Abstract
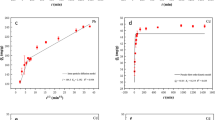
The cheap and efficient heavy metal adsorbents were developed with the forestry and agricultural residues. In this study, three kinds of adsorbents, original ginkgo leaf (GL), NaOH modified ginkgo leaf (NaOH-GL), and KMnO4-modified ginkgo leaf (KMnO4-GL), were prepared and used to adsorb Cd (II) in aqueous solution respectively. The effects of the concentration of Cd (II), absorption time, the dosage of absorbent, and pH of solution on the adsorption process were explored by adsorption experiments. The results showed that the maximum adsorption capacity of Cd (II) by GL, NaOH-GL, and KMnO4-GL was 10.20 mg/g, 39.99 mg/g, and 48.82 mg/g, respectively, under the conditions of room temperature, adsorbent dosage 1g/L, adsorption time 300 min, and pH 6.0. The adsorption of Cd (II) by the three adsorbents accorded with the pseudo-second-order kinetic model and Langmuir isothermal adsorption model, which indicated that the rate-limiting step in the adsorption process was chemical adsorption process and mainly monolayer adsorption. The reason why NaOH-GL and KMnO4-GL could effectively adsorb Cd (II) was that the surface of the modified adsorbent was rough and porous, the number of active groups on the surface increased, and Na and Mn elements could promote the precipitation of Cd (II). The mechanism analysis of KMnO4-GL, which had the best adsorption effect, showed that the adsorption mechanism of Cd (II) might be surface adsorption, ion exchange, and complexing precipitation.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this manuscript.
References
Awwad AM, Salem NM (2014) Kinetics and thermodynamics of Cd(II) biosorption onto loquat (Eriobotrya japonica) leaves. J Saudi Chem Soc 18:486–493
Bulut Y, Tez Z (2007) Adsorption studies on ground shells of hazelnut and almond. J Hazard Mater 149:35–41
Chen, J., Hong, L., Wu, S., and Wang, L. (2002). Elucidation of interactions between metal ions and ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir 18.
Chun C, Shengli W, Jingyang G, Tianqi L, Yingying Z (2016) Adsorption kinetics and mechanism of copper ion on biochar with different pyrolysis condition. Acta Sci Circumst 36:2491–2502
Da P, Chongchen W, Peng W, Huifen F, Chen Z (2020) Efficiently adsorptive removal towards roxarsone with ZIF-67. Environ Chem 39:1451–1463
Donglan H, Jietao Z, Chaofan W, Jianmin L (2016) Preparation of new big banyan leaves absorbent andits adsor ption of Cd2+. J Shaoguan Univ (natural science) 37:42–47
Du X-D, Wang C-C, Zhong J, Liu J-G, Li Y-X, Wang P (2017) Highly efficient removal of Pb2+ by a polyoxomolybdate-based organic-inorganic hybrid material {(4-Hap)4[Mo8O26]}. J Environ Chem Eng 5:1866–1873
Fang J, Wang X, Wang L, Cheng B, Wu Y, Zhu W (2007) Preparation of modified SiO2 colloidal spheres with succinic acid and the assembly of colloidal crystals. Chin Sci Bull 52:461–466
Fang X, Liping Z, Xianzhong C, Xiao W (2016) Study on biosorption performance of Cd ( II ) in waste water by modified mulberry leaves. Rock Min Anal 35:62–68
Fei H, Meng Y, Jianning C, Hongyan C, Haibo Z (2020) Adsorption performance of Cu2+ and Cd2+ in water by different biochars derived from spent mushroom substrate. Environ Chem 39:1116–1128
Huanling W, Sainan W, Shuling C (2006) Introduction and application of adsorption isotherm. Textile Dyeing and Finishing J 12-14:27
Ifthikar J, Wang J, Wang Q, Wang T, Wang H, Khan A, Jawad A, Sun T, Jiao X, Chen Z (2017) Highly efficient lead distribution by magnetic sewage sludge biochar: sorption mechanisms and bench applications. Bioresour Technol 238:399–406
Li X, Wang C, Zhang J, Liu J, Liu B, Chen G (2020) Preparation and application of magnetic biochar in water treatment: a critical review. Science of The Total Environment 711
Lin L, Qiu W, Wang D, Huang Q, Song Z, Chau HW (2017) Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: characterization and mechanism. Ecotoxicol Environ Saf 144:514–521
Lin L, Song Z, Huang Y, Khan ZH, Qiu W (2019) Removal and oxidation of arsenic from aqueous solution by biochar impregnated with Fe-Mn oxides. Water Air Soil Pollut 230:105
Lina D, Ruozhen Y, Haiyan W, Yue L, Zhengtao L (2013) Pollution and toxicity of cadmium: a review of recent studies. J Environ Health 30:167–174
Liu CF, Sun RC, Zhang AP, Ren JL, Geng ZC (2006) Structural and thermal characterization of sugarcane bagasse cellulose succinates prepared in ionic liquid. Polym Degrad Stab 91:3040–3047
Nag S, Mondal A, Roy DN, Bar N, Das SK (2018) Sustainable bioremediation of Cd(II) from aqueous solution using natural waste materials: kinetics, equilibrium, thermodynamics, toxicity studies and GA-ANN hybrid modelling. Environ Technol Innov 11:83–104
Rosales-Cruz P, Dominguez-Perez M, Reyes-Zarate E, Bello-Monroy O, Enriquez-Cortina C, Miranda-Labra R, Bucio L, Gomez-Quiroz LE, Rojas-Del Castillo E, Gutierrez-Ruiz MC, Souza-Arroyo V (2018) Cadmium exposure exacerbates hyperlipidemia in cholesterol-overloaded hepatocytes via autophagy dysregulation. Toxicology 398-399:41–51
Senthilkumar P, Gayathri R (2010) Study on removal of cadmium from aqueous solutions by adsorption on bael tree leaf power. Environ Eng Manag J 9:429–433
Sun C, Chen T, Huang Q, Wang J, Lu S, Yan J (2019) Enhanced adsorption for Pb(II) and Cd(II) of magnetic rice husk biochar by KMnO4 modification. Environ Sci Pollut Res Int 26:8902–8913
Tangjuank S, Insuk N, Tontrakoon J, Udeye V (2009) Adsorption of lead(II) and cadmium(II) ions from aqueous solutions by adsorption on activated carbon prepared from cashew nut shells. Intl J Chem Mol Nucl Mater Metall Eng 3:221–227
Tong Y, McNamara PJ, Mayer BK (2019) Adsorption of organic micropollutants onto biochar: a review of relevant kinetics, mechanisms and equilibrium. Environ Sci Water Res Technol 5:821–838
Usman A, Sallam A, Zhang M, Vithanage M, Ahmad M, Al-Farraj A, Ok YS, Abduljabbar A, Al-Wabel M (2016) Sorption Process of date palm biochar for aqueous Cd (II) removal: efficiency and mechanisms. Water Air Soil Pollut 227
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79:241–244
Wang RZ, Huang DL, Liu YG, Zhang C, Lai C, Zeng GM, Cheng M, Gong XM, Wan J, Luo H (2018) Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour Technol 261:265–271
Wang Y, Liu R (2017) Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process Technol 160:55–63
Wanying W, Diyun C, Xiaofeng Z, Zhumei C, Xiaowen Z, Guangchao Y (2016) Research progress of heavy metal ions-containing wastewater treatment by agricultural and forest wastes. Industr Water Wastew 47:7–11
Wen-wen L, Yingming X, Nong W, Yuebing X (2019) The absorption of Cd (II) on NaOH modified fish bone meal function materials. China Environ Sci 39:3824–3831
Wenxia L, Jiaxin L, Junli W, Xuebin C, Xiangyuan M (2014) Adsorption characteristics of Pb2+ and Cd2+ by modified paulownia Leaf powder from aqueous solution. J Agro-Environ Sci 33:1226–1232
Xiao X, Bo-yu L, Zheng-dong D (2010) Analysis of decisive parameters in activated carbon’s adsorption of heavy metals. Energy Environ Protect 24:48–50
Xiaoqin N, Faqin D, Mingxue L, Mingxue L, Ning L, Wei Z, Xueying Y (2013) Characteristics of U (VI) biosorption by biologicaladsorben of platanus leaves. Spectrosc Spectr Anal 33:1290–1294
Xu LQ, Wan D, Gong HF, Neoh KG, Kang ET, Fu GD (2010) One-pot preparation of ferrocene-functionalized polymer brushes on gold substrates by combined surface-initiated atom transfer radical polymerization and "click chemistry". Langmuir 26:15376–15382
Yang F, Liu H, Qu J, Paul Chen J (2011) Preparation and characterization of chitosan encapsulated Sargassum sp. biosorbent for nickel ions sorption. Bioresour Technol 102:2821–2828
Yanrong C, Yang S, Chun C (2020) Review of the falling leaveas adsorption material to remove the pollutant from waste water. New Chem Mater 48:48–53
Yin G, Bi L, Song X, Luo H, Ji P, Lin Q, Liu Q, Tang G (2019) Adsorption of Cd(II) from aqueous solution by Pennisetum sp. straw biochars derived from different modification methods. Environ Sci Pollut Res Int 26:7024–7032
Zama EF, Zhu Y-G, Reid BJ, Sun G-X (2017) The role of biochar properties in influencing the sorption and desorption of Pb(II), Cd(II) and As(III) in aqueous solution. J Clean Prod 148:127–136
Funding
This work was financially supported by LiaoNing Revitalization Talents Program (XLYC1907173), the Science and Technology General Project of Liaoning Provincial Education Department (LQ2019003, LQ2020014, LJ2020014), the Open Fund of State Environmental Protection Key Laboratory of Coastal Ecosystem (202103), and the Open Fund of Institute of Ocean Research (BDHYYJY 2021006, BDHYYJY2020014).
Author information
Authors and Affiliations
Contributions
YC: writing, methodology, review and editing, supervision. YS: writing original draft, data curation. CC: writing, methodology, review and editing, supervision. All authors had read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cai, Yr., Song, Y. & Chang, C. Adsorption properties and mechanism of ginkgo biloba leaf-based materials for Cd (II) in aqueous solution. Environ Sci Pollut Res 29, 78499–78508 (2022). https://doi.org/10.1007/s11356-022-21310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21310-x