormones post-infection compared to their levels in non-infected control snails (p < 0.05) (Fig. 4B).
Impact of S. mansoni with B. alexandrina and S. haematobium with B. truncatus on Comet Assay at 1st Cercarial Shedding Stage
The comet assay was employed to assess DNA damage in B. alexandrina and B. truncatus snails infected with Schistosoma mansoni and S. haematobium, respectively. The parameters of tailed % and tailed length, which indicate cellular malformation, exhibited significant increases in infected B. alexandrina snails compared to the uninfected groups (refer to Fig. 5 and Plate 1). Additionally, there was an increase in the percentage of normal DNA in the tail, indicating migration from the head in infected B. alexandrina snails. The tail moment, which represents the combination of tail length and the percentage of DNA migrated from the head, may serve as an indicator of genotoxicity and negative effects on the cellular resistance system (Fig. 5 and Plate 1). Moreover, the olive tail moment, a marker of DNA fragmentation, showed a significant increase in infected B. alexandrina snails compared to the control group (p < 0.05). Similar adverse impacts on comet assay parameters were observed in infected B. truncatus snails with S. haematobium during the shedding stage (Table 3, Fig. 5A, B, Plate 1).
Impact of S. mansoni with B. alexandrina and S. haematobium with B. truncatus on the Snails’ Digestive and Hermaphrodite Glands Histology at 1st Cercarial Shedding Stage
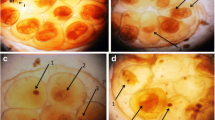
Infection of B. alexandrina and B. truncatus snails with S. mansoni and S. haematobium, respectively, can have destructive effects on the snail tissues. Histological studies were conducted on sections from the digestive and hermaphrodite glands of both infected and uninfected snails. The normal histological structure of the digestive gland in both species includes two main cell types: the columnar digestive cells with rounded apices and the pyramidal-shaped secretory cells (Plate 2A & C).
A Light micrographs show the normal digestive glands and normal hermaphrodite gland of B. alexanderina and C normal digestive glands and normal hermaphrodite gland of B. truncatus snails. Digestive cells (blue arrow), secretory cells (dark red arrow), Lumen (head dark red arrow) (H&E; × 100; × 200). Mature ovum (red arrow), Oocytes (black arrow), Sperms (yellow arrow). B and D show infected digestive and hermaphrodite gland where red arrow (s) sporocysts of cercariae species at 1st cercarial shedding stage compared with uninfected snails.
Histological examination of the sections from the digestive gland of infected snails at the shedding stage revealed detrimental effects, including swelling and deformation of the secretory cells, rupturing and disintegration of the digestive cells, as well as the presence of sporecysts containing cercariae species (Plate 2, B & D).
In the hermaphrodite gland, responsible for producing both male and female reproductive gametes, mature ova are located at the periphery of the acinus, while bundles of male sperm are arranged in the center. Various stages of sperm and ova development can be observed simultaneously (Plate 2, A & C). Histological sections of this gland from infected snails showed varying degrees of degeneration in ova and sperm, depending on the sporecysts of cercariae species during the experimental shedding period in both species (Plate 2, B & D).
Discussion
Snail’s Infection Rate
Lab observations of B. alexandrina infected with S. mansoni and B. truncatus infected with S. haematobium were consistent with the findings of [41], which reported a 30% infection rate (IR) in B. pfeifferi snails with S. mansoni. However, the observations differed from those of [42, 43], who reported higher IR in B. pfeifferi snails infected with S. mansoni. In the case of B. truncatus, a 50.5% IR was observed in snails aged one to seven days, and a 19.9% IR was observed in snails aged one and a half to 5 weeks under laboratory conditions [44].
Prepatent Period and Duration of Cercarial Shedding in Snails
The mean pre-patent and Snail’s duration periods for positive B. alexandrina and B. truncatus observed in this study are consistent with previous findings regarding the time interval between miracidial infection of the intermediate host and the subsequent release of cercariae. Previous research by [45] reported that S. mansoni exhibits the fastest rate of development, taking approximately 33 days at 25 °C, while S. haematobium takes around 50 days. These findings highlight the significance of prepatency periods in the epidemiology of schistosomiasis, as acknowledged by [46].
Furthermore, [45] emphasized the crucial role of the latent period (prepatent period) in determining the prevalence of infection within snails. The latent period refers to the time interval between snail infection by a miracidium (the larval stage of a parasitic trematode) and the initiation of cercarial shedding (the subsequent larval stage that is infective to the final host). The mean total number of cercariae per snail observed in this study differs from the findings reported by [47] in B. glabrata.
However, it aligns with previous studies on Bulinus truncatus infected with miracidia, which reported a range of 29–65 days for cercarial production at 24–26 °C [27, 48]. In the present study, the number of cercariae shed weekly by positive B. alexandrina was greater than the number of cercariae shed weekly in positive Bulinus truncatus. This difference can be attributed to the varying doses of miracidia given to the two species. Massoud [49] demonstrated that the numbers of cercariae shed daily by single snails exposed to one or two miracidia were significantly lower than those exposed to 5, 10, or 20 miracidia.
Mean Total Number of Cercariae Per Positive Snails
The examination of cercariae in the present study revealed that 90–100% of mature S. mansoni and S. haematobium cercariae were shed within 45–60 min of exposure to light. Pflüger [27] documented that the standard stimulation period for mature S. haematobium cercariae was limited to 5 h. While cercariae production typically increased after the first week of patency, it often decreased significantly towards the end of the snails' lifespan.
Snail’s Mean Life Span Comparing with Uninfected Snails
The lifespan of Schistosoma-positive snails in B. alexandrina was found to range from 45 to 81 days (with a mean of 44.1 ± 0.24 days), while in B. truncatus, it ranged from 55 to 91 days (with a mean of 65.9 ± 1.6 days) compared to uninfected snails in both species. The differences between the mean lifespan of infected snails and non-infected snails in the control group in both species were statistically significant (p < 0.05). Chu et al. [50] demonstrated that the cercaria-shedding period and the lifespan of infected snails were shorter than those of the non-infected controls.
The longer lifespan observed in B. truncatus may be attributed to the higher doses of S. haematobium miracidia compared to S. mansoni miracidia within B. alexandrina. Notably, [51] reported observations on the development of the parasite in relation to tissue changes and mortality among infected snails. It was concluded that the extensive migration of large numbers of cercariae, along with the intense tissue reactions associated with trapped and degenerating cercariae, are significant factors contributing to the death of the snails. Furthermore, laboratory studies conducted by [50, 52] clearly demonstrate that infection with any of the three principal species of human schistosomes adversely affects the survival of the molluscan host.
Snail’s Survival Rate at at 1st Cercarial Shedding Stage Comparing with Uninfected Snails
This study reported a decrease in the survival rate of two snail species after shedding cercariae, which supports the findings of [53]. The aforementioned study observed lower survival rates in snails exposed to S. mansoni miracidia compared to unexposed snails. Previous laboratory studies have shown a wide range of mortality rate increases in schistosoma-infected snails compared to uninfected ones, with some estimates reaching up to 0.100 [45]. Additionally, [54] discovered that patent infections of S. species led to higher per capita mortality rates in Bulinus globosus and B. pfeifferia, including mortalities during the prepatent period in the two infected species. The reduction in this biological parameter may be attributed to potential competition between the parasites and the host for essential haemolymph-borne nutrients [55]. Additionally, it could be a result of histopathogenic effects on the snail host and depletion of nutrients by the parasite, particularly around the time of infection maturation and cercariae shedding [56].
Impact of Schistosoma Infection on Feeding, Fecundity and Reproductive Rate Comparing with Uninfected Snails
Infected B. alexandrina and B. truncatus snails exhibited a tendency to feed more frequently compared to uninfected snails. This finding aligns with [57], who observed that freshwater snails infected with larval trematodes displayed increased feeding behavior during the light period under laboratory conditions. Parasite infection often leads to alterations in host behavior, indicating adaptive manipulation of the host behavior by the parasite to enhance its transmission success [58,59,60].
Increased feeding behavior in infected individuals has been interpreted as a compensatory response to nutrient deprivation caused by parasites or as a modification of the host's growth rate, such as gigantism [15, 16]. Other researchers have described the reduced fecundity in infected snails as castration, suggesting that the trematode parasite alleviates the energetic demands of reproduction, allowing the host to allocate this energy towards other life-history traits, such as growth and survival [61, 62]. Another possible explanation for increased feeding is starvation autolysis, which occurs due to the compression of digestive tubules at various locations, hindering the passage of food into the tubules. This can lead to intracellular digestion, and heavy infection can result in the atrophy of digestive tubules [63, 64]. Infection with S. mansoni or S. haematobium miracidia has been observed to cause B. alexandrina and B. truncatus snails to cease egg-laying after exposure, resulting in a reduction in reproduction [8, 10, 15].
The development of the hermaphrodite reproductive system in L. stagnalis infected with T. ocellata was severely hindered, resulting in a near absence of egg production [8, 10, 15, 65]. Reductions in fecundity were also observed in three Bulinus species infected with S. haematobium [66]. The decrease in egg-laying could be attributed to nutrient deprivation caused by the parasite or the dual burden of producing both eggs and parasites, which is not borne by the snail [9, 67,68,69].
In our present study, infected snails ceased egg-laying in the early weeks of infection, leading to a significant reduction in the average number of eggs per snail in both species. This finding aligns with [69], who attributed the suppression of egg-laying to the indirect effect of trematode larvae on oogenesis, potentially caused by nutrient withdrawal by the parasite or the burden of producing eggs and parasites [67, 69]. Nutrient deprivation may be responsible for the decline in egg-laying, coinciding with the development of sporocysts in the digestive gland [70].
Even a small number of mother sporocysts present during the infection stage could be sufficient to disrupt reproductive processes in the two species. Finally, it should be noted that the molluscan host experiences partial or complete castration following infection [71].
Impact of Schistosoma Infection on Oxidative Stress Parameters at 1st Cercarial Shedding Stage Comparing with Uninfected Snails
Increasing the level of TAO in infected B. alexandrina and B. truncatus snails may explain the increase in the number of haemocytes and the generation of large volumes of ROS for defensive purposes to damage or kill the parasite's larvae [72,73,74,75].
Gornowicz et al. [76] found significant differences in TAS between control and P. elegans-infected Lymnaea stagnalis during the initial period of the experiments. TAS was influenced by infection with trematodes in Biomphlaria galabrata with S. mansoni [77].
Biomphalaria alexandrina snails infected with Schistosoma mansoni showed a significant reduction in the levels of lipid peroxidation (LPO) and nitric oxide (NO) compared to uninfected snails. This reduction may be attributed to the developing schistosome larvae scavenging nutrients from the snail's hemolymph, resulting in a decrease in the amount of nutrients circulating to the nervous system [78].
Furthermore, another study [79] reported a significant decrease in catalase (CAT) and glutathione (GSH) levels, along with an increase in malondialdehyde (MDA) levels, in the tissues and hemolymph of B. alexandrina following infection with S. mansoni. However, B. truncatus infected with Schistosoma haematobium exhibited a significant increase in the levels of LPO and NO compared to uninfected snails at the shedding stage. In another investigation [80], it was observed that B. alexandrina snails infected with S. mansoni and B. truncatus snails infected with S. haematobium showed a significant elevation in the activities of glutathione reductase (GR), catalase, and superoxide dismutase (SOD). Changes in the infected snail tissue homogenates were also reported [81]. Upon treatment with sodium fluoride, these altered biochemical parameters were restored to their values in control uninfected snails, indicating the ability of sodium fluoride to inhibit oxidative stress and apoptosis in Schistosoma-infected snails [81]. In response to parasitic infection, both B. alexandrina and B. truncatus snails increase the activity of their defensive haemocytes, which generate significant amounts of reactive oxygen species (ROS) to damage or kill parasite larvae.
Impact of Schistosoma infection on 17β-Esteradiol and Testosterone Hormones in Tissues at 1st Cercarial Shedding Stage Comparing with Uninfected Snails
Steroid hormones, such as testosterone and estradiol, were found to be elevated in Biomphalaria snails during the shedding stage. According to [82], serum estradiol levels in male mice susceptible to Taenia crassiceps (TC) infection increased to levels 200 times higher than their normal values. The authors suggested that the parasite affects the immunoendocrine mechanism, creating a highly permissive environment for its rapid growth. In Biomphalaria alexandrina, larval trematode infection disrupts normal reproductive activity. This may explain why S mansoni snails increase the activity of steroid hormones, creating a highly permissive environment in ova and sperm, resulting in adverse effects on their physiological activities and defense mechanisms. Consequently, infected snails may cease laying eggs. However, infected positive Bulinus snails, the hormones were suppressed. De Jong-Brink [83] illustrated that Schistosomin, a peptide produced by the nervous system of infected snails following schistosome infection, interferes with the host's neuroendocrine system, inhibiting the action of reproductive hormones. Steroid hormones have been documented in various molluscs, including B. alexandrina [84,85,86,87] and B. truncates [88].
Hormonal reductions observed in Bulinus truncatus and increases in Biomphalaria alexandrina may contribute to fecundity loss in these infected snails [89]. Steroid hormones play an important role in gonad development in snails [90]. Hormone administration, including testosterone, estradiol, and progesterone, has been shown to stimulate spermatogenesis and oogenesis in various molluscan species [91,92,93,94].
Impact of Schistosoma Infection on Comet Assay at 1st Cercarial Shedding Stage Comparing with Uninfected Snails
The study revealed that B. alexandrina and B. truncatus infected with positive cercariae at the 1st cercarial shedding stage exhibited a statistically significant increase in DNA fragmentation and migration in molluscan tissues compared to the control group. These findings are consistent with previous studies that have reported an increase in tail length (length of DNA migration) in the digestive gland cells of infected snails due to larval trematode infections. Furthermore, the percentage of apoptosis was significantly elevated (58.80%) in the snails infected with larval trematodes compared to uninfected snails (39.59%). The DNA damage and increased apoptosis in the digestive glands of infected snails may result in a decrease in 5-HT (serotonin) and DA (dopamine) concentrations in all tissues throughout the course of infection [80]. DNA has long been recognized as a primary target of age-related cellular damage, and its damage can potentially contribute to the aging process [97]. Additionally, DNA damage has been observed in the hemocytes of Biomphalaria alexandrina [95] and Bulinus truncatus [96]. In response to parasitic infection, both B. alexandrina and B. truncatus snails increase the activity of their defensive hemocytes, which generate significant amounts of reactive oxygen species (ROS) to damage or kill parasite larvae. These ROS can potentially be toxic to DNA, leading to DNA oxidation and/or strand breaks.
Impact of Schistosoma Infection on Digestive and Hermaphrodite Glands at 1st Cercarial Shedding Stage Comparing with Uninfected Snails
The study revealed severe damage to the cell constituents of the digestive and hermaphrodite glands in infected B. alexandrina and B. truncatus snails caused by trematode larvae. Changes in the digestive glands and ovotestis induced by larval digenean trematode parasites have been reported to depend on the severity of infection, larvae size, and types of larvae [98]. Possible explanations for these alterations include mechanical damage resulting from the migration, feeding, growth, and multiplication of trematode larvae, as well as physiological changes such as autolysis and/or necrosis. Previous studies have shown that redial stages cause more mechanical and physiological damage compared to sporocysts [64, 99].
Rediae engulf the host's digestive cells and utilize hydrolases for extracellular digestion, contributing to physiological damage [100]. It can be assumed that spore larval species observed within the two host cell constituents' tissues in the digestive and hermaphrodite glands are more destructive for the two hosts. Parasitic secretions and excretory products that produce toxic effects may also be contributory factors [101, 102].
References
Ibrahim AM, Ghazy M, El-sayed H et al (2023) In silico molecular docking study of fungal-mediated selenium oxide nanoparticles on Biomphalaria alexandrina (Ehrenberg, 1831) snails. Microorganisms 11:811
WHO (2023) World Health Organization: Schistosomiasis, sheet facts. https://www.who.int/news-room/fact-sheets/detail/s. Accessed 01 Feb 2023
WHO (2022) World Health Organization GUIDELINE on control and elimination of human schistosomiasis. 142
Ibrahim AM, El-karim RMG, Ali RE, Nasr SM (2023) Toxicological effects of Saponin on the free larval stages of Schistosoma mansoni, infection rate, some biochemical and molecular parameters of Biomphalaria alexandrina snails. Pestic Biochem Physiol 191:105357. https://doi.org/10.1016/j.pestbp.2023.105357
World Health Organization (2013) Schistosomiasis: progress report 2001–2011 and strategic plan 2012–2020. WHO, Geneva, p 2
Obare B, Yole D, Nonoh J, Lwande W (2016) Evaluation of cercaricidal and miracicidal activity of selected plant extracts against larval stages of Schistosoma mansoni. JNSR 6(22):24–31
Mansour SM, Ibrahim AM (2023) Differentiation between Bulinus truncatus and Bulinus hexaploidus by morphological characters, chromosomal study and compatibility with Schistosoma haematobium. Exp Parasitol. https://doi.org/10.1016/J.EXPPARA.2023.108502
Joosse J, Van Elk R (1986) Trichobilharzia ocellata: physiological characterization of giant growth, glycogen depletion, and absence of reproductive activity in the intermediate snail host, Lymnaea stagnalis. Exp Parasitol 62:1–13
McClelland G, Bourns TKA (1969) Effects of Trichobilharzia ocellata on growth, reproduction and survival of Lymnaea stagnalis. Exp Parasitol 24:137–146
Sluiters JF, Brussaard-Wiist CCM, Meuleman EA (1980) The relationship between miracidial dose, production of cercariae, and reproductive activity of the host in the combination Trichobilharzia ocellata and Lymnaea stagnalis. Parasitenkd Z 63:13–26
Swennen C (1969) Crawling-tracks of trematode infected Macoma balthica (L.). Neth J Sea Res 4:376–379
Curtis LA (1990) Parasitism and the movements of intertidal gastropod individuals. Biol Bull 179:105–112
Curtis LA (1993) Parasite transmission in the intertidal zone: vertical migrations, infective stages, and snail trails. J Exp Mar Biol Ecol 173:197–209
Levri EP, Lively CM (1996) The effects of size, reproductive condition, and parasitism on foraging behaviour in a freshwater snail, Potamopyrgus antipodarum. Anim Behav 51:891–901
Minchella DJ (1985) Host life-history variation in response to parasitism. Parasitology 90:205–216
Hurd H (1990) Physiological and behavioural interaction between parasites and invertebrate hosts. Adv Parasitol 29:271–318
Pavlica M, Klobucar GIVM, Mojas N, Erben R, Papes D (2001) Detection of DNA damage in haemocytes of zebra mussel using comet assay. Mutat Res 490:209–214
Ibrahim AM, Ahmed AK, Bakry FA, Abdel-Ghaffar F (2018) Hematological, physiological and genotoxicological effects of Match 5% EC insecticide on Biomphalaria alexandrina snails. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2017.09.059
Morad MY, El-Sayed H, Elhenawy AA, Korany SM, Alofi AS, Ibrahim AM (2022) Myco-synthesized molluscicidal and larvicidal selenium nanoparticles: a new strategy to control Biomphalaria alexandrina snails and Larvae of Schistosoma mansoni with an in silico study on induced oxidative stress. J Fungi 8:262. https://doi.org/10.3390/JOF8030262
Choubisa SL, Sharma PN (1986) Incidence of larval trematodes infection and their seasonal variation in the freshwater molluscs of southern Rajasthan. Rec Zool Surv India 83:69–83
Abdel-Tawab H, Ibrahim AM, Hussein T, Mohamed F (2022) Mechanism of action and toxicological evaluation of engineered layered double hydroxide nanomaterials in Biomphalaria alexandrina snails. Environ Sci Pollut Res Int. https://doi.org/10.1007/S11356-021-16332-W
Huffman J, Fried B (1985) Histopathological and histochemical effects of larval trematodes in Goniobasis virginica (Gastropoda: Pleuroceridae). Veliger 27(3):273–281
Soomro NM, Arijo TA, Qureshi NW, Runham MJ (2005) Pathology of Schistosome infection on host tissue during developmental stages of parasite in vector snails. Int J Agric Biol 7:133–141
Huffman JE, Klockars J, Keeler SP, Fried B (2009) Histopathological effects of the intra molluscan stages of Zygocotyle lunata, Echinostoma trivolvis, and Ribeiroia ondatrae on Helisoma trivolvis and observations on keratin in the trematode larvae. Parasitol Res 105:1385–1389
Van der Steen WJ, Van den Hoven NP, Jager JC (1969) A method for breeding and studying freshwater snails under continuous water change, with some remarks on growth and reproduction in Lymnaea stagnalis. Neth J Zool 19:131–139
Coles GC (1973) The effect of diet and crowding on the shedding of Schistosoma mansoni cercariae by Biomphalaria glabrata. Ann Trop Med Parasitol 67:419–423
Pflüger W, Roushdy MZ, El Eman M (1984) The prepatent period and cercarial production of Schistosoma haematobium in Bulinus truncatus (Egyptian field strains) at different constant temperatures. Z Parasitenkd 70:95–103
Valarmathi V (2017) Food preference and feeding behaviour of the land snail Cryptozona bistrialis in nagapattinam, Tamil nadu, India. Int J Zool Appl Biosci 2(2):90–94
Colpaert R, Petitdit Grézériat L, Louzon M et al (2021) Polyethylene microplastic toxicity to the terrestrial snail Cantareus aspersus: size matters. Environ Sci Pollut Res 29:29258–29267. https://doi.org/10.1007/s11356-021-15824-z
Gawish FA, El-Sherbini SA, Aly HF (2009) Effect of photosensitization process of carbamide perhydrate on Biomphalaria alexandrina snails and their infection with Schistosoma mansoni. J Appl Sci Res 5(1):46–56
Damerval C, De Vienne D, Zivy M, Thiellement H (1986) Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 7:52–54. https://doi.org/10.1002/elps.1150070108
Beutler E (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61:882–888
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Montgomery HAC, Dymock JF (1962) The rapid determination of nitrate in fresh and saline waters. Analyst. https://doi.org/10.1039/an9628700374
Koracevic D, Koracevic G, Djordjevic V et al (2001) Papers method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361. https://doi.org/10.1136/jcp.54.5.356
Ibrahim AM, Al-Fanharawi AA, Dokmak HA (2023) Ovicidal, immunotoxic and endocrine disrupting effects of saponin on Bulinus truncatus snails with special emphasize on the oxidative stress parameters, genotoxicological, and histopathological alterations. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-27668-w
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage individual cells. Exp Cell Res 175:184–191
Grazeffe VS, De Freitas TL, De Sa PA et al (2008) Establishment of the comet assay in the freshwater snail Biomphalaria glabrata (Say, 1818). Mutat Res Toxicol Environ Mutagen 654:58–63
Mohamed SH, Saad AA (1990) Histological studies on the hermaphrodite gland of Lymnaea caillaudi and Biomphalaria alexandrina upon infection with certain larval trematodes. Egypt J Histol 13:47–53
Sokal RR, Rohlf FJ (1995) Introduction to biostatistics. W.H. Freeman and Co., San Francisco, pp 271–273
Makanga B (1981) The effect of varying the number of Schistosoma mansoni miracidia on the reproduction and survival of Biomphalaria pfeifferi. J Invertebr Pathol 37(1):7–10
Southgate VR, Tchuenté LA, Théron A, Jourdane J, Ly A, Moncrieff CB, Gryseels B (2000) Compatibility of Schistosoma mansoni Cameroon and Biomphalaria pfeifferi Senegal. Parasitology 121(5):501–505
Ibikounlé MG, Mouahid R, Nguéma M, Sakiti NG, Kindé-Gasard D, Massougbodji A, Moné H (2012) Life-history traits indicate local adaptation of the schistosome parasite, Schistosoma mansoni, to its snail host, Biomphalaria pfeifferi. Exp Parasitol 132:501–507
Southgate VR, Tchuem Tchuenté LA, The Ron A, Jourdan J, Ly A, Moncrieff CB, Gryseels B (2000) Compatibility of schistosoma mansoni cameroon and biomphalaria pfeifferi senegal. Parasitol 121:501–505
Anderson RM, Mercer JG, Wilson RA, Carter NP (1982) Transmission of Schistosoma mansoni from man to snail: experimental studies of miracidial survival and infectivity in relation to larval age, water temperature, host size and host age. Parasitology 85:339–360
Anderson RM, May RM (1979) Prevalence of schistosome infections within molluscan populations: observed patterns and theoretical predictions. Parasitology 79:63–94
Kechemir N (1985) Schistosoma haematobium (Bilharz, 1852) Développement larvaire, clonage, polymorphisme, caractères de la transmission dans les foyers algériens. Doctoral Thesis, Perpignan University
Lo CT (1972) Compatibility and host-parasite relationships between species of the genus Bulinus (Basommatophora: Planorbidae) and an Egyptian strain of Schistosoma haematobium (Trematoda: Digenea). Malacologia 11:225–280
Massoud J (2009) The effect of variation in miracidial exposure dose on laboratory infections of Ornithobilharzia turkestanicum in Lymnaea gedrosiana. Cambridge University Press, Cambridge
Chu KY, Sabbaghian H, Massoud J (1966) Host^parasite relationship of Bulinus truncatus and Schistosoma haematobium in Iran. 2. Effect of exposure dosage of miracidia on the biology of the snail host and the development of the parasites. Bull World Health Organ 34:131–133
Pan CT (1965) Studies on the host-parasite relationship between Schistosoma mansoni and the snail Australorbis glabratus. Am J Trop Med Hyg 14:931–976
Pesigan TP, Haibston KG, Jaubequi JJ, Garcia EG, Santos AT, Santos BC, Besa AA (1958) Studies on Schistosoma japonicum infection in the Philippines the molluscan host. Bull World Health Organ 18:481–578
Mangal TD, Paterson S, Fenton A (2010) Effects of snail density on growth, reproduction and survival of Biomphalaria alexandrina exposed to Schistosoma mansoni. J Parasitol Res 2010:186792
Woolhouse HEJ (1989) The effect of schistosoma infection on mortality rate of Bulinus globosus and Biomphlaria pfeifferia. Ann Trop Med Parasitol 83:137–141
Becker W (1980) Microcolorimetric studies in Biomphalaria glabrata: the influence of Schistosoma mansoni on basal metabolism. Comp Biochem Physiol 135(B):101
El-Sayed K, El-Dafrawy S, Sharaf El-Din A (1999) Influence of Schistosoma mansoni infection on Biomphalaria alexandrina snails under laboratory conditions. J Zool Egypt 33:343–354
Shinagawa K, Urabe M, Nagoshi M (2001) Effects of trematode infection on metabolism and activity in a freshwater snail, Semisulcospira libertine. Dis Aquat Organ 45(2):141–144
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, New York
Poulin R (2010) Parasite manipulation of host behavior: an update and frequently asked questions. Adv Study Behav 41:151–186
Thomas F, Adamo S, Moore J (2005) Parasitic manipulation: where are we and where should we go? Behav Process 68:185–199
Poulin R (2006) Strategies of host exploitation. Evolutionary ecology of parasites, 2nd edn. Princeton University Press, New Jersey, pp 94–131
Lafferty KD, Kuris AM (2009) Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol 25:564–572
Mohandas A (1974) The pathological effect of larval trematodes on the digestive glands of four species of gastropods. Folia Parasitol (Prague) 21:219–224
Choubisa SL (1988) Histological and histochemical observations on the digestive gland of Melanoides tuberculatus (Gastropoda) infected with certain larval treamatodes and focus on their mode of nutrition. Proc Indian Acad Sci (Anim Sci) 97(3):251–262
Thornhill JA, Jones JT, Kusel J (1986) Increased oviposition and growth in immature Biomphalaria glabrata after exposure to Schistosoma mansoni. Parasitology 93:443–450 ([PubMed: 3797059])
Fryer SE, Oswald RC, Probert AJ, Runham NW (1990) The effect of Schistosoma haematobium infection on the growth and fecundity of three sympatric species of bulinid snails. J Parasitol 76:557–563
Neuhaus W (1949) Hungerversuche zur Frage der parasitih’en Kastration bei Bithynia tentaculata. Z Parasitenk 14:300–319
Meier M, Meier-Brook C (1981) Schistosoma mansoni: effect on growth, fertility, and development of distal male organs in Biomphalaria glabrata exposed to miracidia at different ages. Z Parasitenkd 66(121):131
Alberto-Silva AC, Santos EGN, Santos CP, Mello-Silva CC (2015) Changes in the locomotory and reproductive behavior of Biomphalaria glabrata infected with Schistosoma mansoni. Exp Parasitol 153:68–74 ([PubMed: 25765559])
Looker DL, Etges FJ (1979) Effect of Schistosoma mansoni infection on fecundity and perivitelline fluid composition in Biomphalaria glabrata. J Parasitol 65(880):885
Sorensen R, Minchella DJ (2001) Snail-trematode lifehistory interactions: past trends and future directions. Parasitology 123:S1–S16
Bikowska EZ (2006) Interakcje w ukl = adzie z_ywiciel – pasoz_yt mie˛dzy bl =otniarkami Lymnaea stagnalis i przywrami z gatunkow: Diplostomum pseudospathaceum, Echinoparyphium aconiatum, Plagiorchis elegans. Wydawnictwo Uniwersytetu Mikol=aja Kopernika, Torun
Saboor-Yaraghi AA, Farahnak A, Eshraghian MR (2011) Haemolymph components of infected & none infected Lymnaea stagnalis with Xiphidiocercariae. Iran J Parasitol 6:86–91
Hadas E, Stankiewicz M (1996) Strategies of biochemical defence mechanisms of parasites against oxidants and free radicals. Acta Parasitol 41:1–6
Mone Y, Ribou AC, Cosseau C, Duval D et al (2011) An example of molecular co-evolution: reactive oxygen species (ROS) and ROS scavenger levels in Schistosoma mansoni/Biomphalaria glabrata interactions. Int J Parasitol 41:721–730
Gornowicz D, Dmochowska K, Bikowska EZ, Towska KZOL (2013) Total antioxidative status and the activity of peroxidase and superoxide dismutase in the haemolymph of Lymnaea stagnalis (L.) naturally infected with digenean trematodes. J Molluscan Stud 79:225–229
Jong-Brink KM, Oene JM (2005) Parasite manipulation beyond behavior. Behav Process 68:229–233
Habib MR, Ghonamea SI, Alia RE, Gad El-Karima RM, Youssefa AA, Crollb RP, Millerc MW (2020) Biochemical and apoptotic changes in the nervous and ovotestis tissues of Biomphalaria alexandrina following infection with Schistosoma mansoni). Exp Parasitol 213:107887. https://doi.org/10.1016/j.exppara.2020.107887
Mossalem HS, Habib MR, Ghareeb MA (2018) Control of infection of Biomphalaria alexandrina (Ehrenberg, 1831) with Schistosoma mansoni sambon, 1907 using Eucalyptus camaldulensis. Folia Malacol 26:155–165
ElT R, Hamada SF, Abd-ElGhany SR, Ramez AM (2018) Biological investigations on the freshwater snail Pirenella conica (Blainville, 1829) infected with the developmental stages of Heterophyes sp. J Basic Appl Zool 79:4
Koriem KM, Shamsuri RB, Ubaidillah AM (2016) Evaluation of sodium fluoride toxicity in Schistosoma infected snails: assessment of antioxidants, antiapoptotic, hypoprotein and hypocholesterol activities. J Parasit Dis 40:1451–1458 ([PubMed: 27876966])
Pane C, Larralde J, Morales I, Terrazas T, Govezensky MC (1995) Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J Steroid Biochem Mol Biol 52(6):575–580
De Jong-Brink M (1995) How schistosomes profit from the stress responses they elicit in their hosts. Adv Parasitol. https://doi.org/10.1016/S0065-308X(08)60072-X
Oehlmann J, Schulte-Oehlmann U (2003) Endocrine disruption in invertebrates. Pure Appl Chem 75:2207–2218
Croll RP, Wang C (2007) Possible roles of sex steroids in the control of reproduction in bivalve molluscs. Aquaculture 272:76–86
Omran NEE (2012) Testosterone, gonadotropins and androgen receptor during spermatogenesis of Biomphalaria alexandrina snails (Pulmonata: basommatophora). Reprod Biol 12:301–308 ([PubMed: 23153701])
Ragheb M, El-Tayeb TA, El-Emam MA, Amer MA, Bashtar MA (2018) Fecundity, sex hormones and release of cercariae of Schistosoma mansoni in Biomphalaria alexandrina (Ehrenberg, 1831) treated with copper and magnesium chlorophyllin. Folia Malacol 26:17–24
Dokmak HAAS, El-Emam MA, Mossalem HS et al (2021) Impact of carbamide perhydrate on the snail Bulinus truncatus, the intermediate host of Schistosoma haematobium. Egypt J Aquat Biol Fish 25(3):85–99
Ibrahim AM, Hussein AAA (2022) Toxicological impact of organophosphorus Chlorpyrifos 48%EC pesticide on hemocytes, biochemical disruption, and molecular changes in Biomphalaria alexandrina snails. Pestic Biochem Physiol 186:105154. https://doi.org/10.1016/j.pestbp.2022.105154
Alon G, Laureuce SS, Sleinberge Y (2007) Correlation between levels of sex hormones (progesterone, testosterone, and estrogen) and ecophysiological-behavior stages in two species of desert snails (Sphincterochila zonata and Sphincterochila prophetarum) in the Northern Negev Desert. General Comp Endocrinol 151(1):122
Ibrahim AM, Abdel-Tawab H (2020) Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schis. Biologia (Bratisl) 75:1–10
Hamdi SAH, Ibrahim AM, Ghareeb MA, Fol MF (2021) Chemical characterization, biocidal and molluscicidal activities of chitosan extracted from the crawfish Procambarus clarkii (Crustacea: Cambaridae). Egypt J Aquat Biol Fish 25:355–371. https://doi.org/10.21608/EJABF.2021.200336
Sakr A, Osman G, Abo-Shafey A (1992) Effect of testosterone on the ovotestis of the land snail Theba pisana. Funct Dev Morphol 2:99–101 ([PubMed: 1450465])
Wang C, Croll RP (2004) Effects of sex steroids on gonadal development and gender determination in the sea scallop, Placopecten magellanicus. Aquaculture 238:483–498
Mohamed AZ (2011) Sublethal toxicity of roundup to immunological and molecular aspects of Biomphalaria alexandrina to Schistosoma mansoni infection. Ecotoxicol Environ Saf 74(4):754–760
Saad AH, Varjabedian KG, Abdel-gaber R, Hassan HM, Abdel-halim NT (2013) Immunological and molecular detection of digenetic infections in different species of Egyptian freshwater snails. J Egypt Soc Parasitol 43(1):167–182
Gorbunova V, Seluanova A (2016) DNA double strand break repair aging and the chromatin connection. Mutat Res 788:2–6
Choubisa SL, ZulfiyaSheikh Jaroli VJ (2012) Histopathological effects of larval trematodes on the digestive gland of freshwater snail species, Vivipara bengalensis and Lymnaea acuminate. J Parasit Dis 36(2):283–286
Mohandas A (1977) On two new species of cercariae and the histopathology of the digestive gland of their host Digoniostoma pulchella (Benson). Acta Parasitol Pol 25:17–24
Choubisa SL (2008) Mode of nutrition in pathogenic trematode larvae (redia and cercaria) which infect hepatopancreas of fresh water snails (Mollusca: Gastropoda). J Parasit Dis 32(1):68–73
Erasmus DA (1972) The biology of trematodes. University Press, Oxford
Belfast Frank GH (1963) Some factors affecting the fecundity of Biomphalaria pfeifferi (krauss). Bull World Health Organ 29:531–537
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final draft of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dokmak, HA.A., Hammam, O.A. & Ibrahim, A.M. Impact of Schistosoma sp., Infection on Biological, Feeding, Physiological, Histological, and Genotoxicological Aspects of Biomphalaria alexandrina and Bulinus truncatus Snails. Acta Parasit. 69, 648–663 (2024). https://doi.org/10.1007/s11686-023-00760-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00760-4