Abstract
Nattokinase (NK), a protease enzyme produced by Bacillus subtilis, has various biological effects such as lipid-lowering activity, antihypertensive, antiplatelet/anticoagulant, and neuroprotective effects. Exposure to environmental toxicants such as bisphenol A (BPA) or γ-radiation (IR) causes multi-organ toxicity through several mechanisms such as impairment of oxidative status, signaling pathways, and hepatic and neuronal functions as well as disruption of the inflammatory responses. Therefore, this study is designed to evaluate the ameliorative effect of NK against BPA- or IR-induced liver and brain damage in rats. Serum ammonia level and liver function tests were measured in addition to brain oxidative stress markers, amyloid-beta, tau protein, and neuroinflammatory mediators. Moreover, relative quantification of brain nuclear factor-erythroid 2-related factor-2 (Nrf2)/heme oxygenase-1 (HO-1) genes, as well as apoptotic markers in brain tissue, was carried out in addition to histopathological examination. The results showed that NK improved liver functions, impaired oxidative status, the cholinergic deficits, and minified the misfolded proteins aggregates. Furthermore, NK alleviated the neuroinflammation via modulating NF-κB/Nrf2/HO-1 pathway and glial cell activation in addition to their antiapoptotic effect. Collectively, the current results revealed the protective effect of NK against hepatic and neurotoxicity derived from BPA or IR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The proven efficacy of medicinal plants in treating various diseases and their safe impact on human health elevates their consumption and use in the manufacture of drugs globally (Michel et al. 2020; Ullah et al. 2020). Various medicinal plants such as Phoenix dactylifera have anti-inflammatory, anti-hepatotoxicity, and anti-neurotoxicity (El-far et al. 2019). Others have anti-oxidative and anti-apoptotic effects against toxicity such as thymoquinone (Hassan et al. 2019). Nattokinase (NK) is an alkaline protease enzyme produced by Bacillus subtilis during the fermentation of typical Japanese food natto. NK has been used in the prevention of cardiovascular diseases due to its potent fibrinolytic and antithrombotic effects (Huang et al. 2021). Notably, NK possesses a potent lipid-lowering activity, antihypertensive, and neuroprotective effects and is considered a valuable natural product with various pharmaceutical, health, and medical applications (Pham et al. 2020; Zhang et al. 2020). Furthermore, previous studies showed that NK offers multiple health benefits such as the treatment of hemorrhoids, diabetes, muscle spasms, chronic inflammation, cardiovascular diseases, Alzheimer’s disease, stroke, etc. (Nguyen and Nguyen 2020; Takagaki et al. 2020).
Excessive exposure to high environmental temperatures (Abdelnour et al. 2019) and a wide range of environmental pollutants such as toxic chemicals, UV radiation, heavy metal ions, and others elevates the harmful consequences on human health (Alkhalf and Khalifa 2018) via activation of the inflammatory signaling in tissues. Radiation exposure either through natural, radiotherapy, medical, or accidental exposure is associated with various complications like immunosuppression, endocrine dysfunction, and organ injury (Hanedan Uslu et al. 2019). A cascade of radiation-induced tissue injury in living organisms initiates after exposure to relatively high-dose radiation which may cause the death of the organism within a short period due to acute effects (Chiba et al. 2002). The early response of the immune system to ionizing radiation is via inflammatory response and secretion of many inflammatory mediators (Najafi et al. 2018). Ionizing radiation triggers a state of oxidative stress and production of reactive oxygen species (ROS) through radiolysis of water molecules, the main component of tissues and consequently impairs various biochemical processes and molecular signaling pathways (Wei et al. 2019; Tiziana Cervelli et al. 2021).
Considering the wide application of bisphenol A (BPA) in the manufacture of many consumable foods and beverage containers, toys, and medical equipment, it is considered a prevalent environmental and industrial toxin (An et al. 2021). The prolonged exposure and excessive use of BPA in daily life through environmental pollution, ingestion, inhalation, or dermal contact boost detrimental impacts on human health (Thoene et al. 2017). Previous studies revealed that even low doses of BPA induce mutagenicity, hepatotoxicity, neurotoxicity, and immunotoxicity by inducing oxidative stress (Basit et al. 2020; Eweda et al. 2020).
Moreover, there is a relationship between the functional status of the liver and brain known as hepatic encephalopathy (HE) resulting from insufficiency of the liver to remove toxins from the blood leading to brain dysfunction. It is manifested via a wide spectrum of neurologic abnormalities ranging from increased circulating neurotoxin levels (such as ammonia), impaired neurotransmission, changes in brain energy metabolism, and systemic inflammatory response (Jiménez-Torres et al. 2021). Collectively, this study was designed to evaluate the possible ameliorative effect of nattokinase on hepatic and neurotoxicity as well as the histopathological changes induced in rats either by BPA or γ-irradiation.
Materials and methods
Chemicals
Bisphenol A (BPA) was purchased from Merck KGaA, Darmstadt, Germany, CAS Number: 80–05-7, Cat. No.: 803546. Nattokinase (NK) was purchased from Doctor’s Best (USA), Item Code: 286,737, UPC: 75,395,000,253.
Ethics statement
This experiment was carried out according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (No. 85:23, 1996) and in compliance with the principles and guidelines of the animal care committee of the National Center for Radiation Research & Technology (NCRRT), Cairo, Egypt. Every attempt was made to keep animal suffering to a minimum.
Animals
Forty-eight adult female Swiss albino rats (120–150 g) were obtained from the animal farm of the Egyptian Holding Company for Biological Products and Vaccines (VACSERA), Cairo, Egypt. The rats were housed in a conditioned atmosphere (20–22 °C) in suitable cages and maintained on tap water and a standard diet (Clarke et al. 1977).
Radiation facility
Rats were exposed to whole-body gamma-radiation (IR) at The National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt, using the cesium-137 biological irradiator source (gamma-cell-40), Atomic Energy of Canada Limited (Chalk River, ON, Canada).
Experimental design
Rats were randomly divided into 6 equal groups as follows:
-
Control group: Healthy control animals (vehicle group): Rats were given orally corn oil by gavage.
-
NK group: Rats were orally gavaged with NK at a dose of 720 FU/kg BW/day for 5 weeks according to Fadl et al. (2013).
-
BPA group: Rats were injected intraperitoneally with BPA at a dose of 100 mg/kg BW/day which was dissolved in corn oil for 5 weeks according to Bilgi et al. (2019).
-
BPA + NK group: Rats were injected with BPA as in group 3 and treated orally gavaged with NK as in group 2.
-
IR group: Rats were exposed to whole-body IR at a dose of 3 Gy/week for 4 weeks according to Trivedi et al. (2012).
-
IR + NK group: Rats were exposed to whole-body IR as in group 5 and were treated with NK as in group 2.
Collection of samples
After the last dose of NK, rats fasted overnight before scarification under light ether anesthesia. Blood was drawn from the vena cava and the sera were separated by centrifugation for 10 min at 3000 × g for biochemical parameter estimation. Following blood sample collection, the liver and the brain were excised, cleaned, and washed with saline (0.9% sodium chloride). Some parts of the brain were stored at − 80 °C for biochemical parameter assessment, while the other parts of tissues (brain and liver) were rinsed in 10% neutralized formalin for histopathological examination.
Biochemical estimations
Using Thermo Spectronic Helios Delta Visible spectrophotometer, the levels of ammonia and total protein were determined according to the method described by Konitzer and Voigt (1963) and Gornall et al. (1949), respectively. Also, liver alanine transaminase, aspartate transaminase, and alkaline phosphatase activities were measured using commercial kits from Bio-diagnostic, Egypt, according to the previously described methods (Reitman and Frankel 1957; Moss 1982). The oxidative stress markers in the brain tissues malondialdehyde (MDA) and glutathione (GSH) levels were estimated according to previous studies of Ohkawa et al. (1979) and Beutler et al. (1963), respectively.
Estimation of brain inflammatory markers
The levels of interleukin-6 (IL-6) were measured in brain tissue using an enzyme-linked immunosorbent assay (ELISA) kit obtained from Thermo Fisher Scientific Inc. (USA), catalog no. ERA32RB. Also, interleukin-10 (IL-10) levels were measured in brain tissue using an ELISA kit purchased from Abcam (UK), catalog no. ab100765. TECAN (Magellan™) was used as a plate reader and data analysis software for ELISA.
Estimation of neurotoxicity markers: amyloid β, tau proteins, and acetylcholine
Amyloid β-protein (Aβ) level was determined by using an ELISA kit purchased from MyBiosource, Inc. (USA), catalog no. MBS726579; phosphorylated tau protein (p-tau) level was measured using an ELISA kit purchased from MyBiosource, Inc. (USA), catalog no. MBS725098. Moreover, acetylcholine level was measured using an ELISA kit purchased from MyBiosource, Inc. (USA), catalog no. MBS728879, according to the manufacturer’s instruction. Readings and analysis of ELISA plate results were carried out using TECAN (Magellan™).
Western blotting
Brain tissue homogenate was prepared, and western blot analysis was carried out as previously stated by Chen et al. (2005) and Omar et al. (2009) using anti-beta actin antibody (ab8227), anti-GFAP antibody (ab7260), and anti-NF-kB p65 (ab76302) obtained from Abcam (UK). Immunoblot visualization was done using the Amersham detection kit, according to the manufacturer’s protocols, followed by exposure to X-ray film. Auto-radiograms were scanned for quantification of protein levels using a scanning laser densitometer (Biomed Instrument Inc., USA) using Image J analysis of the autoradiograms. Calculation of results was performed following normalization by housekeeping protein (β-actin).
Quantitative real-time polymerase chain reaction
Total RNA was isolated from the brain tissue homogenates according to instructions of the manufacturer using RNeasy Micro Kit purchased from QIAGEN (Germany), catalog no. 74004. The extracted RNA was reversely transcribed to complementary DNA using an RT-PCR kit (Applied Biosystem, Waltham, MA, USA). Quantitative RT-PCR of Nrf2, HO-1, Bcl-2, and caspase-3 was performed using SYBR Green PCR Master Mix (Qiagen, Germany) as described by the manufacturer and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. The primer sequences are described in Table 1. The amplification and analysis were performed using an Applied Biosystems with software version 3.1 (StepOne™, USA). The relative expression of target genes was obtained using the ΔΔCt method (Livak and Schmittgen 2001).
Detection of apoptosis by annexin V staining
For the quantitative evaluation of apoptosis, the annexin V-fluorescein isothiocyanate (FITC) and PI dual staining techniques were employed. Briefly, the peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood using the method of Böyum (1968). Cells were collected and the suspension was made in the binding buffer (Becton Dickinson). Subsequently, the cells (approximately 4 × 105 cells) were stained using a BD Pharmingen FITC Annexin V apoptosis detection kit (BD Bioscience) for 15 min in the dark according to the manufacturer’s instructions. After that, the samples were analyzed within 1 h using a BD Accuri Tm C6 Plus flow cytometer, and the degree of apoptosis was quantified as a percentage of the annexin V-positive and PI-negative (annexin V + /PI −) cells.
Histopathological assessment
Liver and brain tissue specimens were fixed in 10% formalin saline, then trimmed off, washed, and dehydrated in ascending grades of alcohol. The dehydrated specimens were then cleared in xylene, embedded in paraffin blocks, and sectioned at 4–6 µm thick. The obtained tissue sections were deparaffinized using xylol and stained using hematoxylin and eosin (H&E) for histopathological examination using the light microscope according to Downie (1990). The frequency and severity of liver lesions were assessed semi-quantitatively as previously stated by Plaa et al. (2014) and Khafaga et al. (2019) in Table 2A. The severity of cerebral histopathological changes in terms of nuclear pyknosis and degeneration in neurons, focal gliosis, and encephalomalacia was scored from 0 to 3 as reported by Abdel-latif et al. (2018) and Khafaga et al. (2021) in Table 2B.
Statistical analysis
Using one-way analysis of variance (ANOVA) followed by a post hoc test (least significant difference (LSD)), the obtained data were expressed as the mean ± SD. The level of significance between the mean values was set at p ≤ 0.05. Statistical Package for Social Science (SPSS) version 20 for Windows (SPSS® Chicago, IL, USA) software was used to analyze the data.
Results
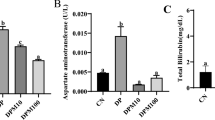
Effect of nattokinase on ammonia level
As shown in Table 3, the intoxication with BPA and exposure to γ-IR resulted in a marked increase in the level of ammonia compared to the control group, while the oral administration of NK showed an opposite effect through a notable reduction of ammonia level.
Effect of nattokinase on parameters of liver
The results of the present study showed that the intoxication with BPA and exposure to γ-IR induced extraordinarily liver damage which was evident through impaired liver functions accompanied by extensive leakage of the hepatic enzymes (ALT, AST, and ALP) to the bloodstream, and this was confirmed with the histopathological examination of liver tissue. Meanwhile, treatment with NK markedly ameliorates the increment in these serum liver function parameters as illustrated in Table 3.
Additionally, a notable decline in the total protein levels was observed in BPA-intoxicated rats and gamma-irradiated rats. On the other hand, oral supplementation with NK significantly increased total protein levels (Table 3).
Effect of NK treatment on oxidative stress biomarkers in brain tissue
The data illustrated in Fig. 1 shows a significant increase in the levels of lipid peroxidation (MDA) together with a marked decline in the levels of GSH in the brain tissues of rats injected with BPA or exposed to γ-irradiation in comparison to control rats. However, upon treatment with NK, the GSH levels were ameliorated along with a notable reduction of lipid peroxidation.
Effect of NK on the amyloid-β, tau protein, and acetylcholine
Alteration in the levels of the amyloid-β (Aβ) and p-tau in the brain tissues is illustrated in Fig. 2, which indicates that both BPA intoxication and exposure to IR significantly augmented the levels of both Aβ and p-tau in the brain tissues as compared to the control group. Alternatively, supplementation with NK markedly declined the Aβ and p-tau protein aggregation in the brain tissues of rats. Besides, the current results reported a significant reduction in the levels of acetylcholine (ACh) in the brain tissues of the BPA or IR groups compared to the control. Conversely, treatment with NK significantly improved the brain’s ACh levels (Fig. 2).
Influence of NK on inflammatory markers in the brain tissue of rats
Inflammation is the response of tissues when stimulated or exposed to a harmful hazard such as toxins, chemicals, radiation or injured by pathogens. Both pro- and anti-inflammatory signaling pathways are involved. Furthermore, there was a significant increase in the levels of pro-inflammatory IL-6 with a concomitant decrease in the levels of the anti-inflammatory IL-10 in the brain tissues of rats in the BPA group or IR group compared to the control. Meanwhile, NK alleviated the inflammatory response via increasing the levels of IL-10 and suppressing the IL-6 (Fig. 3).
Effect of nattokinase on NF-κB and GFAP protein expression
Western blot analysis was used to detect the protein levels of phosphorylated nuclear factor kappa B (p-NF-κB) and glial fibrillary acidic protein (GFAP) in the brain tissues of rats. As illustrated in Fig. 4, BPA and IR resulted in marked upregulation (p ≤ 0.001) of the p-NF-κB and GFAP levels as compared to the control group. Conversely, administration of NK to BPA or IR rats effectively reverted the results and caused a remarkable downregulation (p ≤ 0.001) in the expression levels of p-NF-κB and GFAP proteins.
Effect of nattokinase on Nrf2 and HO-1 gene expression
Nrf2-related/heme oxygenase 1 (Nrf2/HO-1) is an important signaling pathway that plays an important role in inflammatory-derived diseases such as liver damage and neurodegenerative disease. Quantitative RT-PCR was used to measure the effect of NK on the gene expression of Nrf2 and HO-1 genes. The obtained results showed that the gene expression of Nrf2 and HO-1 in the brain tissues was downregulated upon intoxication with BPA or exposure to gamma radiation compared to the control. However, NK treatment caused a remarkable upregulation in the expression of both genes Nrf2 and HO-1 (Fig. 5).
Influence of NK on the brain apoptosis induced by BPA or IR
Apoptosis is regulated by the protein caspase. As shown in Fig. 6, the expression of caspase-3 was significantly increased, whereas the expression of the anti-apoptotic marker Bcl-2 was significantly decreased in the BPA and IR groups in comparison to the control group. Meanwhile, the caspase-3 expression was downregulated and that of the Bcl-2 was upregulated upon NK supplementation.
Supporting these results, the results obtained from flow cytometry indicated that rats injected with BPA or exposed to γ-radiation showed a significant decrease in the live cells population accompanied by a significant increase in the percentages of early and late apoptotic and necrotic cells as compared with the control group. Conversely, treatment with NK increased the percentage of the live cells and decreased the percentages of late apoptotic cells as shown in Fig. 7.
Dot plot chart of the percentages of live (Q4), necrotic (Q1), late apoptotic (Q2), and early apoptotic (Q3) cells representative of one sample, a control group, b NK group, c BPA group, d BPA + NK group, e IR group, and f I R + NK group. g Mean values of three samples of the percentages of live, early apoptotic, late apoptotic, and necrotic cells after injection of BPA or exposure to γ-radiation, and treatment with NK. FL1-H: a detector for fluorescence height for annexin V; FL2-H: a detector for fluorescence height for PI. Q1 represents necrosis, Q2 represents late apoptosis, Q3 represents early apoptosis, and Q4 represents live cells
Histopathological studies
Liver tissue
The liver tissue section of control and NK rats showed normal histological architecture of hepatocytes with a prominent central hepatic vein. Sinusoids are lined by a discontinuous layer of fenestrated endothelial cells with a fine arrangement of Kupffer cells (grade 0) (Fig. 8a, b).
Photomicrograph of hepatic tissue section showing a normal histological structure of hepatic lobules in control group (a), strands of polygonal cells with prominent round nuclei, and eosinophilic cytoplasm (arrow) in NK group (b). BPA group (c): apoptosis of hepatocytes and intracellular fat droplets. BPA + NK group (d): ballooning degeneration of hepatocytes and narrowing of sinusoids with hyperplasia of Kupffer cells (arrow). IR group (e): few number hepatocytes with pyknotic nuclei and intracellular fat droplets. IR + NK group (f): mild swelling of hepatocytes and narrowing of hepatic sinusoids (arrow) (H&E × 200)
The liver section of rats intoxicated with BPA showed severe effects ranging from ballooning degeneration with intracellular fat droplets of hepatocytes, especially in the centrilobular zone, coagulative necrosis, disorganization of hepatic cords, narrowing of hepatic sinusoids, and hyperplasia of Kupffer cells were also noticed (grade 4) (Fig. 8c). However, upon treatment of BPA with NK, the liver section showed ballooning degeneration of hepatocytes, narrowing of sinusoids with hyperplasia of Kupffer cells, large numbers of binucleated cells in the centrilobular zone in addition to mild to moderate swelling and narrowing of hepatic sinusoids (grade 2) (Fig. 8d). The hepatic tissue section of rats exposed to IR showed severe effects represented by disorganization of hepatic cords and necrobiotic changes of hepatocytes characterized by focal necrotic foci and hydropic degeneration of hepatocytes, some micro-vesicular steatosis and apoptotic bodies, nuclear pyknosis and granular cytoplasm of hepatocytes and narrowing of hepatic sinusoids and hyperplasia of Kupffer cells (grade 4) (Fig. 8e). Conversely, the liver tissue section of IR and NK rats showed mild swelling of hepatocytes and narrowing of hepatic sinusoids. Regenerated hepatic cells appeared deeply basophilic with marked hyperplasia of Kupffer cells were seen as grade 1 (Fig. 8f).
Brain tissue
The brain sections of the control and NK groups display the normal architecture of the brain consisting of the cerebral cortex, cerebral (neuronal cells with oval or rounded nuclei arranged with no sharp boundaries in association with small blood vessels in between) (Fig. 9a and b), and hippocampus which appeared as layers of compact granular cells with dark nuclei (Fig. 10a and b). However, the injection of BPA resulted in moderate neuronal degeneration and apoptosis of some neuronal cells which is associated with focal gliosis and satellitosis, neuronophagia, and perivascular edema in the cerebral cortex, score (+ +) (Fig. 9c). Additionally, cellular disorganization, shrinkage in the size of large pyramidal cells, with darkened nuclei, and marked vacuolation in the granular cell layers were observed in the hippocampus (Fig. 10c). In contrast, the co-administration of NK decreased the numbers of degenerated neuronal cells, ameliorated gliosis, satellitosis, and neuronophagia in the cerebral cortex (mild effect), score ( +) (Fig. 9d) along with improving the hippocampal cellular organization (Fig. 10d). Similarly, the exposure of rats to γ-radiation resulted in severe cerebral cortex neuronal degeneration (darkly stained surrounded by per-cellular haloes spaces), apoptosis of neuronal cells (eosinophilic bodies associated with focal gliosis and satellitosis), and marked perivascular edema and congestion in the cerebral blood vessels, score (+ + +) (Fig. 9e). Additionally, cellular disorganization, shrinkage in the size of large pyramidal cells, with darkened nuclei, and marked vacuolation in the granular cell layers were observed in the hippocampus (Fig. 10e). However, NK supplementation decreased the apoptotic neuronal cells, gliosis, satellitosis, and the perivascular edema, score (+ +) (Fig. 9f) with a slight improvement in the hippocampal cellular organization, pyramidal cells, and the granular cell layers (Fig. 10f).
Photomicrograph of the cerebral cortex of control and NK groups (a, b) showing a normal arrangement of neuronal cells in association with small blood vessels in between (arrow) (H&E × 200). BPA: Photomicrograph of the cerebral cortex (c) showing eosinophilic apoptotic bodies and perivascular edema (arrow) (H&E × 200). BPA + NK: Photomicrograph of the cerebral cortex (d) showing few numbers of degenerated neuronal cells with pyknotic nuclei (arrow) (H&E × 200). IR: Photomicrograph of the cerebral cortex (e) showing eosinophilic apoptotic bodies and perivascular edema with congestion (arrow) (H&E × 200). IR + NK: Photomicrograph of the cerebral cortex (f) showing few numbers of apoptotic neuronal cells with focal gliosis (arrow) (H&E × 200)
The photomicrograph of the hippocampus of the control and NK groups (a, b) showed the normal histological structure of compact granular cells with dark nuclei (arrow) (H&E × 200). BPA: Photomicrograph of the hippocampus (c) showing marked vacuolations of granular cell layers (arrow) (H&E × 200). BPA + NK: Photomicrograph (d) showing cellular organization and shrinkage in the size of pyramidal cells, with darkened nuclei in the hippocampus (arrow) (H&E × 200). IR: Photomicrograph (e) showing hippocampus cellular disorganization and shrinkage in the size of large pyramidal cells (arrow) (H&E × 200). IR + NK: Photomicrograph (f) showing small size of hippocampus large pyramidal cells, with darkened nuclei (arrow) (H&E × 200)
Discussion
The current study showed that exposure to the environmental toxicants BPA and γ-radiation causes multi-organ toxicity through several mechanisms such as impairment of oxidative status, signaling pathways, and hepatic and neuronal functions as well as disruption of the inflammatory responses (Ma et al. 2019; Bolbol et al. 2021).
The liver is one of the most radiosensitive organs (Kim et al. 2017) where the BPA is metabolized to form BPA-glucuronide which is accumulated in the liver leading to disturbing liver integrity. Herein, the intoxication with BPA resulted in hepatic damage evidenced by the impaired activity of liver enzymes along with the reduced levels of serum total protein and confirmed with the histopathological examinations. Our results are parallel to that of Kamel et al. (2018) and Lebda et al. (2020), who ensured the toxic effects of BPA on the liver via disrupting the liver tissue architecture and increasing the permeability of the hepatocellular plasma membrane leading to the leakage of the liver enzymes to the blood and elevated activities of ALT, AST, and levels of ALP. Additionally, our results are in accordance with that of El-Shahat et al. (2021) who reported that the rise in the serum transaminases activities and alkaline phosphatase after γ-irradiation may be due either to the release of enzymes from radiosensitive tissues or to changes in its synthesis and may be related to the extensive breakdown of liver parenchyma. However, co-administration of nattokinase significantly ameliorates the liver function by reducing the activities of ALT and AST as well as the levels of ALP (Hideaki Suzuki et al. 2017).
Furthermore, impaired hepatic function and loss of liver ability to detoxify toxins are associated with lower levels of total protein and higher levels of ammonia. This agrees with Abdelgawad et al. (2019) and El-Baz et al. (2021) who indicated that impaired liver function decreases the total protein and elevates the levels of ammonia in the blood that crosses the blood-brain barrier and is metabolized in the brain astrocytes to glutamine. Indeed, ammonia interacts with other factors to disturb brain function (Butterworth 2019).
Both gamma irradiation and BPA-mediated neurotoxicity via several mechanisms, including production of reactive oxygen species (ROS), mitochondrial degeneration, neuroinflammation, and protein degradation, lead to apoptotic cell death (Wang et al. 2017; Sharma et al. 2019). The brain is very sensitive to oxidative stress and ROS due to the extensive consumption of oxygen and high content of polyunsaturated fatty acids along with low antioxidant levels (Li et al. 2013; Cheignon et al. 2018).
In the present study, consistent with the proposed mode of action, the exposure to BPA or γ-irradiation was accompanied by significant deleterious changes in antioxidant status, as a response to the induced oxidative stress and production of ROS. In the present study, an increase in the levels of lipid peroxidation and a decline in the levels of GSH were noticed in the brain tissues. These results are consistent with El Morsy and Ahmed (2020) who revealed that BPA intoxication disturbed the brain’s redox state which can easily penetrate the blood–brain barrier and induce its toxicity through oxidative stress. Furthermore, Kale et al. (2019) reported that radiation increased lipid peroxidation in the brain especially neuronal cells via ROS production, thus impairing both the structure and function of neurons.
Previous studies had shown that oxidative stress promotes Aβ aggregation and tau hyperphosphorylation leading to neuronal toxicity and injury as well as impaired cognitive function (Khandelwal et al. 2020). The accumulation of both Aβ and phosphorylated tau is one of the main pathological hallmarks of Alzheimer’s disease. Moreover, the disruption of the cholinergic system is accompanied by cognitive impairments in neurodegenerative diseases (Bensalem et al. 2016). Herein, both BPA and IR resulted in brain toxicity and impaired cognitive functions manifested through the higher levels of the misfolded proteins Aβ and p-tau besides the decline in the acetylcholine levels (ACh). Our results are in harmony with that of Hawas et al. (2020) who indicated that γ-radiation reduces ACh and increases Aβ in the brain. Li et al. (2014) demonstrated that oxidative stress induced by ionizing radiation mediates the increased tau phosphorylation. Moreover, Sharma et al. (2018) showed that high-dose radiation promotes misfolding and aggregation of proteins involved in the progression of the neurodegenerative disease. This may be due to neural loss and demyelination, vascular abnormalities and changing the brain microenvironment consequently, cognitive defects and impaired neurogenesis. Additionally, our results are consistent with that of Wang et al. (2017) who showed that BPA enhanced the level of Aβ and the expression of phosphorylated tau and Sukjamnong et al. (2020) who reported that maternal exposure to BPA disrupts brain functions and induces cognitive deficits and learning-memory impairment in offspring mice. Administration of BPA leads to the death of cerebellar granule cells, olfactory bulb neurons, and basal forebrain cholinergic neurons. Furthermore, BPA causes choline toxicity, resulting in decreased acetylcholine transferase activity. This demonstrates that administration of BPA induced the death of forebrain cholinergic neurons leading to a reduction of the acetylcholine and cognitive dysfunction (Mahdavinia et al. 2019).
Conversely, supplementation with nattokinase markedly declined the levels of Aβ and p-tau and alleviated the levels of acetylcholine. These results are parallel to that of Bhatt et al. (2018) and Chen et al. (2018) who reported that treating rats suffering from Alzheimer’s disease (AD) with nano-nutraceuticals containing NK ameliorated the impaired cognitive functions and enhanced the clearance of Aβ along with the inhibition of the BACE-1 activity, thus suggesting a neuroprotective efficacy of NK. Moreover, Fadl et al (2013) showed that NK supplementation treats AD via modulating brain acetylcholinesterase activity and IL-6 levels. Additionally, Wang et al. (2012) reported that the neuroprotective effects of NK were through its ability to scavenge the free radicals.
Interestingly, the accumulation of Aβ aggregates in the brain tissues activates non-neuronal cells, especially microglia cells and astrocytes that mediate neuroinflammation. These activated cells release inflammatory cytokines causing impaired neurogenesis and increased susceptibility to neurodegenerative disorders such as AD (Heneka et al. 2015; Kinney et al. 2018). Moreover, the astrocytes (the major glial cells) were reactivated through the overexpression of an intermediate filament protein glial fibrillary acidic protein (GFAP) (Schiffer et al. 1986; Nahirnyj et al. 2013). Consequently, the upregulated expression of the GFAP during neuroinflammation may be a reliable marker of brain injury.
The overproduction of oxidative stress activates the nuclear factor kappa B (NF-ĸB) a transcription factor involved in various processes such as apoptosis and inflammation, therefore, the release of various pro-inflammatory cytokines (Kaulmann and Bohn 2014; Ahmed et al. 2017). Accordingly, in response to the intoxication with the BPA or the exposure to γ-radiation, the present study showed marked upregulated levels of the neuroinflammatory mediators IL-6 and NF-ĸB and GFAP along with the abolished levels of the anti-inflammatory marker IL-10. Our data are in line with that of Acaroz et al. (2019) who reported that BPA induced neuroinflammation via the overexpression of the brain pro-inflammatory cytokines (IL-1β and IL-6) and decreased anti-inflammatory IL-10. Additionally, Zhu et al. (2015) showed that BPA significantly upregulated the phosphorylated NF-κB p65 levels as well as the GFAP levels confirming the involvement of the microglial cells and NF-κB in the inflammatory response and impaired neurogenesis after BPA intoxication (Tiwari et al. 2015). Moreover, radiation triggers neuroinflammation and significantly increased the brain levels of the pro-inflammatory mediators IL-1β, IL-6, and NF-κB (Lee et al. 2010; Yang et al. 2017). Besides, the astrocytes proliferate and exhibit hypertrophic nuclei/cell bodies with an apparent elevation in the GFAP expression upon radiation injury (Greene-Schloesser et al. 2012; Gao et al. 2021).
Nrf2/HO-1 signaling pathway is an important endogenous protective system for multi-organ against different stimuli and environmental stressors. It is involved in the anti-inflammation, anti-oxidative, and antiapoptotic response and thus can be targeted for the treatment of the diseases linked to oxidative stress and inflammation (Li et al. 2020). Nrf2 and NF-ĸB signaling pathways interact to regulate the function of downstream target proteins. Previous studies reported that the depletion of Nrf2 promotes the NF-κB–mediated proinflammatory reactions (Ahmed et al. 2017). Based on the aforementioned data, our results revealed that BPA or γ-irradiation concomitant with oxidative stress and neuroinflammation, there is a remarkable suppression in the gene expression of both Nrf2 and HO-1. These results work in with that of Chiang et al (2022) who observed downregulation of the Nrf2 and HO-1 gene transcription upon intoxication with BPA. Moreover, Zhang et al. (2021) demonstrated that irradiation impedes the protein and gene expression of the Nrf2 and HO-1 via ROS the main mediators of radiation-induced damage, therefore, inducing cell apoptosis and stimulating numerous inflammatory factors.
According to the previous studies, the impaired oxidative status and overproduction of ROS by both BPA and IR trigger mitochondrial dysfunction and promote the mitochondrial apoptotic signaling pathway. In addition to the results from flow cytometry, this was evidenced through the upregulation of caspase-3 expression accompanied by the downregulation of the antiapoptotic Bcl-2 gene expression. Accordingly, BPA and IR promote neurotoxicity, neuronal degeneration, and apoptosis (Huang et al. 2018; El Morsy and Ahmed 2020).
Contrarily, the co-treatment with NK significantly increased the anti-inflammatory IL-10 levels, upregulated the expression of Nrf2 and its downstream target HO-1 as well as the antiapoptotic Bcl-2 expression with concomitant suppression of the proinflammatory mediators IL-6 levels, NF-ĸB, GFAP expression, and the apoptotic caspase-3 activity. The anti-inflammatory and anti-apoptotic effects of NK coincided with the results of Bhatt et al. (2018) and Gallelli et al. (2021) who confirmed that NK inhibits the pro-inflammatory IL-6 and elevates the anti-apoptotic Bcl-2 activity, therefore, protecting neurons from apoptosis and ameliorates the neurological diseases. Moreover, NK notably declined the GFAP expression consequently, alleviating the neuroinflammation by switching the proinflammatory microglia into an anti-inflammatory (Huang et al. 2021). Additionally, NK attenuated ischemia–reperfusion injury and improved neurological function via the activation of the Nrf2/HO-1 axis (Loboda et al. 2016). Furthermore, Li et al. (2020) confirmed that modulation of the Nrf2/HO-1 signaling pathway ameliorates neuronal damage and inhibits the oxidation tension contributing to inflammation and apoptosis.
Conclusion
In conclusion, our results showed that NK extraordinarily enhanced the impaired oxidative status along with the clearance of misfolded proteins. Furthermore, NK alleviated the neuroinflammation via suppressing the NF-κB, modulating Nrf2/HO-1 pathway and astrocyte/glial cell activation. Additionally, NK ameliorated both neuropathology and neurological function by improving the cholinergic deficits. Collectively, NK may be used to protect the body not only from hepatic damage but also from neurotoxicity and neurodegenerative diseases derived from environmental toxicants. However, further studies are required to determine the exact underlying mechanisms.
Data availability
All data obtained from this study are included in the current manuscript.
Change history
11 April 2024
Editor’s Note: Readers are alerted that the concerns have been raised with this article. Editorial action will be taken as appropriate once this matter is resolved and all parties have been given an opportunity to respond in full.
References
Abdelgawad MH, Awad MM, Eraba KMT (2019) Study of the effects of naturally occurring radioactive materials on blood indices in blood’s rats. Egypt J Biophys Biomed Engng 20:1–7
Abdel-Latif RG, Heeba GH, Taye A, Khalifa MMA (2018) Lixisenatide, a novel GLP-1 analog, protects against cerebral ischemia/reperfusion injury in diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 391(7):705–717. https://doi.org/10.1007/s00210-018-1497-1
Abdelnour SA, Abd El-Hack ME, Khafaga AF, Arif M, Taha AE (2019) Noreldin AE (2019) Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J Therm Biol 79:120–134. https://doi.org/10.1016/j.jtherbio.2018.12.013
Acaroz U, Ince S, Arslan-Acaroz D, Gurler Z, Demirel HH, Kucukkurt I, Eryavuz A, Kara R, Varol N, Zhu K (2019) Bisphenol-A induced oxidative stress, inflammatory gene expression, and metabolic and histopathological changes in male Wistar albino rats: protective role of boron. Toxic Res 8(2):262–269. https://doi.org/10.1039/c8tx00312b
Ahmed SM, Luo L, Namani A, Wang XJ (1863) Tang X (2017) Nrf2 signaling pathway: pivotal roles in inflammation. Biochim et biophys acta Mol basis dis 2:585–597. https://doi.org/10.1016/j.bbadis.2016.11.005
Alkhalf MI, Khalifa FK (2018) Blueberry extract attenuates γ-radiation-induced hepatocyte damage by modulating oxidative stress and suppressing NF-κB in male rats. Saudi J Biol Sci 25(7):1272–1277. https://doi.org/10.1016/j.sjbs.2018.07.002
An SJ, Yang EJ, Oh S, Park KJ, Kim T, Hong YP, Yang YJ (2021) The association between urinary bisphenol A levels and nonalcoholic fatty liver disease in Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ Health Prev Med 26(1):91. https://doi.org/10.1186/s12199-021-01010-7
Basit F, Akhtar T, Hameed N, Abbasi MH, Sheikh N (2020) Subchronic toxicity of bisphenol A on the architecture of spleen and hepatic trace metals and protein profile of adult male Wistar rats. Hum Exp Toxicol 39(10):1355–1363. https://doi.org/10.1177/0960327120921440
Bensalem J, Dal-Pan A, Gillard E, Calon F, Pallet V (2016) Protective effects of berry polyphenols against age-related cognitive impairment. Nutr Aging 3:89–106. https://doi.org/10.3233/NUA-150051
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Bhatt PC, Pathak S, Kumar V, Panda BP (2018) Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacol 26(1):105–118. https://doi.org/10.1007/s10787-017-0381-9
Bilgi A, Abalı R, Bilgi PT, Şahin M, Tunçdemir M, Boran AB (2019) The apoptotic effects of bisphenol A exposure on the rat ovary: an experimental study. Environ Sci Pollut Res Int 26(10):10198–10203. https://doi.org/10.1007/s11356-019-04487-6
Bolbol SA, Zaitoun MF, Abou El-Magd SA, Mohammed NA (2021) Healthcare workers exposure to ionizing radiation: oxidative stress and antioxidant response. Indian J Occup Environ Med 25(2):72–77. https://doi.org/10.4103/ijoem.IJOEM_198_20
Böyum A (1968) Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl 97:7
Butterworth RF (2019) Hepatic encephalopathy in cirrhosis: pathology and pathophysiology. Drugs 79(Suppl 1):17–21. https://doi.org/10.1007/s40265-018-1017-0
Cervelli T, Basta G, Del Turco S (2021) Chapter 30 - effects of antioxidant nutrients on ionizing radiation-induced oxidative stress, Editor(s): Vinood B. Patel, Victor R. Preedy, Toxicology, Academic Press, Pages 307–316, https://doi.org/10.1016/B978-0-12-819092-0.00030-3.
Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464. https://doi.org/10.1016/j.redox.2017.10.014
Chen CS, Weng SC, Tseng PH, Lin HP, Chen CS (2005) Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem 280(46):38879–38887. https://doi.org/10.1074/jbc.M505733200
Chen H, McGowan EM, Ren N, Lal S, Nassif N, Shad-Kaneez F, Qu X, Lin Y (2018) Nattokinase: a promising alternative in prevention and treatment of cardiovascular diseases. Biomarker Insights 13:1177271918785130. https://doi.org/10.1177/1177271918785130
Chiang YW, Su CH, Sun HY, Chen SP, Chen CJ, Chen WY, Chang CC, Chen CM, Kuan YH (2022) Bisphenol A induced apoptosis via oxidative stress generation involved Nrf2/HO-1 pathway and mitochondrial dependent pathways in human retinal pigment epithelium (ARPE-19) cells. Environ Toxicol 37(1):131–141. https://doi.org/10.1002/tox.23384
Chiba S, Saito A, Ogawa S, Takeuchi K, Kumano K, Seo S, Suzuki T, Tanaka Y, Saito T, Izutsu K, Yuji K, Masuda S, Futami S, Nishida M, Suzuki G, Gale RP, Fukayama M, Maekawa K, Hirai H (2002) Transplantation for accidental acute high-dose total body neutron- and gamma-radiation exposure. Bone Marrow Transplant 29(11):935–939. https://doi.org/10.1038/sj.bmt.1703568
Clarke HE, Coates ME, Eva JK, Ford DJ, Milner CK, O’Donoghue PN, Scott PP, Ward RJ (1977) Dietary standards for laboratory animals: report of the Laboratory Animals Centre Diets Advisory Committee. Lab Anim 11(1):1–28. https://doi.org/10.1258/002367777780959175
Downie T (1990) Theory and practice of histological techniques edited by JD Bancroft & A. Stevens, Churchill Livingstone, Edinburgh, 740 pages, £ 55.00. Histopathology 17:386
El Morsy E, Ahmed M (2020) Protective effects of lycopene on hippocampal neurotoxicity and memory impairment induced by bisphenol A in rats. Hum Exp Toxicol 39(8):1066–1078. https://doi.org/10.1177/0960327120909882
El-Baz FK, Elgohary R, Salama A (2021) Amelioration of hepatic encephalopathy using Dunaliella salina microalgae in rats: modulation of hyperammonemia/TLR4. Biomed Res Int 2021:8843218. https://doi.org/10.1155/2021/8843218
El-Far AH, Oyinloye BE, Sepehrimanesh M, Allah MAG, Abu-Reidah I, Shaheen HM, Razeghian-Jahromi I, Alsenosy AEA, Noreldin AE, Al Jaouni SK, Mousa SA (2019) Date palm (Phoenix dactylifera): novel findings and future directions for food and drug discovery. Curr Drug Discov Technol 16(1):2–10. https://doi.org/10.2174/1570163815666180320111937
El-Shahat AN, Hamza RG, Mounir AM, Al-Seeni MN (2021) Ameliorative effect of graviola fruit juice on the damaged tissues of gamma-irradiated male rats. Pakistan J. Zool., pp 1–8. https://doi.org/10.17582/journal.pjz/20201113181133.
Eweda SM, Newairy A, Abdou HM, Gaber AS (2020) Bisphenol A-induced oxidative damage in the hepatic and cardiac tissues of rats: the modulatory role of sesame lignans. Exp Ther Med 19(1):33–44. https://doi.org/10.3892/etm.2019.8193
Fadl NN, Ahmed HH, Booles HF, Sayed AH (2013) Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum Exp Toxicol 32(7):721–735. https://doi.org/10.1177/0960327112467040
Gallelli G, Di Mizio G, Palleria C, Siniscalchi A, Rubino P, Muraca L, Cione E, Salerno M, De Sarro G, Gallelli L (2021) Data recorded in real life support the safety of nattokinase in patients with vascular diseases. Nutrients 13(6):2031. https://doi.org/10.3390/nu13062031
Gao XH, Zheng J, Ma L, Ma LB, Zhai YJ, Yang FF, Wang SJ, Fan QS, Wen J, Wang HL, Wu XH, Chen SS, Liu CM (2021) Mitigation of acute radiation-induced brain injury in a mouse model using anlotinib. Ann palliat med 10(1):312–322. https://doi.org/10.21037/apm-20-2284
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD (2012) Radiation-induced brain injury: a review. Front Oncol 2:73. https://doi.org/10.3389/fonc.2012.00073
Hanedan Uslu G, Canyilmaz E, Serdar L, Ersöz Ş (2019) Protective effects of genistein and melatonin on mouse liver injury induced by whole-body ionising radiation. Mol Clin Oncol 10(2):261–266. https://doi.org/10.3892/mco.2018.1790
Hassan E, El-Neweshy M, Hassan M, Noreldin A (2019) Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: possible mechanisms are involved. Life Sci 1(230):132–140. https://doi.org/10.1016/j.lfs.2019.05.067
Hawas AMM, Rashed LA, Mohamed MA (2020) Evaluation of glucosamine effect against heart and brain damage induced by Y-radiation or aluminium chloride in female rats., Braz Arch Biol Technol 63(7). https://doi.org/10.1590/1678-4324-2020180687.
Heneka MT, Carson MJ, El Khoury J et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Huang FM, Ch ang YC, Lee SS, Ho YC, Yang ML, Lin HW, Kuan YH (2018) Bisphenol A exhibits cytotoxic or genotoxic potential via oxidative stress-associated mitochondrial apoptotic pathway in murine macrophages. Food Chem Toxicol: an Int J Published for the Br Ind Biol Res Assoc 122:215–224. https://doi.org/10.1016/j.fct.2018.09.078
Huang Z, Ng TK, Chen W, Sun X, Huang D, Zheng D, Yi J, Xu Y, Zhuang X, Chen S (2021) Nattokinase attenuates retinal neovascularization via modulation of Nrf2/HO-1 and glial activation. Invest Ophthalmol vis Sci 62(6):25. https://doi.org/10.1167/iovs.62.6.25
Jiménez-Torres C, El-Kehdy H, Hernández-Kelly LC, Sokal E, Ortega A, Najimi M (2021) Acute liver toxicity modifies protein expression of glutamate transporters in liver and cerebellar tissue. Front Neurosci 6(14):613225. https://doi.org/10.3389/fnins.2020.613225
Kale A, Pişkin Ö, Baş Y, Aydın BG, Can, Büyükuysa Ç (2019) Ameliorative effects of Hesperidin on radiation induced brain injury in rats. Int J Radiat Res 17(2):229–236. https://doi.org/10.18869/acadpub.ijrr.17.2.229
Kamel AH, Foaud MA, Moussa HM (2018) The adverse effects of bisphenol A on male albino rats. J Basic Appl Zool 79:6. https://doi.org/10.1186/s41936-018-0015-9
Kaulmann A, Bohn T (2014) Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr res (New York, N Y) 34(11):907–929. https://doi.org/10.1016/j.nutres.2014.07.010
Khafaga AF, Noreldin AE, Taha AE (2019) The adaptogenic anti-ageing potential of resveratrol against heat stress-mediated liver injury in aged rats: role of HSP70 and NF-kB signalling. J Therm Biol 83:8–21. https://doi.org/10.1016/j.jtherbio.2019.04.012
Khafaga AF, El-Kazaz SE, Noreldin AE (2021) Boswellia serrata suppress fipronil-induced neuronal necrosis and neurobehavioral alterations via promoted inhibition of oxidative/inflammatory/apoptotic pathways. Sci Total Environ 785:147384. https://doi.org/10.1016/j.scitotenv.2021.147384
Khandelwal M, Manglani K, Gupta S, Tiku AB (2020) Gamma radiation improves AD pathogenesis in APP/PS1 mouse model by potentiating insulin sensitivity. Heliyon 6(7):e04499. https://doi.org/10.1016/j.heliyon.2020.e04499
Kim HG, Jang SS, Lee JS, Kim HS, Son CG (2017) Panax ginseng Meyer prevents radiation-induced liver injury via modulation of oxidative stress and apoptosis. J Ginseng Res 41(2):159–168. https://doi.org/10.1016/j.jgr.2016.02.006
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s dement (New York, N Y) 4:575–590. https://doi.org/10.1016/j.trci.2018.06.014
Konitzer K, Voigt S (1963) Clinica chimica acta; Int J Clin Chem 8:5–11. https://doi.org/10.1016/0009-8981(63)90192-x
Lebda MA, Hashem AS, Taha NM, Mandour A, Edres HA (2020) L-carnitine mitigates bisphenol A-induced hepatic toxicity via activation of Nrf2 and inhibition of pro-inflammatory cytokine gene expression in rats. Veterinarski arhiV 90(1):57–68. https://doi.org/10.24099/vet.arhiv.0438
Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW (2010) Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol 86(2):132–144. https://doi.org/10.3109/09553000903419346
Li J, W O, Li W, Jiang ZG, Ghanbari HA (2013) Oxidative stress and neurodegenerative disorders. Int j mol sci 14(12):24438–24475. https://doi.org/10.3390/ijms141224438
Li L, Wang W, Welford S, Zhang T, Wang X, Zhu X (2014) Ionizing radiation causes increased tau phosphorylation in primary neurons. J Neurochem 131(1):86–93. https://doi.org/10.1111/jnc.12769
Li B, Nasser MI, Masood M, Adlat S, Huang Y, Yang B, Luo C, Jiang N (2020) Efficiency of traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed pharmacother = Biomed pharmacotherapie 126:110074. https://doi.org/10.1016/j.biopha.2020.110074
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci: CMLS 73(17):3221–3247. https://doi.org/10.1007/s00018-016-2223-0
Ma Y, Liu H, Wu J et al (2019) The adverse health effects of bisphenol A and related toxicity mechanisms. Environ Res 176:108575. https://doi.org/10.1016/j.envres.2019.108575
Mahdavinia M, Ahangarpour A, Zeidooni L, Samimi A, Alizadeh S, Dehghani MA, Alboghobeish S (2019) Protective effect of naringin on bisphenol A-induced cognitive dysfunction and oxidative damage in rats. Int j mol cell med 8(2):141–153. https://doi.org/10.22088/IJMCM.BUMS.8.2.141
Michel J, Abd Rani NZ, Husain K (2020) A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front Pharmacol 11:852. https://doi.org/10.3389/fphar.2020.00852
Moss DW (1982) (1982) Alkaline phosphatase isoenzymes. Clin Chem 28(10):2007–2016. https://doi.org/10.1093/clinchem/28.10.2007
Nahirnyj A, Livne-Bar I, Guo X, Sivak JM (2013) ROS detoxification and proinflammatory cytokines are linked by p38 MAPK signaling in a model of mature astrocyte activation. PLoS ONE 8(12):e83049. https://doi.org/10.1371/journal.pone.0083049
Najafi M, Motevaseli E, Shirazi A, Geraily G, Rezaeyan A, Norouzi F, Rezapoor S, Abdollahi H (2018) Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol 94(4):335–356. https://doi.org/10.1080/09553002.2018.1440092
Nguyen T, Nguyen CH (2020) Determination of factors affecting the protease content generated in fermented soybean by Bacillus subtilis 1423. Energy Rep 6:831–836. https://doi.org/10.1016/j.egyr.2019.11.011
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Omar HA, Sargeant AM, Weng JR, Wang D, Kulp SK, Patel T, Chen CS (2009) Targeting of the Akt-nuclear factor-kappa B signaling network by [1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol (OSU-A9), a novel indole-3-carbinol derivative, in a mouse model of hepatocellular carcinoma. Mol Pharmacol 76(5):957–968. https://doi.org/10.1124/mol.109.058180
Pham PT, Han B, Hoang BX (2020) Nattospes as effective and safe functional supplements in management of stroke. J Med Food 23(8):879–885. https://doi.org/10.1089/jmf.2019.0183
Plaa G, Charbonneau M, Plante I (2014) Detection and evaluation of chemically induced liver injury. In book: Hayes’ principles and methods of toxicology, sixth edition.; chapter 29,1445–1488. https://doi.org/10.1201/b17359-33.
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56
Schiffer D, Giordana MT, Migheli A, Giaccone G, Pezzotta S (1986) Mauro A (1986) Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res 374:110–118
Sharma NK, Sharma R, Mathur D, Sharad S, Minhas G, Bhatia K, Anand A, Ghosh SP (2018) Role of ionizing radiation in neurodegenerative diseases. Front Aging Neurosci 10:134. https://doi.org/10.3389/fnagi.2018.00134
Sharma NK, Stone S, Kumar VP, Biswas S, Aghdam SY, Holmes-Hampton GP, Fam CM, Cox GN, Ghosh SP (2019) Mitochondrial degeneration and autophagy associated with delayed effects of radiation in the mouse brain. Front Aging Neurosci 11:357. https://doi.org/10.3389/fnagi.2019.00357
Sukjamnong S, Thongkorn S, Kanlayaprasit S, Saeliw T, Hussem K, Warayanon W, Hu VW, Tencomnao T, Sarachana T (2020) Prenatal exposure to bisphenol A alters the transcriptome-interactome profiles of genes associated with Alzheimer’s disease in the offspring hippocampus. Sci Rep 10(1):9487. https://doi.org/10.1038/s41598-020-65229-0
Suzuki H, Kobayashi K, Kosuge Y, Sasaoka Y, Tajika Y, Sugiyama T, Ichikawa T and Ishihara K (2017) Nattoesse inhibits the high-fat diet-induced increase in serum lipid levels in ApoE-deficient mice. Int J Anal Bio-Sci.; Vol. 5, No 1.
Takagaki S, Suzuki M, Suzuki E, Hasumi K (2020) Unsaturated fatty acids enhance the fibrinolytic activity of subtilisin NAT (nattokinase). J Food Biochem 44(8):e13326. https://doi.org/10.1111/jfbc.13326
Thoene M, Rytel L, Dzika E, Włodarczyk A, Kruminis-Kaszkiel E, Konrad P, Wojtkiewicz J (2017) Bisphenol A causes liver damage and selectively alters the neurochemical coding of intrahepatic parasympathetic nerves in juvenile porcine models under physiological conditions. Int J Mol Sci 18(12):2726. https://doi.org/10.3390/ijms18122726
Tiwari SK, Agarwal S, Seth B, Yadav A, Ray RS, Mishra VN, Chaturvedi RK (2015) Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-catenin pathway. Mol Neurobiol 52(3):1735–1757. https://doi.org/10.1007/s12035-014-8940-1
Trivedi R, Khan AR, Rana P, Haridas S, Hemanth Kumar BS, Manda K, Rathore RK, Tripathi RP, Khushu S (2012) Radiation-induced early changes in the brain and behavior: serial diffusion tensor imaging and behavioral evaluation after graded doses of radiation. J Neurosci Res 90(10):2009–2019. https://doi.org/10.1002/jnr.23073
Ullah R, Alqahtani AS, Noman OMA, Alqahtani AM, Ibenmoussa S, Bourhia M (2020) A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J Bio Sci 27(10):2706–2718. https://doi.org/10.1016/j.sjbs.2020.06.020
Wang JM, Chen HY, Cheng SM, Chen SH, Yang LL (2012) Cheng FC (2012) Nattokinase reduces brain infarction, fibrinogen and activated partial thromboplastin time against cerebral ischemia-reperfusion injury. J Food Drug Anal 20(3):686–691. https://doi.org/10.6227/jfda.2012200317
Wang T, Xie C, Yu P, Fang F, Zhu J, Cheng J, Gu A, Wang J, Xiao H (2017) Involvement of insulin signaling disturbances in bisphenol A-induced Alzheimer’s disease-like neurotoxicity. Sci Rep 7(1):7497. https://doi.org/10.1038/s41598-017-07544-7
Wei J, Wang B, Wang H, Meng L, Zhao Q, Li X, Xin Y, Jiang X (2019) Radiation-induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev 2019:3010342. https://doi.org/10.1155/2019/3010342
Yang L, Yang J, Li G, Li Y, Wu R, Cheng J, Tang Y (2017) Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol Neurobiol 54(2):1022–1032. https://doi.org/10.1007/s12035-015-9628-x
Zhang X, Lyu X, Tong Y, Wang J, Ye J, Yang R (2020) Chitosan/casein based microparticles with a bilayer shell-core structure for oral delivery of nattokinase. Food Funct 11(12):10799–10816. https://doi.org/10.1039/d0fo02349c
Zhang T, Shi L, Li Y, Mu W, Zhang H, Li Y, Wang X, Zhao W, Qi Y, Liu L (2021) Polysaccharides extracted from Rheum tanguticum ameliorate radiation-induced enteritis via activation of Nrf2/HO-1. J Radiat Res 62(1):46–57. https://doi.org/10.1093/jrr/rraa093
Zhu J, Jiang L, Liu Y, Qian W, Liu J, Zhou J, Gao R, Xiao H, Wang J (2015) MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells. Inflammation 38(2):637–648. https://doi.org/10.1007/s10753-014-9971-5
Acknowledgements
The authors want to send a special thanks to Prof. Dr. Ahmed Osman (Department of Pathology, Faculty of Veterinary Medicine, Cairo University) for his unappreciated effort in the histopathological examination and for writing the comments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mustafa M. M. Elbakry: conceptualization, methodology. Somaya Z. Mansour: data curation, methodology. Hamed Helal: conceptualization, writing—reviewing and editing, visualization, methodology. Esraa S. A. Ahmed: conceptualization, writing—original draft preparation, investigation, data curation.
Corresponding author
Ethics declarations
Ethics approval
This experiment was carried out according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (No. 85:23, 1996) and in compliance with the principles and guidelines of the animal care committee of the National Center for Radiation Research & Technology (NCRRT), Cairo, Egypt. Every attempt was made to keep animal suffering to a minimum.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elbakry, M.M.M., Mansour, S.Z., Helal, H. et al. Nattokinase attenuates bisphenol A or gamma irradiation-mediated hepatic and neural toxicity by activation of Nrf2 and suppression of inflammatory mediators in rats. Environ Sci Pollut Res 29, 75086–75100 (2022). https://doi.org/10.1007/s11356-022-21126-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21126-9