Abstract
Wastewater treatment plants (WWTPs) continuously release a complex mixture of municipal, hospital, industrial, and runoff chemicals into the aquatic environment. These contaminants are both legacy contaminants and emerging-concern contaminants, affecting all tissues in a fish body, particularly the liver. The fish liver is the principal detoxifying organ and effects of consistent pollutant exposure can be evident on its cellular and tissue level. The objective of this paper is thus to provide an in-depth analysis of the WWTP contaminants’ impact on the fish liver structure, physiology, and metabolism. The paper also gives an overview of the fish liver biotransformation enzymes, antioxidant enzymes, and non-enzymatic antioxidants, their role in metabolizing xenobiotic compounds and coping with oxidative damage. Emphasis has been placed on highlighting the vulnerability of fish to xenobiotic compounds, and on biomonitoring of exposed fish, generally involving observation of biomarkers in caged or native fish. Furthermore, the paper systematically assesses the most common contaminants with the potential to affect fish liver tissue.
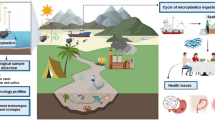
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wastewater treatment plants (WWTP) continuously release a complex mixture of municipal, hospital, industrial, and runoff chemicals into the aquatic environment. These contaminants are of both long-standing and emerging concern (Sauvé and Desrosiers 2014). The WWTP effluents also comprise microorganisms and excess nutrients, along with engineered nanomaterials, microplastic, metals, pharmaceutically active compounds, organic pollutants, endocrine-disrupting chemicals, and a plethora of other compounds (Mehdi et al. 2021; Lapointe et al. 2020; Tran et al. 2018; Neale et al. 2013). We know that many of these contaminants individually affect fish health, but the collective impacts of chemical mixtures and associated bacteria on fish are still unclear (Restivo et al. 2021).
WWTPs have a significant role in the water cycle and pollutant removal through primary, secondary, and advanced treatments since pollutants circulate via effluents, sludge, and air emissions (Ribeiro et al. 2020; Papa et al. 2016). These treatments may alter the state and stability of pollutants. The primary treatment mostly involves flocculation/coagulation and sedimentation. The secondary one comprises biological treatment, aggregation/sedimentation, sludge treatment, and disinfection. The advanced treatment involves membrane filtration and advanced oxidation processes, where applicable (Neale et al. 2013). The contaminants in effluents are therefore found at relatively low concentrations, and they get further diluted in receiving waters (Wigh et al. 2018). In addition, after WWTP treatment, some of these chemicals may be of altered stability. They might interact with household and industrial surfactants (Jarvie et al. 2009), or be adsorbed onto naturally occurring aquatic colloids or particulate matter, for example (Worms et al. 2010; Klaine et al. 2008). Most WWTPs are not equipped with technology to effectively remove contaminants of emerging concern, including many consumer products (e.g., pharmaceuticals and personal care products), which present a risk to water quality, leading to the input of partially treated effluents to surface waters and exposure of aquatic organisms (Franco et al. 2020). Thus, the contaminants might be taken up into the tissues of fish and other aquatic organisms where they can elicit adverse biological effects. In comparison with mollusc and crustacean models, fish have a similar development, anatomy, and physiology to higher vertebrates, which makes them ideal models for monitoring of chemicals in the aquatic environment (Schujit et al. 2021).
The routes of xenobiotic uptake depend on the dietary and ecological circumstances of a particular fish species, as the contaminants may occur in sediments, suspended material, and food sources (James and Kleinow 2018; Livingstone 2001). They also depend on the season of the year, relative to water temperature, and fish reproductive status (Soler et al. 2020). Responding to stressors through exposure to wastewater, fish undergo physiological and biochemical tissue processes in order to compensate for the imposed xenobiotic challenge (Blahova et al. 2014). The consequences of these processes can be observed across multiple levels of biological organization, from metabolic to behavioral, to population and community-level responses (Mehdi et al. 2021; Topić Popović et al. 2015a). Fish may react with acute responses to contaminants, but as latter often occur at low environmental levels, chronic effects are more prominent in contaminated waters (Franco et al. 2020).
The liver of fish and other vertebrates is the principal detoxifying organ and effects of consistent pollutant exposure can be evident on its cellular and tissue level (Minarik et al. 2014). The liver is also the major organ for the storage of heavy metals (Ardeshir et al. 2017). Fish liver biomarkers, conjugation enzymes, carboxylesterase activities, and antioxidant defenses can confirm chemical exposure to lipophilic microcontaminants, from dioxin-like chemicals to drugs of broader nature (Soler et al. 2020). Biomarkers, as biological endpoints, are used for the evaluation of tissues at the cellular, biochemical, and molecular levels. They are important as short-term indicators of long-term biological effects and indicate the presence of contaminants (exposure biomarkers) or the magnitude of the organismal response (effects biomarkers) (Garmendia et al. 2015).
The objective of this paper is thus to provide an in-depth analysis of the WWTP contaminants’ impact on the fish liver structure, physiology, and metabolism. Furthermore, it systematically assesses the most common contaminants with the potential to affect fish liver tissue.
Vulnerability of fish systems to wastewater outfalls and environmental stressors
Fish vulnerability to xenobiotics is not a constant and largely varies between individual organisms, but also within an organism in relation to its genetic makeup, life stage, development, gender, gonadal maturation, diseases, infestations, energy reserves, etc. Vulnerability also resides in the sensitivity and resilience of the system exposed to various hazards (Rudneva et al. 2020; Turner et al. 2003). Besides xenobiotics and pollutants, there is also many environmental stressors, which may affect fish communities: climate change, coastal or riverine development, erosion, alterations of hydrological systems, physicochemical changes of water and sediment, pathogen burden, toxic algae, invasive species, intra- and interspecific competitions, eutrophication, fishing, and aquaculture leading to the altered food web and habitat changes (Couillard et al. 2008). All these factors contribute to the cumulative effects of the vulnerability burden.
WWTP outfalls can alter physicochemical properties of water, depending on the inflowing sources. For example, the sugarplant wastewaters and municipal wastewaters differ in many physicochemical parameters which can dramatically affect fish (liver) health (Topić Popović et al. 2015a). The sugarplant wastewaters mostly surpass the raw municipal wastewaters inflowing the WWTP in values of many parameters, such as suspended solids, chemical oxygen demand (COD), permanganate index (COD-Mn), biochemical oxygen demand (BODn), nitrate, nitrite. Namely, increased nitrogen may result in overgrowth of algae, which can decrease the dissolved oxygen content of the water, thereby harming or killing fish (Topić Popović et al. 2016, 2015a). Besides, continuous exposure to high levels of ammonia can cause oxidative damage to the fish liver, where the degree of liver damage increases with the prolongation of exposure (Liu et al. 2021).
Wastewater outfalls generally enhance the presence of omnivorous, non-native, and tolerant fish of a few species. They may be attracted to the effluent or outfall habitat because of nutrients, organic particulate matter, and increased food availability (McCallum et al. 2019). However, the combination of a constant nutrient supply and temperature of the effluent, particularly in the winter months, might create an ecological trap for such fish, threatening their reproduction and survival (Mehdi et al. 2021). The toxic injury of such fish is based on the duration of exposure and concentration of xenobiotic chemicals at the target site, the number of available target sites, and the recovery capacity of fish (Couillard et al. 2008). The toxicokinetic processes such as uptake, distribution, metabolism, and excretion control the concentration of xenobiotic chemicals at the target sites. On the other hand, the toxicodynamic processes such as the interaction between chemicals and target sites, and repair mechanisms, modify their responses (Couillard et al. 2008; Heinrich-Hirsch et al. 2001).
The most vulnerable period in fish development is the early life stage when they are more sensitive to xenobiotics. In such a critical life stage, fish absorb higher concentrations of xenobiotics, have undeveloped detoxification capacity, and are less capable of avoiding contaminated areas (Andersen et al. 2003; Vosyliene et al. 2003). Several fish species are able to survive in environments with high levels of multiple anthropogenic pollutants such as wastewater outfalls. However, the synergistic impact of complex pollutants in interaction with environmental factors, physical or biological stressors, significantly exerts their effects on fish health. Furthermore, deleterious effects of multiple stressors on fish communities are challenging to detect in native populations, which tend to be under constant or recurring exposure causing a prolonged physiological response during their acclimation (Topić Popović et al. 2016).
Caged or native fish
For evaluation of the impact of WWTP effluents on fish, a variety of strategies is used. Biomonitoring of environmentally exposed fish generally involves observation of biomarkers in caged or native fish. Laboratory studies, on the other hand, often use the extreme exposure concentration of a single xenobiotic or its mixtures (Gagnon and Rawson 2017). Laboratory studies can thus overestimate or sometimes underestimate the effects in wild populations. As the concentrations used in laboratory studies may not relate to their levels in field situations, the observations validated with field studies are generally more reliable (van der Oost et al. 2003).
Environmental exposures are often variable in both duration and magnitude, but in standard acute and chronic effects testing, they are maintained (Hamer et al. 2019). For example, the avoidance response is one form of phenotypic adaptation allowing fish to survive in the altered environment. Such behavior represents the final integrated fish response to xenobiotic stress. The active withdrawal of fish from polluted areas can affect their physiological migration, distribution, and survival patterns (Vosyliene et al. 2003). Interestingly, non-migratory species have higher interspecies variability in contaminant burdens than fish that move extensively (Marcogliese et al. 2015).
The native fish biomarker responses to effluents are affected by a number of complex factors, such as the age of the individual fish, length/weight ratio, sex, migration pattern, adaptation mechanisms to chronic pollution, and presence of sentinel or endangered species (Catteau et al. 2021). The variations in biological and physicochemical parameters may influence the endpoint measurement unless applying multiple collection times over seasons (Gagnon and Rawson 2017). In addition, fluctuations in the food supply related to season, spawning, and migration affect food abundance or deprivation, and the feeding rates of native fish. Therefore, native fish with insufficient energy stores could have adverse reactions to acute or chronic effluent exposures (Weinrauch et al. 2021). Another problem of the field surveys is extrapolating from observations on a few individuals in a limited number of species to groupings of many individuals and species and the need to consider how the relative sensitivity of their individual responses reflect the response of populations, communities, and ecosystems (Galloway et al. 2004).
By choosing to use the caged fish for biomonitoring studies, individual fish are selected according to their particular characteristics such as sex, age, or size. No fishing effort allocated to catching the same species in the different sampling sites is needed. Fish mobility is avoided which removes a source of variability in the field study. The use of fish from a hatchery avoids the possibility of sampling pollution-adapted fish (Kosmala et al. 1998). Variability of the responses can be reduced by controlling the distance of the cage from the effluent source, the position of the cage, season, or caging duration (Catteau et al. 2021). Therefore, the use of cages offers precise information about their location and exposure duration, gives control of the selection of representative species and their particular developmental stage and genetic background, yielding comparable results over various sites (Cazenave et al. 2014). The most comprehensive evaluation of sublethal toxicity of contaminants is achieved when studies are performed on fish in all stages of development (Marcogliese et al. 2015), which advocates the use of cages. However, caged fish may suffer from density stress, which could increase cortisol levels and potentiate induction of hepatic enzyme activity (Kosmala et al. 1998). In any case, selecting the caged or native fish, the utilization of a battery of complementary biomarkers is recommended for the accurate estimation of fish responses during effluent exposure.
Fish liver, oxidative stress, and biomarkers
The fish liver is microanatomically similar to livers of other vertebrates. It is a reticulotubular gland, often with lobular organization, covered with a serous membrane. Its hepatocytes are polygonal cells with round nuclei and a single, prominent nucleolus, often containing lipid and glycogen (Stoskopf 1993). Hepatocytes are in contact with blood perfusing the hepatic parenchyma at their sinusoidal membranes, at which blood-borne chemicals can be taken up (Luckenbach et al. 2014). The liver is a key metabolic organ for the storage and mobilization of energy, with several anabolic and catabolic functions (Bernet et al. 2000). It converts glucose into glycogen and lipids, which provide energy during fasting, but it also produces and secretes glucose through glycogenolysis and gluconeogenesis. The liver converts fatty acids for extrahepatic tissues during fasting. Multiple nutrient, hormonal, and neuronal signals regulate glucose, lipid, and amino acid metabolism in the liver (Rui 2014). Since it has a key role in digestion, storage, and protein production, it is a target tissue for lesions related to wastewater-related contaminants. Upon exposure to contaminants, a metabolic trade-off occurs as hepatic nutrient metabolism is altered (Weinrauch et al. 2021). Energy allocation is disturbed since large proportion of the ingested energy shifts from maintenance to detoxification and repair mechanisms. These processes result in rapid decrease of energy storage such as ATP, phosphocreatine and glycogen, and rapid accumulation of blood (Pi et al. 2016).
Upon exposure, increased levels of oxidative damage occur, which stimulate the production of reactive oxygen species (ROS) and other pro-oxidants (Hu et al. 2021). The ROS generated by liver metabolism are eliminated by activation of a variety of antioxidant defense mechanisms, both direct and indirect. They include redox cycling, redox reactions, autoxidation, oxidative and non-oxidative enzyme induction, disruption of electron transport, depletion of antioxidant defenses (Maria et al. 2009; Livingstone 2001). In addition, when the antioxidant defense system cannot efficiently counteract the ROS, it triggers oxidative stress leading to lipid peroxidation and DNA damage, micronuclei, chromosome aberrations, and sister chromatid exchanges in hepatocytes and other cells (Ardeshir et al. 2017; Livingstone 2001). In short, oxygen toxicity involves the production of superoxide anion radical, hydrogen peroxide, and the hydroxyl radical, leading to enzyme inactivation, protein oxidation, lipid peroxidation (formation of malonaldehyde-like species and 4-hydroxyalkenals), DNA damage, and even cell death (Jia et al. 2021; Winston and Di Giulio 1991). Therefore, under pollution stress, fish liver antioxidant enzymes and non-enzymatic antioxidants may be altered. Their increased activity may be considered as an adaptation in which the organism partially or completely overcame the exposure stress. In addition, the inactivation of the defense system by the chemically reactive species, which act as antioxidant enzyme inhibitors reduce cell protection and the organism’s fitness (Maria et al. 2009). Conversely, in an undisturbed environment, a balance exists between pro-oxidant and antioxidant processes, as the production of ROS and other reactive species is controlled by the antioxidant defense system.
Since the liver is involved in the metabolism of xenobiotic compounds and their excretion (Bernet et al. 2000), a number of hepatic biochemical, physiological, and metabolic biomarkers were developed. They include biomarkers of exposure (biochemical response occurring following exposure to a contaminant), biomarkers of effect (measurable biochemical, physiological or other alterations within hepatic tissue or body fluids that can be associated with health impairment), biomarkers of susceptibility (ability to respond to exposure to a contaminant, including genetic factors and changes in receptors which alter the susceptibility of fish to the exposure), and bioaccumulation markers (analytical/chemical indicators, also referred to as body burden) (Kroon et al. 2017; Hook et al. 2014; van der Oost et al. 2003) (Fig. 1).
It is a challenge to choose a suitable biomarker between a plethora of biomarkers for assessing fish liver impairment. To that end, selection criteria must be applied to refine the extensive biomarker list. Namely, biomarkers of exposure typically comprise biotransformation enzymes of phase I, phase II, and co-factors (Weinrauch et al. 2021; Ogueji et al. 2020; Kroon et al. 2017; Gagnon and Rawson 2017). Oxidative stress parameters include a group of antioxidant enzymes forming a part of a cellular defence system such as superoxide dismutase, catalase, glutathione peroxidase, and glutathione (Faheem and Lone 2018). Biochemical biomarkers of oxidative damage are also frequently used to assess the consequences of oxidative stress, such as lipid peroxidation quantifiable by the measurement of degradation products such as aldehydes, acetone, and malondialdehyde. Additionally, relevant biomarkers related to fish liver function are stress proteins or heat shock proteins which protect and regenerate cells in response to stress. Furthermore, multixenobiotic resistance prevents the accumulation of xenobiotic compounds inside the cell, by removing them via an energy-dependent transport protein (Wang et al. 2022; Kroon et al. 2017; Smital and Ahel 2014; Žaja et al. 2007). Hematological parameters, like serum transaminases, provide general insights of the hepatic tissue health (Wang et al. 2022; Fathy et al. 2019; Cai et al. 2018; Kroon et al. 2017). Endocrine parameters, notably vitellogenin, genotoxic parameters such as DNA adducts, secondary DNA modifications, and irreversible genotoxic events, apoptosis, and micronuclei are also valuable biomarkers (Haque et al. 2022; Laurent et al. 2022; Simó-Mirabet et al. 2018; Casatta et al. 2017). Liver structural alterations, as histopathological biomarkers of effect, are important non-specific indicators of fish health as they bear the effects of exposure to various pollutants (Hinton et al. 1992). Acute alterations are apparent at high contaminant levels, while chronic exposure might induce sublethal aspects of tissue alterations. Many of such alterations are irreversible and can be useful in the determination of prior exposure to wastewater contaminants (Hinton 1994). A list of major biochemical, physiological, and metabolic biomarkers in fish liver reflecting exposure to, or effects of pollutants, is presented in Table 1.
Macroscopic morphological liver changes
Morphological indices are often indicative of pollutant exposure. Liver somatic index (LSI), a ratio between the liver mass and the total fish mass, is one of such non-specific indices (Sloof et al. 1983). The exposure to pollutants, such as bleached sulfite mill effluent, may affect the increase of fish LSI by two to three times in relation to the LSI of control fish. Life cycle and long-term fish exposure result in increased body size and increased liver size (Parrott et al. 2007). Fish exposed to WWTP effluent discharges, heavy metals, pulp mill discharges, urbanized areas, had enlarged livers, and increased LSI (Long et al. 2020; Wang et al. 2019; Bahamonde et al. 2015; Batchelar et al. 2013; Kosmala et al. 1998; Munkittrick et al. 1994). Du et al. (2019) demonstrated that the exposure of fish to a municipal WWTP induced liver enlargement for up to 35% in both native and caged fish. The physiological compensations in the liver offset some of the detrimental impacts in fish after exposure, particularly the increase of the cumulative mitochondrial oxidative capacity of the liver.
Larger liver mass can also be affected by reproductive status since the liver plays a central role in the process of vitellogenesis (Long et al. 2020). However, since vitellogenesis does not usually occur in males, LSI in male fish can be more informative of contaminant exposure (Duarte et al. 2017). The enlarged livers of fish from contaminated waters can stem from hypertrophy (increase in cell size) (Sloof et al. 1983), and hyperplasia (increase in cell number) (van der Oost et al. 2003). They are usually associated with the induction or stimulation of hepatic enzyme activities (Kosmala et al. 1998). Larger liver mass relative to body mass, caused by the increased metabolic activity to detoxify contaminants, may thus be indicative of exposure to contamination, resulting in liver damage that ranges from increased liver weight and fat content to cell necrosis (Samanta et al. 2018). Fatty liver, or storage of large quantities of fats in the fish liver, is a morphological tissue change visible macroscopically as mottling or whitening of its surface. Lipid accumulation results from the imbalance between the synthesis of fatty acids or lipogenesis and fat catabolism or lipolysis in the liver tissue (Wang et al. 2019). Another macroscopic pathology of the liver is the formation of neoplasms. Neoplastic growths in the liver are a common finding in chronically exposed fish. Tumors might appear as small nodules up to lumps of significant size, having a different coloration than the rest of the liver tissue (Peters et al. 1987). Generally, larger/older fish are at higher risk to be affected by liver tumors (Lang et al. 2017).
Physiological and histological changes of livers from exposed fish
A healthy fish liver has a very homogeneous structure in contrast to its heterogeneous physiology. It consists mostly of parenchymal tissue formed from double layers of liver cells, separated by liver sinusoids or capillary-like blood spaces. Liver stores carbohydrates as glycogen and, especially before spawning, fats. The polygonal hepatocytes under normal conditions appear compact. Near the sinusoids, uniformly distributed groups of pigmented phagocytic cells or melanomacrophages can be found (Peters et al. 1987).
Aberrations from the normal structural tissue integrity can provide valuable information on the integrated effects of molecular, biochemical, and physiological changes deriving from exposure to pollutants. Liver physiological and histological parameters represent higher-level responses of exposed fish, generally indicative of irreversible damage (Lang et al. 2017; Oliveira et al. 2015). Histological/histopathological parameters of the fish liver are very useful for assessing the chronic and sublethal effects of persistent contaminants present at very low levels in waters affected by the effluents. Such responses can be used as biomarkers of exposure and effects to contaminants related to the health of individuals which also allow further extrapolation to population/community effects (Chiang and Au 2013). Hepatic lesions of fish exposed to contaminants, sorted by histopathological categories, are listed in Table 2.
The most common histopathological findings in livers of fish exposed to pollutants are macrophage aggregates, also known as melanomacrophage centers, and liver necrosis (Oliveira et al. 2015). Necrosis is the direct toxic effect of a pollutant and could be due to the infiltration of leucocytes (Javed and Usmani 2013). In liver disease associated with hepatic necrosis, serum AST and ALT are elevated even before the clinical signs and symptoms of disease appear (van der Oost et al. 2003). Melanomacrophages can serve as non-specific indicators of environmental stress as they are related to exposure to degraded environments. They usually contain a number of pigments including melanin, with the primary function of iron capture and storage. Melanin is a complex polymer that can absorb and neutralize free radicals, cations, and other potentially toxic agents, deriving from degradation of phagocytosed cellular material (Zuasti et al. 1989). Other pigments usually found in melanomacrophages are lipofuscin (generally the most abundant), and hemosiderin (Agius and Roberts 2003). The increase in melanomacrophage numbers indicates humoral and inflammatory responses, and also detoxification of exogenous and endogenous substances (Herraez and Zapata 1991; Peters et al. 1987). The liver infiltration of defence cells is caused by hyperemia, leading to increased blood flow in the liver, and facilitating the transport of macrophages to the damaged regions, thus improving oxygenation and possibly indicating an auxiliary mechanism in detoxification (Vieira et al. 2019).
Energetic costs linked to exposure to contamination mainly refer to glycogen depletion, which is a stress-induced response. The decrease of glycogen content under effluent stress is greater in liver than in muscle, as liver is the principal site of its synthesis and storage (Cazenave et al. 2014). Lower liver glycogen can be associated with the inability to increase plasma glucose following an acute stress exposure (Javed and Usmani 2013, 2015). Such depletion of energy reserves may have consequences in form of diminished growth, survival and reproduction probability (Du et al. 2019; Sancho et al. 2010).
Biotransformation enzymes and xenobiotic compounds
Most xenobiotic compounds undergo biotransformation before being excreted. The most commonly involved organ in (fish) biotransformation is the liver. In the first phase of biotransformation, the lipophilic substrate is oxidized by the cytochrome P-450 (CYP450) enzyme system, which introduces a single oxygen atom into the molecule (Moutou et al. 1998). In the second phase, the oxidized compound is conjugated with an endogenous molecule such as glucuronic acid, sulfate, glutathione, or amino acid, becoming less toxic and readily excreted (Topić Popović et al. 2012; Andersson and Förlin 1992).
The P-450 (CYP) oxidase enzymes metabolize many xenobiotics, and CYP1A1 and CYP3A4 are important markers of chemical exposure in fish (Table 1). Disruptions of the activities of these enzymes, especially CYP3A4, may have consequences in the metabolism of other compounds. The CYP3A4 is one of the most important enzymes involved in the metabolism of xenobiotics and oxidation of the largest range of substrates (Topić Popović et al. 2007). The CYP1A is best known as a major hydrocarbon-inducible CYP. Members of the CYP1 family play a prominent role in the activation of many drugs and procarcinogens (Topić Popović et al. 2015a). Many studies demonstrated an increase in the CYP1A levels in liver tissues of fish exposed to organic trace pollutants, and particularly to PAHs, PCBs, PCDDs, and PCDFs (Table 1). Single xenobiotic compounds can act as inducers of specific isoenzymes, but also inhibit others, which may result in variation of isoenzyme levels (Oost et al. 2003). However, measuring these proteins or their mRNA levels can be time-consuming and costly for field studies, so the determination of their catalytic activity is more common. Thus, measurement of the CYP1A-catalyzed deethylation of ethoxyresorufin to a fluorescent product resorufin via the activity of EROD, or using benzo(a)pyrene as a substrate in AHH assays, or measuring CYP3A-catalyzed activity of BFCOD (7-benzyloxy-4-[trifluoromethyl]-coumarin-O-debenzyloxylase), allow for their more feasible quantification (Gagnon and Rawson 2017). For example, the increase of EROD, AHH, and BFCOD activity in livers of native fish sampled downstream from the WWTP indicate exposure to dioxin-like compounds, organic trace pollutants, and drugs of broader nature related to WWTPs (Burkina et al. 2021; Catteau et al. 2021; Weinrauch et al. 2021; Soler et al. 2020; Kosmala et al. 1998).
In the second phase of biotransformation, the conjugation with glutathione (realized by the glutathione-S-transferase) is the major pathway for electrophilic compounds and metabolites, while the conjugation with glucuronic acid is the major route for nucleophilic compounds (George 1994). The mechanism of induction for most of the phase II enzymes is also regulated via the Ah-receptor, but their induction responses are generally less prominent (van der Oost et al. 2003). In healthy liver tissues, more than 90% of the total glutathione pool is in the reduced form and less than 10% is in the oxidized form. An increased oxidized-to-reduced ratio is indicative of oxidative stress, resulting from the conversion of glutathione from reduced to oxidized form as radicals with oxidative potential are neutralized. These rations were significantly elevated in livers of fish exposed to WWTP outfalls, showing evidence of oxidative stress (Jasinska et al. 2015). Also, in fish exposed to environmental concentrations of diclofenac, the levels of reduced glutathione and glutathione S-transferase activity increased at all tested concentrations (Guiloski et al. 2017).
Predictors of adverse responses
In the past decades, high‐throughput screening assays and omics-based methods have been developed for studies of the mechanisms of toxicity. Assays were developed for short-term high-content and high-throughput prediction of toxicity of numerous chemicals potentially found in the WWTP outfalls. Responses at the molecular level, transcriptome sequencing, proteomics, and metabolomics, may provide reliable biomarkers of exposure and stress induced by pollutants, as well as their underlying mechanisms (Bai and Tang 2020). In particular, biochemical changes revealed by metabolomics are easily correlated to other measurements and can set a path for unveiling the mechanisms of toxicity of various compounds (Huang et al. 2019). Exposure of fish to pollutants leads to changes in gene expression and protein production, which are subjected to a number of homeostatic and feedback mechanisms. These changes are amplified at the metabolome level, which makes metabolomics analyses a significant tool for assessing toxicity of environmental contaminants (Lankadurai et al. 2013). Such data aid to identify multiple impaired pathways contributing to the overall adverse responses. Fish from the two effluent-receiving sites in the work of Meador et al. (2020) exhibited alteration in 31 different pathways, related to metabolism and biosynthesis of amino acids, lipids, fatty acids, purines, pyrimidines, and sugars. Many of these are important for energy production, antioxidant defenses and ammonia cycling (Meador et al. 2020).
Since molecular markers precede morphological alterations, they are useful for a predictive aspect of toxicity testing. Many of the genes measured in such studies are functional orthologs of human ones involved in processes of toxic responses and thus have an additional applied value (Gonçalves et al. 2020a). Namely, a higher expression of gpx, hsp70, ucp2, and bax genes, as well as a lower expression of Bcl-2, mRNA levels of SOD and CAT genes in fish can indicate to toxic effects of silver oxide and silver carbonate nanoparticles (NP) before other biomarkers, and indicate to a NP hazard (Mahjoubian et al. 2021). The balance between the oxidative and antioxidant processes inside the cells is related to several genes such as coxIV, prdx, ucp, sod, cat, and hsp70 (Espinosa et al. 2017). The increase of the fish liver ucp1 and decrease of hsp90 expression can thus be correlated to microplastics exposure (Espinosa et al. 2017). Microarray studies established that the exposure to the WWTP effluent can significantly affect gene transcription in the fish liver, having altered expression in relation to the unaffected site. These genes are mostly involved in mechanisms of the fish immune system, lipid and retinol metabolism, detoxification processes, cellular proliferation, and membrane transport (Houde et al. 2014). Incorporation of gene expression tools enables characterization of responses even when the stressors are unknown, but calls for validation with a phenotypic trait on an organ or a tissue level (Hook et al. 2014).
There is a number of molecular effects which need to be extensively studied to investigate species-specific molecular responses, response levels and typical inducers. The detrimental effects are often multifactorial and associated with alterations in different pathways or subnetworks (Gonçalves et al. 2020b). Advances in high-content –omics screening and bioinformatics enable surveying of multiple molecular endpoints in single runs and revealing alterations in metabolic pathways in complex ecosystems affected by pollution (Cuevas et al. 2018).
WWTP-associated legacy contaminants and emerging-concern contaminants with affinity to fish liver
Pharmaceutically active compounds
Pharmaceutically active compounds (PhACs) are biologically active compounds designed to interact with specific physiological pathways of humans and livestock in order to evoke a desirable pharmacological response (Corcoran et al. 2010). In recent decades, PhACs have aroused an increasing concern due to their ubiquitous presence in the aquatic environment and negative ecological effects. Currently, over 4000 PhACs are being used all over the world for medical and veterinary health care, as well as growth promotion of livestock (Boxall et al. 2012). Excretion of PhACs occurs via urine and feces, with 50–90% of oral doses generally being excreted as a mixture of the parent compounds and their metabolite forms (Köpping et al. 2020; Celiz et al. 2009). PhACs enter the aquatic environment via numerous routes, including direct discharge of raw and/or treated wastewater from municipal, hospital, industrial WWTPs, where they are often inefficiently removed. Secondary PhACs sources should not be neglected, such as landfill leachate and surface runoff from urban or agricultural areas where treated wastewater is used for irrigation activities and/or sewage sludge as a fertilizer (Tran et al. 2018).
PhACs are designed to be stable, thus the slow degradation rate is usually exceeded by continuous release rate (OECD 2019). For such reason, PhACs are being frequently reported in freshwaters and groundwaters all over the world in concentrations ranging from ng/L to µg/L (Tran et al. 2018; Bielen et al. 2017; Corcoran et al. 2010), and even mg/L (Larsson 2014). Although the occurrence of PhACs and their concentrations in aquatic ecosystems depend on the socioeconomic composition of the population, they are strongly seasonally dependent and linked with increased consumption during seasonal influenza and allergies, but also farming practices (Vieno et al. 2005). Over the past two decades, ecotoxicity studies have revealed a strong positive correlation between the most commonly used classes of PhACs and their occurrence in the environment (Küster and Adler 2014; Corcoran et al. 2010). Regarding human medicinal products, antibiotics, hormones, analgesics, antidepressants, and antineoplastics are the most frequently used/found in the aquatic environment, while among veterinary products, those are hormones, antibiotics, and parasiticides (Corcoran et al. 2010). Although the majority of published prioritization approaches already indicated high environmental relevance of these compounds, in aquatic environments they occur as a mixture of various compounds whose combined toxicity does not necessarily correspond to one of the individual substances due to synergistic, antagonistic, and/or additive interactions (do Amaral et al. 2019; Corcoran et al. 2010).
Although PhACs uptake into fish may vary, i.e., through the gills, dermal absorption from the surrounding water, and dietary intake of food, tissue-specific accumulation can be observed. PhACs are generally nonpolar and lipophilic, so their accumulation mainly occurs in the organs with a high lipid content, i.e., liver, brain, and muscle (Schnell et al. 2009). The liver is the major target and storage of PhACs from distinct therapeutic groups, hence numerous studies reported higher BCFs of such compounds in the liver than in the remaining organs/tissues (Gomez et al. 2010). Supporting this assumption, many authors reported the accumulation of anti-depressants and their metabolites in the liver of the fish inhabiting effluent-dominated streams/rivers in concentrations ranging from 9.1 ng/g of tissue (for norfluoxetine, the primary active metabolite of the antidepressant fluoxetine), 12.94 ng/g (for desmethylsertraline, an active metabolite of the antidepressant sertraline), 80 ng/g (for fluoxetine), up to 545 ng/g (for sertraline) (Ondarza et al. 2019; Ramirez et al. 2009; Brooks et al. 2005). An extensive study by Huerta et al. (2013) focused on evaluating twenty PhACs from seven commonly used therapeutic families and their metabolites in the liver and muscle of eleven fish species from four heavily impacted Mediterranean rivers. The authors claimed the highest levels in trout liver, with a maximum concentration of 18 ng/g for non-steroidal drug carbamazepine, whereas the most ubiquitous and recurring compound was analgesics/anti-inflammatory drug diclofenac. Antibiotics, one of the most concerning PhACs, are not an exception. Recently, Baesu et al. (2021) detected lincomycin, sulfamethoxazole, and azithromycin in fish livers at an average concentration of 30.3, 25.6, and 27.8 ng/g fw, respectively.
Although several analytical methods for the determination of PhACs in biological matrices have been developed in the last decade, there is still a need for improvement toward the higher sensitivity of used instruments and accuracy of analytical methods, particularly for liver samples that are loaded with lipids and proteins that may interfere with analysis (Tanoue et al. 2014). The importance of such studies does not reflect only in the determination of bioaccumulated PhACs in the fish tissue, but indirectly enables an effective screening of emerging contaminants in aqueous ecosystems and determination of wastewater treatment efficiency, which could ultimately result in establishing thresholds of regulation. Erythromycin, a common over-the-counter antibiotic, was due to its ubiquity in the aquatic organisms (up to 5.6 µg/kg in the fish liver; Ondarza et al. 2019) included in the Drinking Water Contaminant Candidate List (CCL3, US-EPA) and was identified as a “priority monitoring substance” by the European Union (Barbosa et al. 2016).
Receptors and signaling pathways are relatively conserved across vertebrate phyla, so it can be concluded that some PhACs purposely designed to induce an effect on humans or livestock to have a high probability to produce a similar or same physiological response in fish (Burkina et al. 2015; Corcoran et al. 2010). Moreover, hepatocytes among vertebrates are responsible for the majority of identical functions, i.e., metabolism of a plethora of endogenous and exogenous compounds, synthesis of blood protein and clotting factors, secretion of bile, storage of glycogen, aminoacids, fat, and iron (Field et al. 2003). Hence, by paralleling PhACs mode of action among fish and humans, this review article further supports the undoubtful value of the pinpointed topic. Moreover, such an approach can be utilized to better interpret the mode of action of a single PhAC and its mixtures.
Due to the growing regulatory restrictions of conducting in vivo studies on vertebrates, the impact of PhAC is frequently assessed in vitro using fish liver cell lines, such as RTL-W1, PLHC-1. Thibaut et al. (2006) investigated the interactions of fibrate, anti-inflammatory, and anti-depressive drugs with CYP catalyzed pathways (CYP1A, CYP3A-, CYP2K-, and CYP2M-like) and Phase II activities (UDP-glucuronosyltransferases and sulfotransferases) involved in both xenobiotic and endogenous metabolism in fish. Gemfibrozil, diclofenac, and three anti-depressive drugs (fluoxetine, fluvoxamine, paroxetine) had the highest potential to interfere with fish metabolic systems. A few years later, Schnell et al. (2009) reported toxicity of eleven pharmaceuticals from different therapeutic classes (anti-inflammatory drugs, serotonin re-uptake inhibitors, and lipid regulators) individually and as a mixture on the rainbow trout liver cell line RTL-W1. In accordance with Thibaut et al. (2006), anti-depressives (fluoxetine and paroxetine, 7–50 µM) showed the highest potential to induce toxicity in RTL-W1 cells. Within a study, the combined toxicity of tested mixtures was multiplied, mostly following the concentration addition concept (Schnell et al. 2009). Additional PhACs group of particular concern are antifungals, such as clotrimazole, ketoconazole, miconazole. It is well-established that compounds containing an imidazole ring system frequently interact with cytochrome P450 enzymes to inhibit drug oxidation. Burkina et al. (2015) emphasized the ability of mentioned antifungal agents to affect the endocrine system, induce a cascade of molecular and cellular events, via stimulation/inhibition of CYP450.
Although in vitro cytotoxicity assays with fish cell lines have multiple advantages, due to insufficient and heterogenous data a correlation between the results obtained with such in vitro studies and in vivo toxicity of pharmaceuticals still needs to be assessed and validated. For this reason, recent studies shift focus from in vitro studies on cell lines to PhACs exposure-directed studies that link to specific biological effects. With the conducted toxico-pathological and stereological study, Madureira et al. (2012) gave a comprehensive overview of the effects caused by non-steroidal pharmaceutical compounds in the zebrafish Danio rerio liver. The mixture of environmentally relevant concentrations of non-steroidal pharmaceuticals (carbamazepine, fenofibrate, propranolol hydrochloride, sulfamethoxazole, and trimethoprim) shown qualitative and quantitative changes in the selected liver cytohistological parameters. The volume-weighted nuclear volume of zebrafish hepatocytes increased upon exposure, which together with the greater cytoplasmic eosinophilia and changes in cytochrome P450 1A immunoreactivity, pointed to an increase in metabolic/detoxification activity. Tested compounds interfered also with the liver mass and normal liver histology, induced liver VTG expression, and altered CYP1A expression, even at low environmental levels (Madureira et al. 2012). Recently, Rojo et al. (2021) reported the ability of carbamazepine, atenolol, enalapril, and sildenafil present in waters influenced by the sewage discharge to increase the EROD, BROD, and GST activity in fish Pimelodus maculatus. The authors showed a positive correlation between the distribution of PhACs in a water body and the higher activity of hepatic enzymes.
While numerous studies employ some variant of comparative methodology, generally they are difficult to compare due to different PhACs class-tested, differences in sensitivity of the test organism, exposure times, concentrations, and also selected endpoints. As a result, currently, it is difficult to elaborate a more detailed and focused discussion toward a mode of action of a single PhACs and their mixture and to prioritize PhACs likely to pose a negative impact on fish liver biotransformation and antioxidant enzymes, as well as non-enzymatic antioxidants. For this reason, further studies on establishing state‐of‐the‐art standardized methods of measurement that may result in a long-term recommendation regarding the presence of PhACs in the environment are encouraged. Moreover, further investigation of the complex interactions between receptors, transcription factors, and other proteins in the regulation of expression and activity of fish CYP 450 isoforms is needed.
Personal care products
Personal care products (PCPs) are an extraordinarily diverse group of chemicals that comprises compounds used in cosmetics, skin and hair products, sunscreens, toothpaste, soaps, etc. The primary classes of PCPs include disinfectants, fragrances, UV filters, insect repellants, preservatives (Pemberthy et al. 2020; Hopkins and Blaney 2016). Contrary to PhACs, PCPs are intended for external use on the human body, thereby bypassing metabolic alterations (Brausch and Rand 2011). Therefore, large quantities of unaltered PCPs are introduced into the environment either directly via wash-off from the skin, or indirectly through (un)treated effluents (Pemberthy et al. 2020). Just as PhACs, conventional WWTPs are not able to completely remove PCPs, which has led to their detection in wastewaters and effluent receiving systems at concentrations from ng/L to µg/L (Roberts et al. 2016; Buchberger 2011). Despite its short environmental half-life, continuous loading of these pollutants into aquatic environments, even at low parts-per-trillion/parts-per-billion concentrations, causes its pseudo-persistence (Mottaleb et al. 2015; Ramirez et al. 2009). Thus, the exponential introduction of new PCPs to the marketplace ultimately results in perpetual life-cycle exposures of aquatic organisms. Accompanied by a currently unknown toxicological mechanism of action, the negative impact of PCPs can undergo undetected until cumulative effects finally result in an irreversible change in non-target aquatic organisms (Schnell et al. 2009).
The use of antimicrobials in PCPs has increased over the last decade, reaching its maximum production/use during the coronavirus disease 2019 (COVID-19) pandemic. Accordingly, the concentration of antimicrobials in the aquatic environment has nowadays become an emerging global concern (Chen et al. 2021). Triclosan is ranked among the ten most commonly detected PCPs in all environmental compartments. This is not surprising since it is also one of the most commonly used antimicrobials worldwide implemented in soaps, toothpaste, deodorants, and hand sanitizers (Overturf et al. 2015). Its concentration in aquatic environments ranges from 0.01 ng/L up to 27 µg/L (Overturf et al. 2015; James et al. 2012). Triclosan, like most antimicrobials, is lipophilic and has demonstrated the ability to bioaccumulate through the food chain. This was supported by Mottaleb et al. (2009) that reported the presence of triclosan (up to 31 ng/g) in tissues of fish sampled from the effluent-dominated stream in Texas. Due to the lipophilic and non-ionic properties of triclosan, systemic absorption and retention are influenced by biotransformation in fish liver and intestine. Triclosan has a phenolic hydroxyl group which is likely to be a substrate for glucuronidation and sulfonation (James et al. 2012). A concerning effect of triclosan on zebrafish was recently shown by Gyimah et al. (2020). Authors reported that sublethal concentrations of triclosan upon subchronic exposure significantly decreased SOD, CAT, GPx, GSH, and GSSH in the liver of adult zebrafish, and simultaneously caused dose-dependent DNA damage in hepatocytes. Following triclosan exposure, Hemalatha et al. (2019) noticed significantly enhanced glutamic oxaloacetic transaminases (GOT), glutamic pyruvic transaminases (GPT), and GST activity in the liver of Indian major carp Catla catla in a dose- and time-dependent manner. Except for triclosan, there are other antimicrobials often found in PCPs (triclocarban, biphenylol, chlorophene, bromophene, etc.; Overturf et al. 2015), despite currently scarce ecotoxicological data.
Fragrances are one of the most widely studied classes of PCPs used to scent various products including deodorants, soaps, detergents, lotions, and perfumed household cleaners (Brausch and Rand 2011). The most commonly used fragrances are synthetic musks, here sorted by frequency of usage—polycyclic (celestolide, galaxolide, toxalide), nitro (musk xylene, musk ketone), and macrocyclic musks (Brausch and Rand 2011). After the application, a major part of the musk compounds is carried by municipal wastewaters, so their high concentration in untreated wastewaters is not a surprise. Due to the worldwide production and usage, they are ubiquitously distributed reaching up to 410 ng/L (for nitro musks) and 10 µg/L (for polycyclic musks) in effluent-dominated water bodies (Brausch and Rand 2011). The high log Kow values (4.0–4.4 for nitro musks and 5.4–5.9 for polycyclic musks) indicate their potential to bioaccumulate in aquatic species, for example up to 10,000 μg/kg fat in freshwater fish (Hopkins and Blaney 2016; Yamauchi et al. 2008). Following the exposure of medaka Oryzias latipes to synthetic polycyclic musks, expression analysis of hepatic VTG protein showed potential estrogenic effects indicative of the induction of VTG synthesis. Furthermore, tonaline and galaxolide induced the expression levels of hepatic ERα and VTG mRNA/protein and modulated expression levels of CYP3A40 mRNA in the livers of male medaka (Yamauchi et al. 2008). Fish consumption could lead to ingestion of musk substances in humans, causing currently unknown consequences. Even though acute oral and dermal toxicity for humans is low, results obtained with laboratory organisms showed concerning negative impacts, including some hints for the carcinogenic potential (Mottaleb et al. 2012).
Growing awareness of the harmfulness of UV radiation led to the implementation of chemical UV filters in a wide range of PCPs, including sunscreens, creams, lotions, and other cosmetic products. Due to the type of application, these compounds enter surface waters directly via washing-off from the skin during recreational activities, or indirectly via wastewater effluents (Overturf et al. 2015; Brausch and Rand 2011). Their occurrence in aquatic environments is ubiquitous, with the highest concentrations found during the summer months (up to 2.7 μg/L). At present, there are 27 UV filters allowed in cosmetics in the European Union, including the most commonly used benzophenones, p-aminobenzoic acid and derivatives, salicylates, and cinnamates (Buchberger 2011). A comprehensive study by O’Malley et al. (2020) reported the presence of UV filters in wastewater effluent samples from 33 WWTPs across Australia. Of the analyzed effluent samples, 95% contained at least one of the target compounds, with the summed concentrations of UV filters ranging from 130 to 8400 ng/L. Based on the removal efficiencies data, the authors estimated that approximately 40% of the total UV filter influent load remains in the effluent, resulting in a total of 20 kg per day of UV filters released into the aquatic environment (mainly 2-phenylbenzimidazole-5sulfonic acid (PBSA) and benzophenone 4 (BP4)). It has been documented that UV filters accumulate in the food chain, therefore posing a potential risk to the reproduction of fish species (Overturf et al. 2015). UV filters such as PBSA, BP4, and 4-aminobenzoic acid (PABA) increased the activity of certain P450 cytochromes and induce severe oxidative stress in the liver of Oncorhynchus mykiss and Danio rerio (Huang et al. 2020; Grabicova et al. 2013).
Although PCPs are positioned on the list of most commonly detected compounds in surface water throughout the world, compared to PhACs, environmental concentrations and toxicity of PCPs have been largely overlooked. One of the most concerning facts is the PCPs’ potential to bioaccumulate and consequently biomagnify at higher trophic levels. Further, most of the studies have focused on testing the toxicity of a single compound, even though PCPs are present in the aquatic environments in very complex mixtures which can produce synergistic or antagonistic effects.
Even though PCPs are being continuously released into the aquatic environments, most studies conducted to date focused on acute and sub-acute toxicity determination. Although valuable, only chronic mixture studies accompanied with multigenerational approaches could accurately identify the potential risk of PCPs. Undoubtedly, biomonitoring of PCPs in environmental compartments and further research focused on toxicity and mode of action are of considerable interest to the scientific community.
Heavy metals
Discharge of industrial, municipal, and agricultural wastewaters and sewage into the aquatic and marine environment is nowadays strictly regulated in order to decrease the levels of pollutants such as heavy metals in discharge effluents (Frémion et al. 2016; Karvelas et al. 2003). WWTPs can remove high amounts of heavy metals using different treatment processes, including physicochemical methods, chemical precipitation, coagulation, flocculation, electrochemical treatments, etc. (Gunatilake 2015; Agoro et al. 2020). Although some heavy metals are essential for fish growth, such as copper (Cu), nickel (Ni), iron (Fe), and zinc (Zn), they can be toxic if their concentration exceeds an optimal limit. Contrarily, nonessential metals with no biological role such as lead (Pb), mercury (Hg), and cadmium (Cd) are toxic even at low concentrations while the most toxic environmentally relevant heavy metals and metalloids include chromium (Cr), arsenic (As), and manganese (Mn) (Ali et al. 2019; Barakat 2011). Additionally, the emerging problem of plastic in the environment also contributes to the accumulation of toxic metals that latch onto plastic surfaces and enter the marine environment and food chains (Rochman et al. 2014).
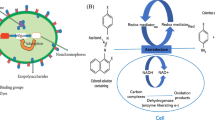
Trace metals are chemically highly reactive so their toxicity in fish is due to the catalytic activities of metalloenzymes (Fig. 2). The toxicity of metals may also be indirect, by binding to other cellular components, which is particularly important for metal ions that show similar chemical behavior, such as Ag+, Cu+, Hg+, Cd2+, and Zn2+ ions (Rainbow 1990). Biota can accumulate various chemicals, and that uptake has been extensively used to measure the effects of metals on aquatic organisms as an essential indicator of water quality (El‐Moselhy et al. 2014). The mollusks (Gupta and Singh 2011) and fish (Stankovic et al. 2014; Hauser-Davis et al. 2012a) are the most used organisms as bioindicators of metal pollution in water.
Representation of essential and pollutant metals’ effects on cellular metabolism with controlled intracellular concentrations of essential trace metals by homeostatic mechanisms, and exceeded concentrations when metal becomes toxic. Inducible tolerance (detoxification systems) raises the inhibitory thresholds, but when exceeded, permanent damage occurs
Metal bioavailability controls their accumulation in aquatic organisms (Gheorghe et al. 2017). External environmental factors like metal speciation, the presence of organic or inorganic complexes, and physicochemical properties of water (pH, temperature, salinity, and redox conditions) (Bonnail et al. 2016) are the main factors that could modulate metal toxicity. The ingestion uptake depends on the rate of feeding, intestinal transit time, and digestion efficiency (Jia et al. 2017) as well as on the age and size (weight and length), feeding habits, and body physiology of fish (Gheorghe et al. 2017). The degree of accumulation of heavy metals in various tissues of fish is generally different depending on the structure and function of tissues. Gills, liver, and kidney are metabolically active tissues with a higher affinity to accumulate heavy metals than other tissues (Ali et al. 2019). This is mostly explained by the induction or occurrence of metal-binding proteins called metallothioneins (MTs) or some other transporters which translocate metals across the plasma membrane in these tissues upon exposure to heavy metals (Ali et al. 2019; Hauser-Davis et al. 2012b).
Liver has a major role in the metabolism of lactate to pyruvate and can store up to one-eighth of the total glycogen stores. Glycogen content in the fish liver is a potent indicator for changes in the activity of functional systems affected by heavy metals (Javed and Usmani 2015). Another indicator in hepatocytes is related to the increase in the number and size of lysosomes to store pollutants and lipids, increased permeability of the lysosomal membrane, and release of lysosomes into the cytoplasm and nucleoplasm (Köhler 1991). Heavy metals enter the fish gall bladder, to be excreted from the organism (Ardeshir et al. 2017). Fish bile might thus also serve as a target tissue for heavy metal exposure (Hauser-Davis et al. 2012a) due to its role in the excretion of many endogenous and exogenous substances (Grosell et al. 2000).
Fish liver damage due to heavy metal exposure has been reported in various geographical sites (Kuton et al. 2021; Rubalingeswari et al. 2021; Weber et al. 2020; Kumar Maurya et al. 2019; Łuczyńskaa et al. 2018; Uysal 2011) indicating the importance of their monitoring and evaluation. Kumar Maurya et al. (2019) revealed that heavy metal accumulation in fish tissues is in the magnitude order of liver > gill > muscle pointing out Ni, Cr, Pb, Cu, Cd, Zn, and Fe as dominant metals influencing toxicity in fish tissues (Rubalingeswari et al. 2021). However, the bioaccumulation magnitude is a species-specific function (Spry and Wiener 1991) so the determination of bio-concentration factors (BCFs) (Orata and Birgen 2016) of heavy metals in fish liver, which are the ratio of the heavy metals in liver to the surrounding water, are needed and often used to evaluate results within different studies. In addition, it was observed that accumulation of heavy metals in liver varies with the season from minimum in winter to the maximum in summer with histological changes such as cytoplasmic vacuolation, necrosis, and sinusoid dilation (Mahboob et al. 2020). Partitioning of the heavy metals was explained with Cd, which was largely bound to heat-stable proteins including MTs and metal-rich granules in two fish species. These elements followed the Cd partitioning pattern, suggesting that they are involved in antioxidant responses against Cd toxicity (Gaël et al. 2019). Most recently, it was revealed that fish exposed to heavy metals show tissue degenerations and alterations become prominent as the duration of exposure increases (Naz et al. 2021). This includes hematological and histopathological changes under different concentrations of Cu and Cd. Various impairments were observed by histopathological examination of liver tissues like karyorrhexis, hepatic cell degeneration, congestion, and hemorrhages. Weber et al. (2020) pointed out that non-treated wastewater from the mining industry with a high load of Pb, Ni, and As is responsible for numerous impairments in fish. Hepatic alterations, such as cytoplasmic vacuolization and necrosis with the positive antibody reactions for cytochrome P450 1A (CYP1A) and MT, are associated with metal contamination. Thus, species more sensitive to environmental modifications may not resist this highly contaminated aquatic environment. Metal concentrations in fish tissues have also shown a species-specific bioaccumulation pattern, with diverse histopathological alterations (Omar et al. 2013). As mentioned, upon exposure to heavy metals, liver metabolism generates ROS, so antioxidant changes in liver are frequently used as a biomarker of metal exposure. A recent study evaluated levels of liver enzymes AST and ALT, with the focus on the antioxidant status of liver (El-Shenawy et al. 2021). They determined the TBARS levels as a direct response to lipid peroxidation, in addition to antioxidant enzymes including SOD, CAT, and GSH and concluded that the elevation of lipid peroxidation in fish is connected to the increased activity of endogenous antioxidants. Another study (Hossain et al. 2021) examined the changes in antioxidant enzymes as well as histopathological damage in liver tissue after exposure to heavy metals. Enzymes SOD, CAT, GPx, GST, reduced glutathione, and MDA levels were significantly increased. Severe hemorrhage, vacuoles, pyknotic space, and evidence of lipid accumulation were also observed.
All provided studies have one main target—evaluation of heavy metal accumulation and its impact on fish tissues by implementing different analysis approaches. For liver damage evaluation, histopathological observation of tissue is the most frequently used one, but since metals cause oxidative stress, measurement of antioxidant enzymes gives valuable insight into acute and chronic toxicity. Such findings need to be continuously introduced to the wider public, i.e., endpoint consumers, to acknowledge existing and further problems regarding the constant heavy metal discharge into the environment.
Conclusions and future directions
This review provided a comprehensive assessment of the impact of WWTP effluents on fish liver damage. Continuous release of (un)processed wastewaters with residual xenobiotics has greatly impacted the environment. The fish liver represents one of the most used models to establish a link between the concentration of pollutants and their bioaccumulation and detoxification within the organism. In this review, a variety of sensitive fish liver biomarkers as suitable indices for health conditions was discussed referring to the cellular, biochemical-physiological, and histopatological levels. Detailed observation of these changes has been made with a specific accent on legacy contaminants and emerging-concern contaminants such as pharmaceutically active compounds, personal care products, and heavy metals. Our results show that it is a challenge to identify a suitable fish liver biomarker for assessing WWTP impacts. Furthermore, suitable fish species need to be selected for such investigations, both for field studies and laboratory exposure studies. In that fashion, their applicability and specificity can be assessed and standardized for targeted determination of the impact of WWTP effluents on fish liver.
Furthermore, engineered nanoparticles occurring in wastewater discharges raise concerns in toxicological assessments. In particular, metal and metal oxide NPs can translocate among organs and penetrate the blood‐brain barrier, accumulating in fish liver. Most studies on such NPs were focused on acute toxicity. Therefore, more chronic toxicity studies are required, particularly co-exposure studies. Additionally, systematic understanding of the transport, distribution and toxicological mechanisms of complex pollutants in fish livers are still not sufficient. The research on toxic effects of such NPs still principally focuses on pathological effects such as body distribution, cell damage and organ damage (Bai and Tang 2020).
Although a significant decrease in the concentration of several persistent contaminants like Hg, PCBs, etc. can be noticed in the last few decades, many other classes of contaminants that are currently being poorly studied are becoming ubiquitous (new pharmaceuticals, personal care products, etc.). For such reasons, current bioaccumulation data do not represent the actual impact of WWTPs on the aquatic ecosystem and consequent impact on non-target organisms. All this indicates that continuous monitoring is essential, while further research and improvement of treatment plants can provide less contaminated discharge effluent with legally controlled release or further treated disposal of effluent sludge. Further research is required to confirm bioaccumulation factor of emerging contaminants in aquatic organisms and to reveal mechanisms of their toxicity. More focus should be also put on determining their potential interaction in water bodies which could enhance their hepatotoxicity. Knowledge gaps, as well as research priorities, emphasized in this review article may help to steer and prioritize future research in this important field.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdel-Latif HMR, Dawood MAO, Mahmoud SE, Shukry M, Noreldin AE, Ghetas HA, Khallaf MA (2021) Copper oxide nanoparticles alter serum biochemical indices, induce histopathological alterations, and modulate transcription of cytokines, hsp70, and oxidative stress genes in Oreochromis niloticus. Animals 11(3):652. https://doi.org/10.3390/ani11030652
Abdel-Moneim AM, Abdel-Mohsen HA (2010) Ultrastructure changes in hepatocytes of catfish Clarias gariepinus from Lake Mariut, Egypt. J Environ Biol 31(5):715–720
Agius C, Roberts RJ (2003) Melano-macrophage centres and their role in fish pathology. J Fish Dis 26(9):499–509. https://doi.org/10.1046/j.1365-2761.2003.00485.x
Agoro MA, Adeniji AO, Adefisoye MA, Okoh OO (2020) Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in Eastern Cape Province, South Africa. Water 12:2746. https://doi.org/10.3390/w12102746
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Hindawi J Chem Article ID 6730305. https://doi.org/10.1155/2019/6730305.
Allen P (1993) Effects of acute exposure to cadmium (II) chloride and lead (II) chloride on the haematological profile of Oreochromis aureus (Steindachner). Comp Biochem Physiol Part C 105(2):213–217. https://doi.org/10.1016/0742-8413(93)90197-S
Andersen L, Holbech H, Gessbo A, Norrgren L, Petersen GI (2003) Effects of exposure to 17alpha-ethiny lestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio). Comp Biochem Physiol c: Toxicol Pharmacol 134:365–374. https://doi.org/10.1016/s1532-0456(03)00006
Andersson T, Förlin L (1992) Regulation of the cytochrome P450 enzyme system in fish. Aquat Toxicol 24:1–20. https://doi.org/10.1016/0166-445X(92)90014-E
Annabi A, Kessabi K, Navarro A, Said K, Messaoudi I, Pina B (2012) Assessment of reproductive stress in natural populations of the fish Aphanius fasciatus using quantitative mRNA markers. Aquat Biol 17:285–293. https://doi.org/10.3354/ab00482
Ardeshir RA, Movahedinia A, Rastgar S (2017) Fish liver biomarkers for heavy metal pollution: A review article. Am J Toxicol 1:1–8
Baesu A, Ballash G, Mollenkopf D, Wittum T, Sulliván SMP, Bayen S (2021) Suspect screening of pharmaceuticals in fish livers based on QuEChERS extraction coupled with high resolution mass spectrometry. Sci Total Environ 783:146902. https://doi.org/10.1016/j.scitotenv.2021.146902
Bahamonde PA, Fuzzen ML, Bennett CJ, Tetreault GR, McMaster ME, Servos MR, MartyniukCJ MKR (2015) Whole organism responses and intersex severity in rainbow darter (Etheostoma caeruleum) following exposures to municipal wastewater in the Grand River basin, ON, Canada. Part a Aquat Toxicol 159:290–301. https://doi.org/10.1016/j.aquatox.2014.11.023
Bai C, Tang M (2020) Toxicological study of metal and metal oxide nanoparticles in zebrafish. J Appl Toxicol 40(1):37–63. https://doi.org/10.1002/jat.3910
Banaei M, Forouzanfar M, Jafarinia M (2022) Toxic effects of polyethylene microplastics on transcriptional changes, biochemical response, and oxidative stress in common carp (Cyprinus carpio). Comp Biochem Physi Part - C: Toxicol Pharmacol 261:109423. https://doi.org/10.1016/j.cbpc.2022.109423
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377. https://doi.org/10.1016/j.arabjc.2010.07.019
Barbosa MO, Moreira NF, Ribeiro AR, Pereira MF, Silva AM (2016) Occurrence and removal of organic micropollutants: an overview of the watch list of EU Decision 2015/495. Water Res 94:257–279. https://doi.org/10.1016/j.watres.2016.02.047
Batchelar KL, Kidd KA, Drevnick PE, Munkittrick KR, Burgess NM, Roberts AP, Smith JD (2013) Evidence of impaired health in yellow perch (Perca flavescens) from a biological mercury hotspot in northeastern north America. Environ Toxicol Chem 32(3):627–637. https://doi.org/10.1002/etc.2099
Benedetti M, Ciaprini F, Piva F, Onorati F, Fattorini D, Notti A, Ausili A, Regoli F (2012) A multidisciplinary weight of evidence approach for classifying polluted sediments: integrating sediment chemistry, bioavailability, biomarkers responses and bioassays. Environ Int 38:17–28. https://doi.org/10.1016/j.envint.2011.08.003
Bernet D, Schmidt-Posthaus H, Wahli T, Burkhardt-Holm P (2000) Effects of wastewater on fish health: an integrated approach to biomarker responses of brown trout (Salmo trutta L.). J Aquat Ecosyst Stress Recover 8:143–151. https://doi.org/10.1023/A:1011481632510
Bielen A, Šimatović A, Kosić-Vukšić J, Senta I, Ahel M, Babić S, Jurina T, González Plaza JJ, Milanković M, Udiković-Kolić N (2017) Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res 126:79–87. https://doi.org/10.1016/j.watres.2017.09.019
Bignell JP, Barber J, Bateman KS, Etherton M, Feist SW, Galloway TS, Katsidaki I, Sabire M, Scott AP, Stentiford GD, Bean TP (2020). Insights into the development of hepatocellular fibrillar inclusions in European flounder (Platichthys flesus) from UK estuaries. Chemosphere 126946. https://doi.org/10.1016/j.chemosphere.2020.126946
Blahova J, Modra H, Sevcikova M, Marsalek P, Zelnickova L, Skoric M, Svobodova Z (2014) Evaluation of biochemical, haematological, and histopathological responses and recovery ability of common carp (Cyprinus carpio L.) after acute exposure to atrazine herbicide. BioMed Res Int 4:980948. https://doi.org/10.1155/2014/980948
Blazer VS, Walsh HL, Braham RP, Hahn CM, Mazik P, McIntyre PB (2016) Tumours in white suckers from Lake Michigan tributaries: pathology and prevalence. J Fish Dis 40(3):377–393. https://doi.org/10.1111/jfd.12520
Bonnail E, Sarmiento AM, DelValls TA, Nieto JM, Riba I (2016) Assessment of metal contamination, bioavailability, toxicity and bioaccumulation in extreme metallic environments (Iberian Pyrite Belt) using Corbicula fluminea. Sci Total Environ 544:1031–1044. https://doi.org/10.1016/j.scitotenv.2015.11.131
Borucinska JD, Morka D, Grabowski Z, Smith H (2017) A follow-up study of selected biomarkers of health in cod Gadus morhua L. collected from the southern Baltic off the Polish coast. J Fish Dis 40:1883–1894. https://doi.org/10.1111/jfd.12663
Boxall AB, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, ..., Van Der Kraak G (2012) Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect 120(9): 1221–1229. https://doi.org/10.1289/ehp.1104477
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82(11):1518–1532. https://doi.org/10.1016/j.chemosphere.2010.11.018
Brooks BW, Chambliss CK, Stanley JK, Ramirez A, Banks KE, Johnson RD, Lewis RJ (2005) Determination of select antidepressants in fish from an effluent-dominated stream. Environ Toxicol Chem: an Int J 24(2):464–469. https://doi.org/10.1897/04-081r.1
Buchberger WW (2011) Current approaches to trace analysis of pharmaceuticals and personal care products in the environment. J Chromatogr A 1218(4):603–618. https://doi.org/10.1016/j.chroma.2010.10.040
Buhler DR (1995) Cytochrome P450 expression in rainbow trout: An overview. In: Arinc E, Schenkman JB, Hodgson E (eds) Molecular aspects of oxydative drug metabolizing enzymes, vol 90. NATO ASI Series. Springer-Verlag, Berlin, Heidelberg, pp 159–177
Buhler DR, Wang-Buhler JL (1998) Rainbow trout cytochrome P450s: Purification, molecular aspects, metabolic activity, induction and role in environmental monitoring. Comp Biochem Physiol Part C 121:107–137. https://doi.org/10.1016/s0742-8413(98)10033-6
Bunton TE (2010) Hepatopathology of diethylnitrosamine in the medaka (Oryzias latipes) following short-term exposure. Toxicol Pathol 18(2):313–323. https://doi.org/10.1177/019262339001800210
Burkina V, Zlabek V, Zamaratskaia G (2015) Effects of pharmaceuticals present in aquatic environment on Phase I metabolism in fish. Environ Toxicol Pharmacol 40(2):430–444. https://doi.org/10.1016/j.etap.2015.07.016
Burkina V, Zamaratskaia G, Sakalli S, Giang PT, Zlabek V, Rasmussen MK (2021) Tissue-specific expression and activity of cytochrome P450 1A and 3A in rainbow trout (Oncorhynchus mykiss). Toxicol Lett 341:1–10. https://doi.org/10.1016/j.toxlet.2021.01.011
Cai Y, Yin Y, Wang L, Leng D, Ge C, Abdallah A, Li Y (2018) Effect on serum parameters and immune responses of Carassius auratus gibelio exposed to dietary lead and Bacillus subtilis. Biol Trace Elem Res. https://doi.org/10.1007/s12011-018-1544-2
Canedo A, Rocha TL (2021) Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: historical review, current status and trends. Sci Total Environ 762:144084. https://doi.org/10.1016/j.scitotenv.2020.1440
Carrola J, Fontaínhas-Fernandes A, Pires MJ, Rocha E (2014) Frequency of hepatocellular fibrillar inclusions in European flounder (Platichthys flesus) from the Douro River estuary, Portugal. Environ Sci Pollut Res 21(4):3116–3125. https://doi.org/10.1007/s11356-013-2248-y
Casatta N, Stefani F, Viganò L (2017) Hepatic gene expression profiles of a non-model cyprinid (Barbus plebejus) chronically exposed to river sediments. Comp Biochem Physiol c: Toxicol Pharmacol 196:27–35. https://doi.org/10.1016/j.cbpc.2017.03.006
Catteau A, Bado-Nilles A, Beaudouin R, Tebby C, Joachim S, Palluel O, Turiès C, Chrétien N, Nott K, Ronkart S, Geffard A, Porcher JM (2021) Water quality of the Meuse watershed: assessment using a multi-biomarker approach with caged three-spined stickleback (Gasterosteus aculeatus L.). Ecotoxicol Environ Saf 208:111407. https://doi.org/10.1016/j.ecoenv.2020.111407
Cazenave J, Bacchetta C, Rossi A, Ale A, Campana M, Parma MJ (2014) Deleterious effects of wastewater on the health status of fish: a field caging study. Ecol Ind 38:104–112. https://doi.org/10.1016/j.ecolind.2013.10.029
Celiz MD, Tso J, Aga DS (2009) Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ Toxicol Chem 28(12):2473–2484. https://doi.org/10.1897/09-173.1
Chen Z, Guo J, Jiang Y, Shao Y (2021) High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ Sci Eur 33(1):1–4. https://doi.org/10.1186/s12302-021-00456-4
Chiang MWL, Au DWT (2013) Histopathological Approaches in Ecotoxicology. Encyclopedia Aquat Ecotoxicol 597–614. https://doi.org/10.1007/978-94-007-5704-2_56
Chupani L, Savari A, Zolgharnein H, Rezaie A, Zeinali M (2013) Enzymatic and histopathologic biomarkers in the flatfish Euryglossa orientalis from the northwestern Persian Gulf. Toxicol Ind Health 32(5):866–876. https://doi.org/10.1177/0748233713513490
Corcoran J, Winter MJ, Tyler CR (2010) Pharmaceuticals in the aquatic environment: a critical review of the evidence for health effects in fish. Crit Rev Toxicol 40(4):287–304. https://doi.org/10.3109/10408440903373590
Couillard CM, Courtenay SC, Macdonald RW (2008) Chemical–environment interactions affecting the risk of impacts on aquatic organisms: a review with a Canadian perspective — interactions affecting vulnerability. Environ Rev 16:19–44. https://doi.org/10.1139/A07-008
Cuevas N, Martins M, Costa PM (2018) Risk assessment of pesticides in estuaries: a review addressing the persistence of an old problem in complex environments. Ecotoxicology 27:1008–1018. https://doi.org/10.1007/s10646-018-1910-z
Da Costa Araújo AP, de Andrade Vieira J E, Malafaia G (2020). Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci Total Environ 140217. https://doi.org/10.1016/j.scitotenv.2020.140217
Dale K, Müller MB, Tairova Z, Khan EA, Hatlen K, Grung M, Yadetie F, Lille-Langøy R, Blaser N, Skaug HJ, Lyche JL, Arukwe A, Hylland K, Karlsen OA, Goksøyr A (2019) Contaminant accumulation and biological responses in Atlantic cod (Gadus morhua) caged at a capped waste disposal site in Kollevåg, western Norway. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2019.02.003
do Amaral QDF, da Rosa E, Wronski JG, Zuravski L, Querol MVM, dos Anjos B, de Andrade CFF, Machado MM, de Oliveira LFS (2019) Golden mussel (Limnoperna fortunei) as a bioindicator in aquatic environments contaminated with mercury: cytotoxic and genotoxic aspects. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.04.108
Du SNN, Choi JA, McCallum ES, McLean AR, Borowiec BG, Balshine S, Scott GR (2019) Metabolic implications of exposure to wastewater effluent in bluegill sunfish. Comp Biochem Physiol, Part C 224:108562. https://doi.org/10.1016/j.cbpc.2019.108562
Duarte RM, Sadauskas-Henrique H, de Almeida-Val VMF, Val AL, Nice HE, Gagnon MM (2017) Biomarker responses and PAH ratios in fish inhabiting an estuarine urban waterway. Environ Toxicol 32(10):2305–2315. https://doi.org/10.1002/tox.22447
El-Moselhy KhM, Othman AI, Abd El-Azem H, El-Metwally MEA (2014) Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egyptian J Basic Appl Sci 1(2):97–105. https://doi.org/10.1016/j.ejbas.2014.06.001
El-Shenawy NS, Gad EL-Hak HN, Ghobashy MA, Mansour FA, Soliman MFM (2021) Using antioxidant changes in liver and gonads of Oreochromis niloticus as biomarkers for the assessment of heavy metals pollution at Sharkia province, Egypt. Reg Stud Mar Sci 46:101863. https://doi.org/10.1016/j.rsma.2021.101863
Espinosa C, Cuesta A, Esteban MÁ (2017) Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 68:251–259. https://doi.org/10.1016/j.fsi.2017.07.006
Faheem M, Lone KP (2018) Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Braz J Pharm Sci 53:3. https://doi.org/10.1590/s2175-97902017000317003
Falfushynska H, Khatib I, Kasianchuk N, Lushchak O, HorynO SIM (2022) Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci Total Environ 833:155236. https://doi.org/10.1016/j.scitotenv.2022.155236
Fathy M, Mohamed IA, Farghal AIA, Temerak SAH, Sayed AEDH (2019) Hemotoxic effects of some herbicides on juvenile of Nile tilapia Oreochromis niloticus. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06280-x
Ferroni HI, Bezerra V, Risso WE, dos Reis B, Martinez C, Delatim Simonato J (2022) Sublethal effects of biogenic silver nanoparticles and silver nitrate in the neotropical fish Prochilodus lineatus: Is the nanoform really less toxic? Water. Air Soil Pollut 233:74. https://doi.org/10.1007/s11270-022-05547-3
Field HA, Ober EA, Roeser T, Stainier DY (2003) Formation of the digestive system in zebrafish I. liver morphogenesis. Developmental Biology 253(2):279–290. https://doi.org/10.1016/s0012-1606(02)00017-9
Finn RN (2007) The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat Toxicol 81(4):337–354. https://doi.org/10.1016/j.aquatox.2006.12.021
Förlin L, Blom S, Celander M, Sturve J (1996) Effects on UDP glucuronosyl transferase, glutathione transferase, DT-diaphorase and glutathione reductase activities in rainbow trout liver after long-term exposure to PCB. Mar Environ Res 42:213–216. https://doi.org/10.1016/0141-1136(95)00061-5
Fournie and Vogelbein (1994) Exocrine pancreatic neoplasms in the mummichog (Fundulus heteroclitus) from a creosote-contaminated site. Toxicol Pathol 22(3):237–247. https://doi.org/10.1177/019262339402200302
Fournie JW, Hawkins WE (2002) Exocrine pancreatic carcinogenesis in the guppy Poecilia reticulata. Dis Aquat Org 52:191–198. https://doi.org/10.3354/dao052191
Franco ME, Burket SR, Sims JL, Lovin LM, Scarlett KR, Stroski K, Steenbeek R, Ashcroft C, Luers M, Brooks BW, Lavado R (2020) Multi-approach assessment for the evaluation of spatio-temporal estrogenicity in fish from effluent-dominated surface waters under low instream flow. Environ Pollut 265:115122. https://doi.org/10.1016/j.envpol.2020.115122
Frémion F, Bordas F, Mourier B, Lenain JF, Kestens T, Courtin-Nomade A (2016) Influence of dams on sediment continuity: a study case of a natural metallic contamination. Sci Total Environ 547:282–294. https://doi.org/10.1016/j.scitotenv.2016.01.023
Fricke NF, Stentiford GD, Feist SW, Lang T (2012) Liver histopathology in Baltic eelpout (Zoarces viviparus)–A baseline study for use in marine environmental monitoring. Marine Environmental Research 82:1–14. https://doi.org/10.1016/j.marenvres.2012.08.012
Gaël LC, Camille L, Sébastien A, Stéphane LF, Jean-Marie M, Jean R, Virginie P, Marie-Laure R, Raymond L, De Morais LT (2019) Metal subcellular partitioning determines excretion pathways and sensitivity to cadmium toxicity in two marine fish species. Chemosphere 217:754–762. https://doi.org/10.1016/j.chemosphere.2018.10.212
Gagnon MM, Rawson CA (2017) Bioindicator species for EROD activity measurements: a review with Australian fish as a case study. Ecol Ind 73:166–180. https://doi.org/10.1016/j.ecolind.2016.09.015
Galloway TS, Brown RJ, Browne MA, Dissanayake A, Lowe D, Jones MB, Depledge MH (2004) A multibiomarker approach to environmental assessment. Environ Sci Technol 38(6):1723–1731. https://doi.org/10.1021/es030570+
Garmendia L, Izagirre U, Soto M, Lermen D, Koschorreck J (2015) Combining chemical and biological endpoints, a major challenge for twenty-first century’s environmental specimen banks. Environ Sci Pollut Res 22:1631–1634. https://doi.org/10.1007/s11356-014-2925-5
George SG (1994) Enzymology and molecular biology of phase II xenobiotic-conjugating enzymes in fish. In: Malins DC, Ostrander GK (eds) Aquatic Toxicology; Molecular, biochemical and cellular perspectives. Lewis Publishers, CRC Press, pp 37–85
Germande O, Beaufils F, Daffe G, Gonzalez P, Mornet S, Bejko M, Errera MH, Lacomme S, Gontier E, Guibert C, Baudrimont I, Baudrimont M (2022) Cellular and molecular mechanisms of NiONPs toxicity on eel hepatocytes HEPA-E1: an illustration of the impact of Ni release from mining activity in New Caledonia. Chemosphere 303(2):135158. https://doi.org/10.1016/j.chemosphere.2022.135158
Ghazaly KS (1992) Hematological and physiological responses to sublethal concentrations of cadmium in a freshwater teleost, Tilapia zilii. Water Air Soil Pollut 64(3–4):551–559. https://doi.org/10.1007/BF00483365
Gheorghe S, Stoica C, Vasile GG, Nita-Lazar M, Stanescu E, Lucaciu IE (2017) Metals toxic effects in aquatic ecosystems: modulators of water quality, Chapter 4 in Water Quality (Ed. Tutu H), IntechOpen. https://doi.org/10.5772/65744
Gilannejad N, Dorafshan S, Heyrati FP, Soofiani NM, Asadollah S, Martos-Sitcha JA, Prat F, Yúfera M, Martínez-Rodríguez G (2016) Vitellogenin expression in wild cyprinid Petroleuciscus esfahani as a biomarker of endocrine disruption along the Zayandeh Roud River, Iran. Chemosphere 144:1342–1350. https://doi.org/10.1016/j.chemosphere.2015.09.106
Gomez CF, Constantine L, Huggett DB (2010) The influence of gill and liver metabolism on the predicted bioconcentration of three pharmaceuticals in fish. Chemosphere 81(10):1189–1195. https://doi.org/10.1016/j.chemosphere.2010.09.043
Gonçalves ÍFS, Souza TM, Vieira LR, Marchi FC, Nascimento AP, Farlas DP (2020a) Toxicity testing of pesticides in zebrafish—a systematic review on chemicals and associated toxicological endpoints. Environ Sci Pollut Res 27:10185–10204. https://doi.org/10.1007/s11356-020-07902-5
Gonçalves C, Marins AT, do Amaral AMB, Nunes MEM, Müller TE, Severo E, Feijó A, Rodrigues CCR, Zanella R, Damian Prestes O, Clasen B, Loro VL (2020) Ecological impacts of pesticides on Astyanax jacuhiensis (Characiformes: Characidae) from the Uruguay river, Brazil. Ecotoxicol Environ Saf 205:111314. https://doi.org/10.1016/j.ecoenv.2020.111314
Grabicova K, Fedorova G, Burkina V, Steinbach C, Schmidt-Posthaus H, Zlabek V, Kroupova HK, Grabic R, Randak T (2013) Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol Environ Saf 96:41–47. https://doi.org/10.1016/j.ecoenv.2013.06.022
Grosell M, O’Donnell MJ, Wood CM (2000) Hepatic versus gallbladder bile composition: in vivo transport physiology of the gallbladder in rainbow trout. Am J Physio Regul Integr Comp Physiol 278:1674–1684. https://doi.org/10.1152/ajpregu.2000.278.6.R1674
Gu Z, Jia R, He Q, Cao L, Du J, Feng W, Jeney G, Xu P, Yin G (2021) Alteration of lipid metabolism, autophagy, apoptosis and immune response in the liver of common carp (Cyprinus carpio) after long-term exposure to bisphenol A. Ecotoxicol Environ Saf 211:111923. https://doi.org/10.1016/j.ecoenv.2021.111923
Guiloski IC, Stein Piancini LD, Dagostim AC, de Morais Calado SL, Fávaro LF, Boschen SL, Cestari MM, da Cunha SC, de Assis HC (2017) Effects of environmentally relevant concentrations of the anti-inflammatory drug diclofenac in freshwater fish Rhamdia quelen. Ecotoxicol Environ Saf 139:291–300. https://doi.org/10.1016/j.ecoenv.2017.01.053
Gunatilake SK (2015) Methods of removing heavy metals from industrial wastewater. J Multidiscip Eng Sci Stud 1:12–18
Gupta SK, Singh J (2011) Evaluation of mollusk as sensitive indicator of heavy metal pollution in aquatic system: a review. IIOAB J 2:49–57
Gyimah E, Dong X, Qiu W, Zhang, Z, Xu H (2020) Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ Sci Pollut Res 1–10. https://doi.org/10.1007/s11356-020-08232-2
Hamer M, Maynard SK, Schneider S (2019) A pulsed-dose study evaluating chronic toxicity of Chlorothalonil to fish: a case study for environmental risk assessment. Environ Toxicol Chem 38(7):1549–1559. https://doi.org/10.1002/etc.4421
Haque N, Nam SE, Han YS, Park HS, Rhee JS (2022) Chronic exposure to sublethal concentrations of saxitoxin reduces antioxidant activity and immunity in zebrafish but does not affect reproductive parameters. Aquat Toxicol 243:106070. https://doi.org/10.1016/j.aquatox.2021.106070
Hartl MGJ, Kilemade M, Sheehan D, Mothersill C, O’Halloran J, O’Brien NM et al (2007) Hepatic biomarkers of sediment-associated pollution in juvenile turbot, Scophthalmus maximus L. Mar Environ Res 64:191–208. https://doi.org/10.1016/j.marenvres.2007.01.002
Hauser-Davis RA, Bastos FF, de Oliveira TF, Ziolli RL, de Campos RC (2012a) Fish bile as a biomarker for metal exposure. Mar Pollut Bull 64(8):1589–1595. https://doi.org/10.1016/j.marpolbul.2012.05.017
Hauser-Davis RA, Calixto de Campos R, Ziolli RL (2012b) Fish metalloproteins as biomarkers of environmental contamination. Rev Environ Contam Toxicol 218:101–123. https://doi.org/10.1007/978-1-4614-3137-4_2
Heinrich-Hirsch B, Madle S, Oberemm A, Gundert-Remy U (2001) The use of toxicodynamics in risk assessment. Toxicol Lett 120:131–141. https://doi.org/10.1016/S0378-4274(01)00291-0
Hemalatha D, Rangasamy B, Nataraj B, Ramesh M (2019) Assessment of triclosan impact on enzymatic biomarkers in an Indian major carp, Catla catla. J Basic Appl Zool 80(1):1–8. https://doi.org/10.1186/s41936-019-0094-2
Herraez MP, Zapata AG (1991) Structural characterization of the melano-macrophage centers (MMC) of goldfish Carrasius auratus. Eur J Morphol 29(2):89–102
Hinton DE, Baumann PC, Gardner GC, Hawkins WE, Hendricks JD, Murchelano RA, Okihiro MS (1992) Histopathologic biomarkers. In: Huggett RJ, Kimerly RA, Mehrle PM, Bergman HL (eds) Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress. Lewis Publishers, Chelsea, MI, USA, pp 155–210
Hinton DE (1994) Cells, cellular responses, and their markers in chronic toxicity of fishes. In: Aquatic Toxicology; Molecular, Biochemical and Cellular Perspectives; Malins DC, Ostrander GK (Eds.), Lewis Publishers CRC Press, pp. 207–240
Hook SE, Gallagher EP, Batley GE (2014) The role of biomarkers in the assessment of aquatic ecosystem health. Integr Environ Assess Manag 10(3):327–341. https://doi.org/10.1002/ieam.1530
Hopkins ZR, Blaney L (2016) An aggregate analysis of personal care products in the environment: identifying the distribution of environmentally-relevant concentrations. Environ Int 92:301–316
Hossain Z, Hossain S, Safa Ema N, Omri A (2021) Heavy metal toxicity in Buriganga river alters the immunology of Nile tilapia (Oreochromis niloticus L). Heliyon 7:e0828. https://doi.org/10.1016/j.heliyon.2021.e08285
Houde M, Giraudo M, Douville M, Bougas B, Couture P, De Silva AO, Spencer C, Lair S, Verreault J, Bernatchez L, Gagnon C (2014) A multi-level biological approach to evaluate impacts of a major municipal effluent in wild St. Lawrence River yellow perch (Perca flavescens). Sci Total Environ 497–498:307–318. https://doi.org/10.1016/j.scitotenv.2014.07.059
Hu J, Liu J, Li J, Lv X, Yu L, Wu K, Yang Y (2021) Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J Hazard Mater 4055. https://doi.org/10.1016/j.jhazmat.2020.124150
Huang Z, Xu B, Huang X, Zhang Y, Yu M, Han X, Song L, Xia Y, Zhou Z, Wang X, Chen M, Lu C (2019) Metabolomics reveals the role of acetyl-L-carnitine metabolism in γ-Fe2O3 NP-induced embryonic development toxicity via mitochondria damage. Nanotoxicology 13(2):204–220. https://doi.org/10.1080/17435390.2018.1537411
Huang X, Li Y, Wang T, Liu H, Shi J, Zhang X (2020) Evaluation of the oxidative stress status in zebrafish (Danio rerio) liver induced by three typical organic UV filters (BP-4, PABA and PBSA). Int J Environ Res Public Health 17(2):651. https://doi.org/10.3390/ijerph17020651
Huerta B, Jakimska A, Gros M, Rodriguez-Mozaz S, Barceló D (2013) Analysis of multi-class pharmaceuticals in fish tissues by ultra-high-performance liquid chromatography tandem mass spectrometry. J Chromatogr A 1288:63–72. https://doi.org/10.1016/j.chroma.2013.03.001
Husøy AM, Myers MS, Willis ML, Collier TK, Celander M, Goksøyr A (1994) Immunohistochemical localization of CYP1A and CYP3A-like isozymes in hepatic and extrahepatic tissues of Atlantic cod (Gadus morhua L.), a marine fish. Toxicol Appl Pharmacol 129(2):294–308. https://doi.org/10.1006/taap.1994.1254
James MO, Marth CJ, Rowland-Faux L (2012) Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat Toxicol 124:72–82. https://doi.org/10.1016/j.aquatox.2012.07.009
James MO, Kleinow KM (2018) Trophic transfer of chemicals in the aquatic environment. In: Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives, CRC Press, pp. 1 – 35. https://doi.org/10.1201/9781351069878
Jarvie HP, Al-Obaidi H, King SM, Bowes MJ, Lawrence MJ, Drake AF, Green MA, Dobson PJ (2009) Fate of silica nanoparticles in simulated primary wastewater treatment. Environ Sci Technol 43(22):8622–8628. https://doi.org/10.1021/es901399q
Jasinska EJ, Goss GG, Gillis PL, Van Der Kraak GJ, Matsumoto J, de Souza MAA, Giacomin M, Moon TW, Massarsky A, Gagné F, Servos MR, Wilson J, Sultana T, Metcalfe CD (2015) Assessment of biomarkers for contaminants of emerging concern on aquatic organisms downstream of a municipal wastewater discharge. Sci Total Environ 530–531:140–153. https://doi.org/10.1016/j.scitotenv.2015.05.080
Javed M, Usmani N (2013) Assessment of heavy metal (Cu, Ni, Fe Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. Springer plus 2:390. https://doi.org/10.1186/2193-1801-2-390
Javed M, Usmani N (2015) Impact of heavy metal toxicity on hematology and glycogen status of fish: A review. Proc Natl Acad Sci India - Section b: Biol Sci 85(4):889–900. https://doi.org/10.1007/s40011-014-0404-x
Jia Y, Wang L, Qu Z, Wang C, Yang Z (2017) Effects on heavy metal accumulation in freshwater fishes: species, tissues, and sizes. Environ Sci Pollut Res 24:9379–9386. https://doi.org/10.1007/s11356-017-8606-4
Jia R, Du J, Cao L, Feng W, He Q, Xu P, Ying G (2021) Immune, inflammatory, autophagic and DNA damage responses to long-term H2O2 exposure in different tissues ofcommon carp (Cyprinus carpio). Science of the Total Environment 757, Article ID 143831. https://doi.org/10.1016/j.scitotenv.2020.143831
Johnsen K, Tana J, Lehtinen KJ, Stuthridge T, Mattsson K, Hemming J, Carlberg GE (1998) Experimental field exposure of brown trout to river water receiving effluent from an integrated newsprint mill. Ecotoxicol Environ Saf 40(3):184–193. https://doi.org/10.1006/eesa.1998.1683
Karvelas M, Katsoyiannis A, Samara C (2003) Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere 53:1201–1210. https://doi.org/10.1016/S0045-6535(03)00591-5
Kaur R, Dua A (2014) Adverse effects on histology of liver and kidney in fish Channa punctata exposed to wastewater from Tung Dhab drain in Amritsar, India. J Environ Biol 35:265–272
Khan RA (2003) Health of flatfish from localities in Placentia Bay, Newfoundland, contaminated with petroleum and PCBs. Arch Environ Contam Toxicol 44:485–492. https://doi.org/10.1007/s00244-002-2063-9
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27(9):1825–1851. https://doi.org/10.1897/08-090.1
Köhler A (1991) Lysosomal perturbations in fish liver as indicators for toxic effects of environmental pollution. Comp Biochem Physiol - Part c: Comp Pharmacol 100(1):123–127. https://doi.org/10.1016/0742-8413(91)90137-i
Köhler A, Ellesat K (2008) Nuclear changes in blood, early liver anomalies and hepatocellular cancers in flounder (Platichthys flesus L.) as prognostic indicator for a higher cancer risk? Mar Environ Res 66(1):149–150. https://doi.org/10.1016/j.marenvres.2008.02.071
Köpping I, McArdell CS, Borowska E, Böhler MA, Udert KM (2020) Removal of pharmaceuticals from nitrified urine by adsorption on granular activated carbon. Water Res X 9:100057. https://doi.org/10.1016/j.wroa.2020.100057
Kosmala A, Migeon P, Flammarion P, Garric J (1998) Impact assessment of a wastewater treatment plant effluent using the fish biomarker ethoxyresorufin-o-deethylase: field and on-site experiments. Ecotoxicol Environ Saf 41:19–28. https://doi.org/10.1006/eesa.1998.1662
Kroon F, Streten C, Harries S (2017) A protocol for identifying suitable biomarkers to assess fish health: a systematic review. Plos One 12(4):e0174762. https://doi.org/10.1371/journal.pone.0174762
Kumar Maurya P, Malik DS, Kumar Yadav K, Kumar A, Kumar S, Kamyabe H (2019) Bioaccumulation and potential sources of heavy metal contamination in fish species in River Ganga basin: Possible human health risks evaluation. Toxicol Rep 6:472–481. https://doi.org/10.1016/j.toxrep.2019.05.012
Küster A, Adler N (2014) Pharmaceuticals in the environment: scientific evidence of risks and its regulation. Phil Trans R Soc b: Biol Sci 369(1656):20130587. https://doi.org/10.1098/rstb.2013.0587
Kuton MP, Ayanda IO, Uzoalu IA, Akhiromen DI, George A, Akinsanya B (2021) Studies on heavy metals and fish health indicators in Malapterurus electricus from Lekki Lagoon, Lagos. Nigeria. Vet Ani Sci 12:100169. https://doi.org/10.1016/j.vas.2021.100169
Lang T, Feist SW, Stentiford GD, Bignell JP, Vethaak AD, Wosniok W (2017) Diseases of dab (Limanda limanda): Analysis and assessment of data on externally visible diseases, macroscopic liver neoplasms and liver histopathology in the North Sea, Baltic Sea and off Iceland. Mar Environ Res 124:61–69. https://doi.org/10.1016/j.marenvres.2015.12.009
Lankadurai BP, Nagato EG, Simpson MJ (2013) Environmental metabolomics: an emerging approach to study organism responses to environmental stressors. Environ Rev 21(3):180–205. https://doi.org/10.1139/er-2013-0011
Lapointe D, Pelletier M, Paradis Y, Armellin A, Verreault J, Champoux L, Desrosiers M (2020) Trophic transfer of polybrominated diphenyl ethers in a recently modified freshwater food web from the St. Lawrence River, Canada. Chemosphere 255:126877. https://doi.org/10.1016/j.chemosphere.2020.126877
Larsson DJ (2014) Pollution from drug manufacturing: review and perspectives. Phi Trans R Soc b: Biol Sci 369(1656):20130571. https://doi.org/10.1098/rstb.2013.0571
Laurent J, Lavergne E, Couteau J, Le Floch S, Ouddane B, Cachot J, Davail B, Cleradeau C, Devin S, Fisson C, Devaux A, Amara R, Diop M, Pichereau V, Laroche J (2022) Impacts of chemical stress, season, and climate change on the flounder population of the highly anthropised Seine estuary (France). Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20000-y
Lerebours A, Chapman E, Lyons BP, Bignell JP, Stentiford GD, Rotchell JM (2017) Hepatocellular adenoma in a European flatfish (Limanda limanda): Genetic alterations in laser-capture micro-dissected tissue and global transcriptomic approach. Mar Pollut Bull 119(2):120–127. https://doi.org/10.1016/j.marpolbul.2017.04.052
Lester SM, Braunbeck TA, The SJ, Stegeman JJ, Miller MR, Hinton DE (1993) Hepatic cellular distribution of cytochrome P-4501A1 in rainbow trout (Oncorhynchys mykiss): An immunohisto- and cytochemical study. Cancer Res 53:3700–3706
Liu MJ, Guo HY, Zhu KC, Liu BS, Liu B, Guo L, Zhang M, Yang JW, Jiang SG, Zhang DC (2021) Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat Toxicol 240:105969. https://doi.org/10.1016/j.aquatox.2021.105969
Livingstone DR (2001) Contaminant stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 8:656–666. https://doi.org/10.1016/s0025-326x(01)00060-1
Long SM, Tull DL, De Souza DP, Kouremenos KA, Dayalan S, McConville MJ, Hassel K, Pettigrove KJ, Gagnon MM (2020) Metabolomics provide sensitive insights into the impacts of low level environmental contamination on fish health—a pilot study. Metabolites 10(1):24. https://doi.org/10.3390/metabo10010024
Luckenbach T, Fischer S, Sturm A (2014) Current advances on ABC drug transporters in fish. Comp Biochem Physiol c: Toxicol Pharmacol 165:28–52. https://doi.org/10.1016/j.cbpc.2014.05.002
Łuczyńskaa J, Paszczyka B, Łuczyński MJ (2018) Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol Environ Saf 153:60–67. https://doi.org/10.1016/j.ecoenv.2018.01.057
Luu I, Ikert H, Craig PM (2021) Chronic exposure to anthropogenic and climate related stressors alters transcriptional responses in the liver of zebrafish (Danio rerio) across multiple generations. Comp Biochem Physiol Part C: Toxicol Pharmacol 240:108918. https://doi.org/10.1016/j.cbpc.2020.108918
Madureira TV, Rocha MJ, Cruzeiro C, Rodrigues I, Monteiro RA, Rocha E (2012) The toxicity potential of pharmaceuticals found in the Douro River estuary (Portugal): evaluation of impacts on fish liver, by histopathology, stereology, vitellogenin and CYP1A immunohistochemistry, after sub-acute exposures of the zebrafish model. Environ Toxicol Pharmacol 34(1):34–45. https://doi.org/10.1016/j.etap.2012.02.007
Mahboob S, Al-Ghanim KA, Al-Balawi HF, Al-Misned F, Ahmed Z (2020) Toxicological effects of heavy metals on histological alterations in various organs in Nile tilapia (Oreochromis niloticus) from freshwater reservoir. J King Saud Univ – Sci 32:970–973. https://doi.org/10.1016/j.jksus.2019.07.004
Mahjoubian M, Naeemi AS, Sheykhan M (2021) Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on zebrafish (Danio rerio). Chemosphere 263:128182. https://doi.org/10.1016/j.chemosphere.2020.128182
Marcogliese DJ, Blaise C, Cyr D, de Lafontaine Y, Fournier M, Gagné F, Gagnon C, Houdon C (2015) Effects of a major municipal effluent on the St. Lawrence River: a case study. Ambio 44:257–274. https://doi.org/10.1007/s13280-014-0577-9
Maria VL, Ahmad I, Oliveira M, Serafim A, Bebianno MJ, Pacheco M, Santos MA (2009) Wild juvenile Dicentrarchus labrax L. liver antioxidant and damage responses at Alveiro Lagoon, Portugal. Ecotoxicol Environ Saf 72:1861–1870. https://doi.org/10.1016/j.ecoenv.2009.06.001
Mauerwerk MT, Wolkweis Zadinelo I, Zanella MC Jr, Balen RE, Bombardelli RA, Rosada Silva LC, Denados Santos L, Meurer F (2021) Glycerol effects on silver catfish (Rhamdia quelen) fingerling feeding: Morphometric, zootechnical and blood parameters. Aquaculture 535:736361. https://doi.org/10.1016/j.aquaculture.2021.736361
McCallum ES, Nikel KE, Mehdi H, Du NN, Bowman JE, Midwood JD, Kidd KA, Scott GR, Balshine S (2019) Municipal wastewater effluent affects fish communities: a multi-year study involving two wastewater treatment plants. Environ Pollut 252:1730–1741. https://doi.org/10.1016/j.envpol.2019.06.075
Meador JP, Bettcher LF, Ellenberger MC, Senn TD (2020) Metabolomic profiling for juvenile Chinook salmon exposed to contaminants of emerging concern. Sci Total Environ 747:141097. https://doi.org/10.1016/j.scitotenv.2020.141097
Mehdi H, Lau SC, Synyshyn C, Salena MG, McCallum ES, Muzzatti MN, Bowman JE, Mataya K, Bragg LM, Servos MR, Kidd KA, Scott GR, Balshine S (2021) Municipal wastewater as an ecological trap: effects on fish communities across seasons. Sci Total Environ 759:143430. https://doi.org/10.1016/j.scitotenv.2020.143430
Mikaelian I, De Lafontaine Y, Harshbarger JC, Lee LLJ, Martineau D (2002) Health of lake whitefish (Coregonus clupeaformis) with elevated tissue levels of environmental contaminants. Environ Toxicol Chem 21(3):532–541. https://doi.org/10.1002/etc.5620210310
Minarik TA, Vick JA, Schultz MM, Bartell SE, Martinovic-Weigelt D, Rearick DC, Schoenfuss HL (2014) On-site exposure to treated wastewater effluent has subtle effects on male fathead minnows and pronounced effects on carp. J Am Water Resour Assoc 50(2):358–375. https://doi.org/10.1111/jawr.12167
Mohamed AS, Gad NS, El Desoky M (2019) Liver enzyme activity of Tilapia zillii and Mugil capito collected seasonally from Qarun Lake, Egypt. Fisheries and Aquaculture Journal 10(1). https://doi.org/10.35248/2150-3508.19.10.265
Montenegro D, González MT, Hickey T, Rahnama M, Green S, Lear G (2022) Assessing integrated biomarkers of triplefin fish Forsterygion capito inhabiting contaminated marine water—a multivariate approach. Chemosphere 288(2):132590. https://doi.org/10.1016/j.chemosphere.2021.132590
Mottaleb MA, Usenko S, O’Donnell JG, Ramirez AJ, Brooks BW, Chambliss CK (2009) Gas chromatography–mass spectrometry screening methods for select UV filters, synthetic musks, alkylphenols, an antimicrobial agent, and an insect repellent in fish. J Chromatogr A 1216(5):815–823. https://doi.org/10.1016/j.envint.2016.04.026
Mottaleb MA, Osemwengie LI, Islam MR, Sovocool GW (2012) Identification of bound nitro musk-protein adducts in fish liver by gas chromatography–mass spectrometry: Biotransformation, dose–response and toxicokinetics of nitro musk metabolites protein adducts in trout liver as biomarkers of exposure. Aquat Toxicol 106:164–172. https://doi.org/10.1016/j.aquatox.2011.11.010
Mottaleb MA, Bellamy MK, Mottaleb MA, Islam MR (2015) Use of LC-MS and GC-MS Methods to measure emerging contaminants pharmaceutical and personal care products (PPCPs) in fish. J Chromatogr Sep Tech 6(3):1. https://doi.org/10.4172/2157-7064.1000267
Moutou KA, Burke MD, Houliha DF (1998) Hepatic P450 monooxygenase response in rainbow trout (Oncorhynchus mykiss (Walbaum)) administered aquaculture antibiotics. Fish Physiol Biochem 18:97–106. https://doi.org/10.1023/A:1007765227501
Munkittrick KR, van der Kraak GJ, McMaster ME, Portt CB, van den Heuvel MR, Servos MR (1994) Survey of receiving-water environment impacts associated with discharges from pulp mills. 2. Gonad size, liver size, hepatic EROD activity and plasma sex steroid levels in white sucker. Environ Toxicol Chem 13:1089–1101. https://doi.org/10.1002/etc.5620130710
Mustafa SA, Davies SJ, Jha AN (2012) Determination of hypoxia and dietary copper mediated sub-lethal toxicity in carp, Cyprinus carpio, at different levels of biological organization. Chemosphere 87(4):413–422. https://doi.org/10.1016/j.chemosphere.2011.12.037
Myers MS, Willis ML, Husøy AM, Goksøyr A, Collier TK (1995) Immunohistochemical localization of cytochrome P4501A in multiple types of contaminant-associated hepatic lesions in English sole (Pleuronectes vetulus). Mar Environ Res 39(1–4):283–288. https://doi.org/10.1016/0141-1136(94)00059-x
Naz S, Hussain R, Ullah Q, Chatha AMM, Shaheen A, Khan RU (2021) Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ Sci Pollut Res 28:6533–6539. https://doi.org/10.1007/s11356-020-10980-0
Neale PA, Jämting ÅK, Escher BI, Hermann J (2013) A review of the detection, fate and effects of engineered nanomaterials in wastewater treatment plants. Water Sci Technol. https://doi.org/10.2166/wst.2013.388
Nilsen BM, Berg K, Goksøyr A (1998) Induction of cytochrome P450 1A (CYP1A) in fish. In: Phillips IR, Shephard EA (eds) Cytochrome P450 protocols, Methods in molecular biology. Humana Press, Totowa, NJ, USA, pp 423–438
O’Malley E, O’Brien JW, Verhagen R, Mueller JF (2020) Annual release of selected UV filters via effluent from wastewater treatment plants in Australia. Chemosphere 247:125887. https://doi.org/10.1016/j.chemosphere.2020.125887
OECD (2019) Pharmaceutical residues in freshwater: hazards and policy responses. OECD Studies on Water, OECD Publishing, Paris. https://doi.org/10.1787/c936f42d-en
Ogueji E, Nwani C, Mbah C, Iheanacho S, Nweke F (2020) Oxidative stress, biochemical, lipid peroxidation, and antioxidant responses in Clarias gariepinus exposed to acute concentrations of ivermectin. Environ Sci Pollut Res 27:16806–16815. https://doi.org/10.1007/s11356-019-07035-4
Okihiro MS, Hinton DE (1999) Progression of hepatic neoplasia in medaka (Oryzias latipes) exposed to diethylnitrosamine. Carcinogenesis 20(6):933–940. https://doi.org/10.1093/carcin/20.6.933
Oliveira HHP, Liebel S, Rossi SC, Azevedo ACB, Barrera EAL, Garcia JRE, Grötzner SR, Neto FF, Randi MAF, Ribeiro CAO (2015) Mixtures of benzo(a)pyrene, dichlorodiphenyltrichloroethane and tributyltin are more toxic to neotropical fish Rhamdia quelen than isolated exposures. Ecotoxicol Environ Saf 122:106–115. https://doi.org/10.1016/j.ecoenv.2015.07.023
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2013) Risk assessment and toxic effects of metal pollution in two cultured and wild fish species from highly degraded aquatic habitats. Arch Environ Contam Toxicol 65:753–764. https://doi.org/10.1007/s00244-013-9935-z
Ondarza PM, Haddad SP, Avigliano E, Miglioranza KS, Brooks BW (2019) Pharmaceuticals, illicit drugs and their metabolites in fish from Argentina: implications for protected areas influenced by urbanization. Sci Total Environ 649:1029–1037. https://doi.org/10.1016/j.scitotenv.2018.08.383
Orata F, Birgen F (2016) Fish tissue bio-concentration and interspecies uptake of heavy metals from waste water lagoons. J Pollut Effects Control 4:1000157. https://doi.org/10.4172/2375-4397.1000157
Otto DME, Moon TW (1996) Endogenous antioxidant systems of two teleost fish, the rainbow trout and the black bullhead, and the effect of age. Fish Physiol Biochem 15:349–358. https://doi.org/10.1007/BF02112362
Overturf MD, Anderson JC, Pandelides Z, Beyger L, Holdway DA (2015) Pharmaceuticals and personal care products: a critical review of the impacts on fish reproduction. Crit Rev Toxicol 45(6):469–491. https://doi.org/10.3109/10408444.2015.1038499
Palace VP, Dick TA, Brown SB, Baron CL, Klaverkamp JF (1996) Oxidative stress in Lake sturgeon (Acipenser fulvescens) orally exposed to 2,3,7,8-tetrachlorodibenzofuran. Aquat Toxicol 35:79–92. https://doi.org/10.1016/0166-445X(95)00051-5
Papa M, Ceretti E, Viola GCV, Feretti D, Zerbini I, Mazzoleni G, Steimberg N, Pedrazzani R, Bertanza G (2016) The assessment of WWTP performance: towards a jigsaw puzzle evaluation. Chemosphere 145:291–300. https://doi.org/10.1016/j.chemosphere.2015.11.054
Parrott JL, McMaster ME, Hewitt LM (2007) A decade of research on the environmental impacts of pulp and paper mill effluents in Canada: development and application of fish bioassays. Toxicol Environ Health, Part B 9(4):297–317. https://doi.org/10.1080/15287390500195752
Pemberthy D, Padilla Y, Echeverri A, Peñuela GA (2020) Monitoring pharmaceuticals and personal care products in water and fish from the Gulf of Urabá. Colombia. Heliyon 6(6):e04215. https://doi.org/10.1016/j.heliyon.2020.e04215
Peters N, Köhler A, Kranz H (1987) Liver Pathology in fishes from the lower Elbe as a consequence of pollution. Dis Aquat Org 2(2):87–97
Pi J, Li X, Zhang T, Li. (2016) Effects of acute exposure to sublethal waterborne cadmium on energy homeostasis in silver carp (Hypophthalmichthys molitrix). Bull Environ Contam Toxicol 97(4):497–503. https://doi.org/10.1007/s00128-016-1896-2
Puttipong T, Senarat S, Kettratad J, Chantangsi C, Kaneko G, Siriwong W (2020) Evaluation of health status of the striped catfish Pangasianodon hypophthalmus (Sauvage, 1878) from Khlong Saen Saep, Thailand: the use of integrated biomarkers. Hum Ecol Risk Assess: An Int J. https://doi.org/10.1080/10807039.2020.1789839
Quattrochi LC, Guzelian PS (2001) CYP3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos: The Biol Fate Chem 29(5):615–622
Rainbow PS (1990) Heavy metal levels in the marine invertebrates, Chapter 5 in Heavy metals in the marine environment, Furness RW, Rainbow PS (Ed.), Rainbow PS, 1 edition, CRC Press Taylor & Francis Group, Boca Raton, US
Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JP, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK (2009) Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environ Toxicol Chem 28(12):2587–2597. https://doi.org/10.1897/08-561.1
Restivo VE, Kidd KA, Surette MG, Servos MR, Wilson JY (2021) Rainbow darter (Etheostoma caeruleum) from a river impacted by municipal wastewater effluents have altered gut content microbiomes. Sci Total Environ 10(751):141724. https://doi.org/10.1016/j.scitotenv.2020.141724
Ribecco C, Baker ME, Šášik R, Zuo Y, Hardiman G, Carnevali O (2011) Biological effects of marine contaminated sediments on Sparus aurata juveniles. Aquat Toxicol 104(3–4):308–316. https://doi.org/10.1016/j.aquatox.2011.05.005
Ribeiro RX, da Brito Silva R, Canedo Pereira A, Borges K, Monteiro KBS, Bastos Gonçalves B, Lopes Rocha T (2020) Ecotoxicological assessment of effluents from Brazilian wastewater treatment plants using zebrafish embryotoxicity test: a multi-biomarker approach. Sci Total Environ 735:139036. https://doi.org/10.1016/j.scitotenv.2020.139036
Roberts J, Kumar A, Du J, Hepplewhite C, Ellis DJ, Christy AG, Beavis SG (2016) Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci Total Environ 541:1625–1637. https://doi.org/10.1016/j.scitotenv.2015.03.145
Rochman CM, Hentschel BT, Teh SJ (2014) The long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. Plos One 9:e85433. https://doi.org/10.1371/journal.pone.0085433
Rojo M, Cristos D, González P, López-Aca V, Dománico A, Carriquiriborde P (2021) Accumulation of human pharmaceuticals and activity of biotransformation enzymes in fish from two areas of the lower Rio de la Plata Basin. Chemosphere 266:129012. https://doi.org/10.1016/j.chemosphere.2020.129012
Roy S, Bhattacharya S (2006) Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol Environ Saf 65(2):218–229. https://doi.org/10.1016/j.ecoenv.2005.07.005
Rubalingeswari N, Thulasimala D, Giridharan L, Gopal V, Magesh NS, Jayaprakash M (2021) Bioaccumulation of heavy metals in water, sediment, and tissues of major fisheries from Dyar estuary, Southeast Coast of India: an ecotoxicological impact of a metropolitan city. Mar Pollut Bull 163:111964. https://doi.org/10.1016/j.marpolbul.2020.111964
Rudneva II, Rudyk MP, Shepelevich VV, Skivka LM, Roslova NN, Shaida VG (2020) Comparative study of histopathological and biochemical biomarkers of two black sea marine fish species, belonging to different ecological groups. In: Marine Environments: Diversity, Threats and Conservation. Chapter 9. Charles L (ed.), (Series: Marine and Freshwater Biology). Nova Science Publishers, Inc, pp. 429–464
Rui L (2014) Energy Metabolism in the Liver. Comp Physiol 4(1):177–197. https://doi.org/10.1002/cphy.c130024
Samanta P, Im NJ, Jung J (2018) Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus. Environ Pollut 233:429–438. https://doi.org/10.1016/j.envpol.2017.10.061
Sancho E, Villaroel MJ, Fernandez C, Andrew E, Ferrando MD (2010) Short-term exposure to sublethal tebuconazole induces physiological impairment in male zebrafish (Danio rerio). Ecotoxicol Environ Saf 73:370–376. https://doi.org/10.1016/j.ecoenv.2009.09.020
Santos C, Bueno dos Reis Martinez C (2021) Multixenobiotic resistance mechanism: organ-specific characteristics in the fish Prochilodus lineatus and its role as a protection against genotoxic agents. Comp Biochem Physiol Part C: Toxicol Pharmacol 243:108996. https://doi.org/10.1016/j.cbpc.2021.108996
Sarasquete C, Segner M (2000) Cytochrome P4501A (CYP1A) in teleostean fishes. a review of immunohistochemical studies. Sci Total Environ 247:313–332. https://doi.org/10.1016/s0048-9697(99)00500-8
Sauvé S, Desrosiers M (2014) A review of what is an emerging contaminant. Chem Cent J 8:15. https://doi.org/10.1186/1752-153X-8-15
Schmidt-Posthaus H, Bernet D, Wahli T, Burkhardt-Holm P (2001) Morphological organ alterations and infections diseases in brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss exposed to polluted river water. Dis Aquat Org 44(3):161–170. https://doi.org/10.3354/dao044161
Schnell S, Bols NC, Barata C, Porte C (2009) Single and combined toxicity of pharmaceuticals and personal care products (PPCPs) on the rainbow trout liver cell line RTL-W1. Aquat Toxicol 93(4):244–252. https://doi.org/10.1016/j.aquatox.2009.05.007
Schreiber EA, Otter RR, Van Der Hurk P (2006) A biomarker approach to measure biological effects of contaminant exposure in largemouth bass from Lake Conestee, South Carolina, USA. Environ Toxicol Chem 25:1926–1932. https://doi.org/10.1897/05-589R.1
Schujit LM, Peng FJ, van den Berg SJP, Dingemans MML, Van den Brink PJ (2021) (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: facts, challenges, and future. Sci Total Environ 795:148776. https://doi.org/10.1016/j.scitotenv.2021.148776
Simó-Mirabet P, Perera E, Calduch-Giner JA, Afonso JM, Pérez-Sánchez J. (2018) Co-expression analysis of sirtuins and related metabolic biomarkers in juveniles of gilthead sea bream (Sparus aurata) with differences in growth performance. Front Physiol 9. https://doi.org/10.3389/fphys.2018.00608
Sloof W, van Kreijl CF, Baars AJ (1983) Relative liver weights and xenobiotic-metabolizing enzymes of fish from polluted surface waters in the Netherlands. Aquat Toxicol 4:1–4. https://doi.org/10.1016/0166-445X(83)90057-7
Smital T, Ahel M (2014) Ecotoxicological characterization of the Sava River: biomarker responses and biological assays. Sava River Handb Environ Chem 31:177–200. https://doi.org/10.1007/978-3-662-44034-6_8
Soler P, Solé M, Bañón R, García-Galea E, Mercè D, Matamoros V, Bayona JM, Vinyoles D (2020) Effects of industrial pollution on the reproductive biology of Squalius laietanus (Actinopterygii, Cyprinidae) in a Mediterranean stream (NE Iberian Peninsula). Fish Bhysiol Biochem 46:247–264. https://doi.org/10.1007/s10695-019-00713-7
Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS (2000) Neoplasia in zebrafish (Danio rerio) treated with n-methyl-n’nitro-n-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol Pathol 28(5):716–725. https://doi.org/10.1177/019262330002800512
Spry DJ, Wiener JG (1991) Metal bioavailability and toxicity to fish in low-alkalinity lakes—a critical review. Environ Pollut 71:243–244. https://doi.org/10.1016/0269-7491(91)90034-t
Stankovic S, Kalaba P, Stankovic RA (2014) Biota as toxic metal indicators. Environ Chem Lett 12:63–84. https://doi.org/10.1007/s10311-013-0430-6
Stegeman JJ (1995) Diversity and regulation of cytochromes P450 in aquatic species. In: ArincSchenkman EJB, Hodgson E (eds) Molecular aspects of oxydative drug metabolizing enzymes, vol 90. NATO ASI Series. Springer-Verlag Berlin, Heidelberg, pp 135–152
Stegeman JJ, Brouwer M, Richard TDG, Förlin L, Fowler BA, Sanders BM, van Veld PA (1992) Molecular responses to environmental contamination: enzyme and protein systems as indicators of chemical exposure and effect. In: Huggett RJ, Kimerly RA, Mehrle PM, Bergman HL (eds) Biomarkers: Biochemical, Physiological and Histological markers of Anthropogenic Stress. Lewis Publishers, Chelsea, MI, USA, pp 235–335
Stoskopf MK (1993) Fish histology. In: Stoskopf MK (ed) Fish Medicine, 1st edn. W.B. Saunders Company, Philadelphia, PA, USA, pp 31–47
Subburaj A, Jawahar P, Jayakumar N, Srinivasan A, Ahilan B (2020) Effects of acute toxicity of chlorpyrifos (ec 50%) and associated histological alterations in gills, liver and kidney of mozambique tilapia, Oreochromis mossambicus (Peters, 1852). Indian J Anim Res 54:12. https://doi.org/10.18805/ijar.B-3876
Tabrez S, Zughaibi TA, Javed M (2021) Bioaccumulation of heavy metals and their toxicity assessment in Mystus species. Saudi Journal of Biological Sciences 28(2):1459–1464. https://doi.org/10.1016/j.sjbs.2020.11.085
Tanoue R, Nomiyama K, Nakamura H, Hayashi T, Kim JW, Isobe T, Shinohara R, Tanabe S (2014) Simultaneous determination of polar pharmaceuticals and personal care products in biological organs and tissues. J Chromatogr A 1355:193–205. https://doi.org/10.1016/j.chroma.2014.06.016
Teh SJ, Adams SM, Hinton DE (1997) Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquat Toxicol 37(1):51–70. https://doi.org/10.1016/S0166-445X(96)00808-9
Tenji D, Micic B, Sipos S, Miljanovic B, Teodorovic I, Kaisarevic S (2020) Fish biomarkers from a different perspective: evidence of adaptive strategy of Abramis brama (L.) to chemical stress. Environ Sci Eur 32:1. https://doi.org/10.1186/s12302-020-00316-7
Thibaut R, Schnell S, Porte C (2006) The interference of pharmaceuticals with endogenous and xenobiotic metabolizing enzymes in carp liver: An in-vitro study. Environmental Science & Technology 40(16):5154–5160. https://doi.org/10.1021/es0607483
Thiyagarajah A, Hartley WR, Abdelghani A (1998) Hepatic hemosiderosis in buffalo fish (Ictiobus spp.). Mar Environ Res 46(l–5):203–207. https://doi.org/10.1016/S0141-1136(98)00015-4
Tomasello B, Copat C, Pulvirenti V, Ferrito V, Ferrante M, Renis M, Sciacca S, Tigano C (2012) Biochemical and bioaccumulation approaches for investigating marine pollution using Mediterranean rainbow wrasse, Coris julis (Linneaus 1798). Ecotoxicol Environ Saf 86:168–175
Topić Popović N, Hacmanjek M, Teskeredžić E (2001) Health status of rudd (Scardinius erythrophthalmus hesperidicus H.) in Lake Vrana on the Island of Cres, Croatia. J Appl Ichthyol 17:43–45. https://doi.org/10.1046/j.1439-0426.2001.00236.x
Topić Popović N, Babish JG, Bowser PR (2007) Observational study of hepatic cytochrome P-450 protein expression and activity in summer flounder (Paralychtys dentatus) after combination ormetoprim-sulfadimethoxine treatment. Chemotherapy 53:313–315. https://doi.org/10.1159/000107689
Topić Popović N, Howell T, Babish JG, Bowser PR (2012) Cross-sectional study of hepatic CYP1A and CYP3A enzymes in hybrid striped bass, channel catfish and Nile tilapia following oxytetracycline treatment. Res Vet Sci 92(2):283–291. https://doi.org/10.1016/j.rvsc.2011.03.003
Topić Popović N, Sauerborn Klobučar R, Strunjak-Perović I, Jadan M, Barišić J, Čož-Rakovac R (2015a) Piscine cytochromes P450 (CYP) and their response to antimicrobial drugs. Aquac Res 46:257–271. https://doi.org/10.1111/are.12197
Topić Popović N, Strunjak-Perović I, Sauerborn Klobučar R, Barišić J, Babić S, Jadan M, Kepec S, Kazazić S, Matijatko V, Beer Ljubić B, Car I, Repec S, Stipaničev D, Klobučar G, Čož-Rakovac R (2015b) Impact of treated wastewater on organismic biosensors at various levels of biological organization. Sci Total Environ 538:23–37. https://doi.org/10.1016/j.scitotenv.2015.08.044
Topić Popović N, Strunjak-Perović I, Barišić J, Kepec S, Jadan M, Beer-Ljubić B, Matijatko V, Palić D, Klobučar G, Babić S, Gajdoš Kljusurić J, Čož-Rakovac R (2016) Native Prussian carp (Carassius gibelio) health status, biochemical and histological responses to treated wastewaters. Environ Pollut 218:689–701. https://doi.org/10.1016/j.envpol.2016.07.063
Tran NH, Reinhard M, Gin KYH (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207. https://doi.org/10.1016/j.watres.2017.12.029
Turner BL, Kasperson RE, Matson PA, McCarthy JJ, Corell RW, Christensen L, Eckley N, Kasperson JX, Luers A, Martello ML, Polsky C, Pulsipher A, Schillerb A (2003) A framework for vulnerability analysis in sustainability science. PNAS 100(14):8074–8079. https://doi.org/10.1073/pnas.1231335100
Uno T, Ishizuka M, Itakura T (2012) Cytochrome P450 (CYP) in fish. Environ Toxicol Pharmacol 34:1–13. https://doi.org/10.1016/j.etap.2012.02.004
Uysal K (2011) Heavy metal in edible portions (muscle and skin) and other organs (gill, liver and intestine) of selected freshwater fish species. Int J Food Prop 14:280–286. https://doi.org/10.1080/10942910903176378
Vali S, Mohammadi G, Tavabe KR, Moghadas F, Naserabad SS (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxicol Environ Saf 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
van der Oost R, Vindimian E, van den Brink P, Satumalay K, Heida H, Vermeulen NPE (1997) Biomonitoring aquatic pollution with feral eel (Anguilla anguilla): III. Statistical analyses of relationships between contaminant exposure and biomarkers. Aquat Toxicol 39:45–75. https://doi.org/10.1016/S0166-445X(96)00851-X
van der Oost R, Lopes SCC, Komen H, Satumalay K, van den Bos R, Heida H, Vermeulen NPE (1998) Assessment of environmental quality and inland water pollution using biomarker responses in caged carp (Cyprinus carpio); use of a bioactivation:- detoxication ratio as biotransformation index (BTI). Mar Environ Res 46:315–319. https://doi.org/10.1016/S0141-1136(97)00096-2
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/s1382-6689(02)00126-6
van Dyk JC (2013) Cholangioma in the Mozambique tilapia, Oreochromis mossambicus (Peters). J Fish Dis 37(9):847–851. https://doi.org/10.1111/jfd.12176
Vethaak AD, Jol JG, Meijboom A, Eggens ML, Rheinallt T, Wester PW, van de Zande T, Bergman A, Dankers N, Ariese F, Baan RA, Everts JM, Opperhuizen A, Marquenie JM (1996) Skin and liver diseases induced in flounder (Platichthys flesus) after long-term exposure to contaminated sediments in large-scale mesocosms. Environ Health Perspect 104(11):1218–1229. https://doi.org/10.1289/ehp.961041218
Vieira CED, Costa PG, Caldas SS, Tesser ME, Risso WE, Venquiaruti Escarrone AL, Primel EG, Bianchini A, dos Reis Martinez CB (2019) An integrated approach in subtropical agro-ecosystems: active biomonitoring, environmental contaminants, bioaccumulation, and multiple biomarkers in fish. Sci Total Environ 666:508–524. https://doi.org/10.1016/j.scitotenv.2019.02.209
Vieira CED, Marques JA, da Silva NG, Ziviani Bevitório L, Zebral YD, Maraschi AC, Rutz Costa S, Gomes Costa P, Medeiros Damasceno E, Castro Monteiro Pirovani J, do Vale-Oliveira M, Marques Souza M, de Martinez Gaspar Martins C, Bianchini A, Zomer Sandrini J (2022) Ecotoxicological impacts of the Fundão dam failure in freshwater fish community: metal bioaccumulation, biochemical, genetic and histopathological effects. Sci Total Environ 832:154878. https://doi.org/10.1016/j.scitotenv.2022.154878
Vieno NM, Tuhkanen T, Kronberg L (2005) Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ Sci Technol 39(21):8220–8226. https://doi.org/10.1021/es051124k
Vosyliene MZ, Kazlauskiene N, Svecevičius G (2003) Effect of a heavy metal model mixture on biological parameters of rainbow trout Oncorhynchus mykiss. Environ Sci Pollut Res 10(2):103–107. https://doi.org/10.1065/espr2002.02.109
Wagenaar GM, Barnhoorn IEJ (2018) Health and chemical burdens of fish species from polluted and hypereutrophic freshwater ecosystems in South Africa. Afr J Aquat Sci 43(3):271–280. https://doi.org/10.2989/16085914.2018.1490245
Wang T, Wei X, Chen T, Wang W, Xia X, Miao J, Yin S (2019) Studies of the mechanism of fatty liver formation in Takifugu fasciatus following copper exposure. Ecotoxicol Environ Saf 181:353–361. https://doi.org/10.1016/j.ecoenv.2019.06.013
Wang H, Feng Y, Ming M, Song J, Chen Z, Xiao Z (2022) Amelioration of Cd-induced bioaccumulation, hematological parameters, and heat shock protein-related genes by vitamin C on common carp. Comp Biochem Physiol Part C: Toxicol Pharmacol 258:109362. https://doi.org/10.1016/j.cbpc.2022.109362
Ward DG, Wei W, Cheng Y, Billingham LJ, Martin A, Johnson PJ, Lyons BP, Feist SW, Stentiford GD (2006) Plasma proteome analysis reveals the geographical origin and liver tumor status of dab (Limanda limanda) from UK marine waters. Environ Sci Technol 40(12):4031–4036. https://doi.org/10.1021/es052436q
Weber AA, Sales CF, de Souza Faria F, Melo RMC, Bazzoli N, Rizzo E (2020) Effects of metal contamination on liver in two fish species from a highly impacted neotropical river: a case study of the Fundão dam, Brazil. Ecotoxicol Environ Saf 190:110165. https://doi.org/10.1016/j.ecoenv.2020.110165
Weinrauch AM, Folkerts DJ, Alessi DS, Goss GG, Blewett TA (2021) Changes to hepatic nutrient dynamics and energetics in rainbow trout (Oncorhynchus mykiss) following exposure to and recovery from hydraulic fracturing flowback and produced water. Sci Total Environ 764:142893. https://doi.org/10.1016/j.scitotenv.2020.142893
Wigh A, Ai-Aissa S, Creusot N, Terrisse H, Muller MLD, Bergé A, Emmanuelle V, Domenjoud B, Gonzalez-Ospina A, Brosselin V, Devaux A, Bony S (2018) Assessment of ozone or not-treated wastewater ecotoxicity using mechanism based and zebrafish embryo bioassays. J Environ Prot 9:325–346. https://doi.org/10.4236/jep.2018.94022
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161. https://doi.org/10.1016/0166-445X(91)90033-6
Worms IAM, Al-Gorani Szigeti Z, Dubascoux S, Lespes G, Traber J, Sigg L, Slaveykova VI (2010) Colloidal organic matter from wastewater treatment plant effluents: characterization and role in metal distribution. Water Res 44(1):340–350. https://doi.org/10.1016/j.watres.2009.09.037
Yamauchi R, Ishibashi H, Hirano M, Mori T, Kim JW, Arizono K (2008) Effects of synthetic polycyclic musks on estrogen receptor, vitellogenin, pregnane X receptor, and cytochrome P450 3A gene expression in the livers of male medaka (Oryzias latipes). Aquat Toxicol 90(4):261–268. https://doi.org/10.1016/j.aquatox.2008.09.007
Younas W, Khan FU, Zaman M, Lin D, Zuberi A, Wang Y (2022) Toxicity of synthesized silver nanoparticles in a widespread fish: a comparison between green and chemical. Sci Total Environ 8451:57366. https://doi.org/10.1016/j.scitotenv.2022.157366
Yu Z, Xu SF, Zhao JL, Zhao L, Zhang AZ, Li MY (2021) Toxic effects of hexavalent chromium (Cr6+) on bioaccumulation, apoptosis, oxidative damage and inflammatory response in Channa asiatica. Environ Toxicol Pharmacol 87:103725. https://doi.org/10.1016/j.etap.2021.103725
Žaja R, Klobučar RS, Smital T (2007) Detection and functional characterization of Pgp1 (ABCB1) and MRP3 (ABCC3) efflux transporters in the PLHC-1 fish hepatoma cell line. Aquat Toxicol 81(4):365–376. https://doi.org/10.1016/j.aquatox.2006.12.015
Zhang Y, Shang Z, Wang G, You K, Mi D (2021) High concentrations of environmental ammonia induced changes in large-scale loach (Paramisgurnus dabryanus ) immunity. Ecol Evol 11(13):8614–8622. https://doi.org/10.1002/ece3.7675
Zuasti A, Jara JR, Ferrer C, Solano F (1989) Occurrence of melanin granules and melano synthesis in the kidney of Sparus auratus. Pigment Cell Res 2:93–99. https://doi.org/10.1111/j.1600-0749.1989.tb00168.x
Acknowledgements
This research was supported by the Scientific Centre of Excellence for Marine Bioprospecting—BioProCro, a project co-financed by the Croatian Government and the European Union through the European Regional Development Fund—the Competitiveness and Cohesion Operational Programme (KK.01.1.1.01). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Topić Popović Natalija]; writing—original draft preparation: [Topić Popović Natalija, Čižmek Lara, Babić Sanja]; writing—review and editing: [Topić Popović Natalija, Čižmek Lara, Babić Sanja, Strunjak-Perović Ivančica, and Čož-Rakovac Rozelindra]; literature search and data analysis: [Topić Popović Natalija, Čižmek Lara, Babić Sanja].
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Topić Popović, N., Čižmek, L., Babić, S. et al. Fish liver damage related to the wastewater treatment plant effluents. Environ Sci Pollut Res 30, 48739–48768 (2023). https://doi.org/10.1007/s11356-023-26187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26187-y