Abstract
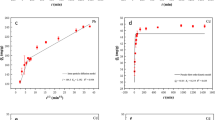
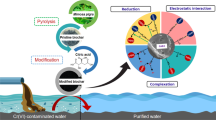
In this study, a novel alkali-modified biochar prepared from ginkgo leaves was produced by simply one-step pyrolysis and was investigated as an adsorbent for the removal of Pb, Cd, and Cu from aqueous solutions. After modified by alkali, ginkgo leaves biochar exhibited abundant surface functional groups and large porosity, resulting in high adsorption capacities for Pb (589.32 mg·g−1), Cd (563.55 mg·g−1), and Cu (81.7 mg·g−1). Simulation results showed that pseudo-second-order model and Langmuir model were better fitted to analyze the heavy metals adsorption process by alkali-BC, indicating that multilayer adsorption was the main reason for heavy metals removal. Besides, effect of pH and coexisting cations on adsorption of Pb, Cd, and Cu by alkali-BC was studied. Moreover, competitive adsorption of the three heavy metal ions by alkali-BC in binary system was also explored. Finally, the removal mechanism of Pb, Cd, and Cu in aqueous solution was analyzed and summarized. Due to the good performance in a wider pH range and high absorption capacity, alkali-BC would be an efficient adsorbent for heavy metals in real environmental samples.






Similar content being viewed by others
References
Subramanian A, Kunisue T, Tanabe S (2015) Recent status of organohalogens, heavy metals and PAHs pollution in specific locations in India. Chemosphere 137:122
D. Xu, R. Fu, H. Liu, et al., Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: a critical review, J. Clean Prod. (2020) 124989.
Jin H, Arazo R, Gao J et al (2014) Leaching of heavy metals from fast pyrolysis residues produced from different particle sizes of sewage sludge. J Anal A ppl Pyrol 109:168
Nanda M, Kumar V, Sharma DK (2019) Multimetal tolerance mechanisms in bacteria: the resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat Toxicol 212:1
Leng L, Xiong Q, Yang L et al (2021) An overview on engineering the surface area and porosity of biochar. Sci Total Environ 763:144204
Singh A, Sharma R, Pant D et al (2021) Engineered algal biochar for contaminant remediation and electrochemical applications. Sci Total Environ 774:145676
Joseph L, Jun B, Flora JRV et al (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere 229:142
Zhang L, Zeng Y, Cheng Z (2016) Removal of heavy metal ions using chitosan and modified chitosan: a review. J Mol Liq 214:175
Gonzalez JM, Boddu VM, Jackson MA et al (2020) Pyrolysis of creosote-treated railroad ties to recover creosote and produce biochar. J Anal Appl Pyrol 149:104826
Kah M, Sigmund G, Xiao F, Hofmann T (2017) Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res 124:673
M.E. Lee, J.H. Park and J.W. Chung, Adsorption of Pb(II) and Cu(II) by ginkgo-leaf-derived biochar produced under various carbonization temperatures and times, Int. J. Environ. Res. Public Health. 14, (2017).
Lee ME, Park JH, Chung JW (2019) Comparison of the lead and copper adsorption capacities of plant source materials and their biochars. J Environ Manage 236:118
Huang W-H, Lee D-J, Huang C (2021) Modification on biochars for applications: a research update. Bioresour Technol 319:124100
Shang H, Li Y, Liu J et al (2020) Preparation of nitrogen doped magnesium oxide modified biochar and its sorption efficiency of lead ions in aqueous solution. Bioresour Technol 314:123708
Yu W, Lian F, Cui G et al (2018) N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193:8
Wu J, Wang T, Wang J et al (2021) A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: enhanced the ion exchange and precipitation capacity. Sci Total Environ 754:142150
Komkiene J, Baltrenaite E (2015) Biochar as adsorbent for removal of heavy metal ions [cadmium (II), copper (II), lead (II), zinc (II)] from aqueous phase. Int J Environ Sci Te 13:471
An Q, Miao Y, Zhao B et al (2020) An alkali modified biochar for enhancing Mn2+ adsorption: performance and chemical mechanism. Mater Chem Phys 248:122895
Aljerf L (2018) High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J Environ Sci Manag 225:120
Jin Q, Wang Z, Feng Y et al (2020) Grape pomace and its secondary waste management: biochar production for a broad range of lead (Pb) removal from water. Environ Res 186:109442
Ding Z, Hu X, Wan Y et al (2016) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. J Ind Eng Chem 33:239
Wang C, Wang H, Cao Y (2018) Pb(II) sorption by biochar derived from Cinnamomum camphora and its improvement with ultrasound-assisted alkali activation. Colloid Surface A 556:177
Jin H, Capareda S, Chang Z et al (2014) Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: batch and column tests. Bioresour Technol 169:622
Gupta S, Sireesha S, Sreedhar I et al (2020) Latest trends in heavy metal removal from wastewater by biochar based sorbents. J Water Process Eng 38:101561
Luo M, Lin H, Li B et al (2018) A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol 259:312
Li H, Dong X, da Silva EB et al (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466
Wu J, Wang T, Zhang Y et al (2019) The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: considering the increased oxygen functional groups by HCl treatment. Bioresour Technol 291:121859
Li F, Wu X, Ji W et al (2020) Effects of pyrolysis temperature on properties of swine manure biochar and its environmental risks of heavy metals. J Anal Appl Pyrol 152:104945
Godwin PM, Pan Y, Xiao H, Afzal MT (2019) Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. Journal of Bioresources and Bioproducts 4:31
Jin H, Hanif MU, Capareda S et al (2016) Copper(II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J Environ Chem Eng 4:365
Xu S, Chen J, Peng H et al (2021) Effect of biomass type and pyrolysis temperature on nitrogen in biochar, and the comparison with hydrochar. Fuel 291:120128
Shang H, Li Y, Liu J et al (2020) Preparation of nitrogen doped magnesium oxide modified biochar and its sorption efficiency of lead ions in aqueous solution. Bioresour Technol 314:123708
Ding W, Dong X, Ime IM et al (2014) Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105:68
Yang B, Yu C, Yu Q et al (2015) N-doped carbon xerogels as adsorbents for the removal of heavy metal ions from aqueous solution. Rsc Adv 5:7182
X. Meng and R. Hu, Nitrogen/phosphorus enriched biochar with enhanced porosity activated by guanidine phosphate for efficient passivation of Pb(II), Cu(II) and Cd(II), J. Mol. Liq. (2020) 115071.
Park J-H, Wang JJ, Kim S-H et al (2019) Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J Colloid Interf Sci 553:298
Lian W, Yang L, Joseph S et al (2020) Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II). Bioresour Technol 317:124011
Peng H, Gao P, Chu G et al (2017) Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ Pollut 229:846
Shan R, Shi Y, Gu J et al (2020) Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chinese J Chem Eng 28:1375
Plazinski W, Rudzinski W, Plazinska A (2009) Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Adv Colloid Interfac 152:2
Saadi R, Saadi Z, Fazaeli R et al (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32:787
Agrafioti E, Kalderis D, Diamadopoulos E (2014) Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J Environ Manage 133:309
Wang YY, Liu YX, Lu HH et al (2018) Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J Solid State Chem 261:53
Lin S, Huang W, Yang H et al (2020) Recycling application of waste long-root Eichhornia crassipes in the heavy metal removal using oxidized biochar derived as adsorbents. Bioresour Technol 314:123749
Wu Z, Chen X, Yuan B et al (2020) A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere 239:124745
Deng J, Liu Y, Liu S et al (2017) Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interf Sci 506:355
Liu S, Liu Y, Tan X et al (2018) The effect of several activated biochars on Cd immobilization and microbial community composition during in-situ remediation of heavy metal contaminated sediment. Chemosphere 208:655
Luo X, Yu L, Wang C et al (2017) Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 169:609
Ding Y, Liu Y, Liu S et al (2016) Competitive removal of Cd(ii) and Pb(ii) by biochars produced from water hyacinths: performance and mechanism. Rsc Adv 6:5223
Ling L, Liu W, Zhang S et al (2017) Magnesium oxide embedded nitrogen self-doped biochar composites: fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ Sci Technol 51:10081
Xiao Y, Xue Y, Gao F et al (2017) Sorption of heavy metal ions onto crayfish shell biochar: effect of pyrolysis temperature, pH and ionic strength. J Taiwan Inst Chem E 80:114
Singh E, Kumar A, Mishra R et al (2021) Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solution. Bioresour Technol 320:124278
Das SK, Ghosh GK, Avasthe R (2021) Applications of biomass derived biochar in modern science and technology. Environ Technol Inno 21:101306
Shan R, Shi Y, Gu J et al (2020) Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin J Chem Eng 28:1375–1383
Q. Hu, J. Jung, D. Chen, et al. Biochar industry to circular economy, Sci. Total Environ. (2020) 143820.
Yaashikaa PR, Senthil Kumar P, Varjani SJ et al (2019) Advances in production and application of biochar from lignocellulosic feedstocks for remediation of environmental pollutants. Bioresour Technol 292:122030
Bandara T, Xu J, Potter ID et al (2020) Mechanisms for the removal of Cd(II) and Cu(II) from aqueous solution and mine water by biochars derived from agricultural wastes. Chemosphere 254:126745
Xu X, Cao X, Zhao L et al (2013) Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut R 20:358
S. Norazlina Abu, I. Che Fauziah and B. Rosenani Abu, Characterization of oil palm empty fruit bunch and rice husk biochars and their potential to adsorb arsenic and cadmium, American Journal of Agricultural and Biological Sciences. 9, (2014).
Zhao L, Cao X, Mašek O et al (2013) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J Hazard Mater 256–257:1
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22006121) and Sichuan Science and Technology Program (2020YFH0048). We would like to thank Mr. Weizhen Fang and Miss Zhangmei Hu at Analytical and Testing Center of Southwest Jiaotong University for their assistance with SEM and BET analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, D., Luo, W., Zhu, J. et al. Potential of removing Pb, Cd, and Cu from aqueous solutions using a novel modified ginkgo leaves biochar by simply one-step pyrolysis. Biomass Conv. Bioref. 13, 8277–8286 (2023). https://doi.org/10.1007/s13399-021-01732-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01732-2