Abstract
This research evaluates the effect of dietary zinc oxide nanoparticles’ (ZnO NPs) supplementation on growth performance, immunity, oxidative antioxidative properties, and histopathological picture of broiler chicken reared in the summer season. A total of 224 1-day-old male Cobb chicks were randomly allocated to seven groups of dietary treatments (n = 32). Seven isocaloric and isonitrogenous diets were formulated. ZnO NPs were added to the basal diet at seven different levels, 0, 5, 10, 20, 40, 60, and 80 ppm/kg diet, respectively, for 35 days. Results indicated that live body weight (g) did not differ significantly (P > 0.05) between treatment groups, whereas compared to control, the 5 ppm ZnO NPs/kg diet recorded the highest live body weight at 21 and 35 days. No significant effects for the feed consumption (g/bird/period) and feed conversion ratio (g feed/g gain) among treated and control birds were observed. Hematological and immunological variables showed significant (P ≤ 0.05) dose-dependent modulations by ZnO NP supplementation. Significant (P ≤ 0.05) differences were observed in the phagocytic activity, phagocytic index, and IgM and IgG between the treatment groups, with the 5 and 10 ppm ZnO NPs/kg diet recording the best values, followed by the 20 ppm ZnO NPs/kg diet. Different supplementations had nonsignificant effects on the digestibility of nutrients (P ≤ 0.05). Histopathological pictures of the kidney, liver, and lymphoid organs, ultrastructural examination of muscle tissues, and expression of inflammatory cytokines showed dose-dependent morphological and structural changes. In conclusion, the ZnO NP supplementation in broiler diet to eliminate the heat stress hazards in summer season is recommended in dose level of not more than 10 ppm/kg diet.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In tropical regions, high ambient temperature is considered one of the crucial factors inducing stress in birds. Due to worldwide warming, high temperature has lately become one of the greatest important stressors influencing the poultry industry (Jadhao et al. 2020; Lara and Rostagno 2013). Once the chicks are subjected to constant high temperatures, this exposure to heat negatively affects the performance and specific immune response and can lead to death, causing a large economic loss in the poultry industry (Abdel-Latif et al. 2018; Rao et al. 2016; Rossi et al. 2007).
The immune response and the antioxidant system of poultry can be enhanced using several feed additives, especially during heat stress, such as trace minerals, vitamins, and probiotics (Abdel-Latif et al. 2018; Dawood et al. 2019a, 2019b; Saeed et al. 2020; Zahin et al. 2020). Recently, more attention has been paid to adding zinc (Zn) supplementation in bird feed to improve the growth performance in broilers. This additive is increasingly required in bird diets during heat stress (Chand et al. 2021; Chand et al. 2020; De Grande et al. 2020; Hafez et al. 2020; Naz et al. 2016; Sahin et al. 2003; Shah et al. 2020) as it positively impacts the performance of chicken subjected to thermal challenges (Rao et al. 2016), enhancing the physiological responses, performance parameters, and immune response of heat-stressed birds (Shah et al. 2020). Moreover, it was reported that Zn and probiotics clearly modulated the intestinal microstructures of birds reared under high temperatures.
Zn is an essential trace mineral and vital for poultry’s metabolic functions, growth, and glandular improvement. It is a cofactor for the activity of up to 300 enzymes in birds (Salim et al. 2008). Moreover, it plays a role in gene transcription and cell division, among other processes (Feng et al. 2010b; Herrera et al. 2017). Therefore, the United Nations considered Zn as a “life-saving commodity,” and this element cannot be stored in the bird’s body (Swain et al. 2016). According to Applegate and Angel (2014) and Council (1994), the Zn requirements in bird diets range from 40 to 75 mg/kg. To meet the Zn requirements, the amount of added Zn should be almost 20- to 30-folds more than that in the normal diets of animals based on the low utilization level of Zn (Bratz et al. 2013). Furthermore, exposure to heat stress conditions increases the need for Zn. Furthermore, a high level of Zn could lead to excess Zn in the fecal matter, causing environmental pollution (Broom et al. 2002). Additionally, it could have a major effect on the balance between the other trace elements in the birds body and reduce the vitamins and nutrients stability (Sahin and Kucuk 2003; Sundaresan et al. 2008; Zhang et al. 2018).
Newly, trace minerals, including Zn nanoparticles, can be successfully used to fulfill the needs of minerals in bird diets (Dosoky et al. 2021; El-Seedi et al. 2019; Mohammed et al. 2020; Sizova et al. 2020) based on their extremely small size and specific physical properties (Biria et al. 2020; Fouda et al. 2021; Radi et al. 2021). Nanoparticles can successfully supply minerals in birds and enhance the growth rate and feed utilization (Abdel-Daim et al. 2019, Abdelsalam et al. 2019, Bhattacharya et al. 2021, Fouda et al. 2020, Gangadoo et al. 2016, Hafez et al. 2017, Ibrahim et al. 2021, Kandeil et al. 2019, Mohammed and Safwat 2020, Oberdörster et al. 2005).
Zinc oxide nanoparticles (ZnO NPs) outweigh conventional Zn sources and positively affect the performance and antioxidant defense of chickens (Ali et al. 2017, El-Katcha et al. 2017b, Eskandani et al. 2021, Mohammadi et al. 2015, Mohammed and Safwat 2013). It could be used as an alternative to antibiotics or as growth promoters (Schmidt 2009). On the other hand, some studies have investigated the toxicity of ZnO NPs in several biological systems, such as bacteria (Sinha et al. 2011) and mammalian cells (Wang et al. 2010). Another study has demonstrated a significantly reduced growth rate of some marine phytoplankton species when ZnO NPs are used in their diet (Miller et al. 2010), explaining the ZnO NPs toxicity in phytoplankton due to the uptake of free Zn ions. In mammalian cells, the ZnO NP toxic effects, like membrane injury, DNA damage, and apoptosis, have been proven (Abdel-Daim et al. 2021; Gojova et al. 2007; Samak et al. 2018). Hence, this study evaluates the efficacy and the possible toxicity of ZnO NPs supplemented in a broiler diet. A feeding trial was taken to investigate their effects on the growth performance, oxidative status, immunologic parameters, and histopathological pictures of internal organs. To the best of the author’s knowledge, this is the first study that evaluates the histopathologic picture of lymphoid organs of ZnO NP-supplemented broiler.
Materials and methods
This investigation was done at the Faculty of Agriculture (Saba Basha), Poultry Research Laboratory, Alexandria University, Egypt, under the approval of ethical standards of scientific research № AU: 14/19/12/19/01/06 from Alexandria University, 2019.
Preparation and characterization of ZnO NPs in powder form
ZnO NPs were produced via the wet chemical technique by utilizing the naturally occurring polysaccharide, namely, sodium alginate, and the precursor, zinc nitrate, Zn(NO3)2.4H2O in the existence of alkaline solution of sodium hydroxide (NaOH) as reported in previous work (Desai et al. 2019; Ishak et al. 2019). At first, 0.5 g of sodium alginate was liquified in 100 ml of dH2O comprising 0.2 g of NaOH. The reaction is kept under stirring for about 10 min until complete solubilization. To this end, a solution of 0.1 M of Zn(NO3)2.4H2O was added dropwise, with continuous high-speed stirring for another 30 min. In the end, the formed colloidal solution is left overnight to settle down, and the remarked supernatant solution is carefully separated. The remaining solution was subjected to centrifugation; then the precipitate was collected and washed 3 times with ddH2O and C2H6O to remove the undesired products that may have linked with the formed nanoparticles. The obtained powder is subjected to drying at 80 °C for 24 h, followed by calcination at 600 °C for another hour to warrant the complete conversion of Zn(OH)2 to ZnO NPs. For the characterization of ZnO NPs, X-ray diffraction (XRD) was measured under ambient conditions via Siemens D-500 X-ray diffractometer (from 30 mA to 40 kV) bearing a copper (Cu) tube. The morphological description of the ZnO NPs was detected during transmission electron microscopy (TEM) on a JEOL (JEM-1230, Japan); the instrument was with an acceleration voltage of 120 kV.
Chicken and dietary treatments
A total of 224 1-day-old chicks (male Cobb 500 chicks, white feather chicks) were utilized in this current study. Chicks weighed 42 g on average and were divided into seven groups randomly with 32 birds in each group, which were allotted into 4 replicates (8 birds in each) in a complete randomized design. Chicks were assigned to twelve pens (1.35 × 1.45 m) (Jang et al. 2008). The birds were vaccinated according to Cobb’s company protocol. The starter and growing diets are found in Table 1 according to the National Research Council (Council 1994; Fouda et al. 2021). The treatments were as follows: T1, control, and T2 to T7, the control diet plus the ZnO NPs levels of 5, 10, 20, 40, 60, and 80 ppm/kg diet, respectively. The dose range of ZnO NPs was chosen according to several relevant studies (El-Haliem et al. 2020; Hafez et al. 2020; Ramiah et al. 2019). The dietary experiment started when the chicken was 1-day-old and ended at 35 days of age. The experiment was conducted over a 5-week duration. Feed and water were given ad libitum. The quantity of feed was weighed before being distributed, and the remaining feed in the next week was used to calculate the feed intake of each group. Throughout, the ambient temperature ranged between 28.8 and 33.7 °C, and the relative humidity ranged between 58.0 and 79.01% during the summer of 2019.
Estimation of growth performance
Chickens were weighed at 1 day of age (the beginning of the test) and weekly for 35 days (the end of the test). Feeding was stopped 12 h before weighing (Li et al. 2007; Marcu et al. 2013). The feed for the day was weighed, and the remaining feed was collected and weighed the next week to calculate the average feed intake (Aydin et al. 2014). The ratio of feed consumption to weight gain was calculated based on the ratio of average feed intake to average gain. Performance index (PI) was determined using the following method: PI = BWG (g) × FER, where BWG is the body weight gain and FER is the feed efficiency ratio (Kalantar et al. 2011). The relative growth rate was estimated according to the following equation (Aggrey 2004):
Growth rate (%) = (W2 – W1) × 100/0.5 (W1 + W2), where Wl is the body weight at the beginning of the test and W2 is the body weight at the last week of the test for which the rate was determined.
Blood sampling and biochemical index
Eight birds from every treatment (from 4 replicates) were randomly selected and slaughtered. The blood was divided into two parts equally. The first part was stored on ethylenediaminetetraacetic acid (EDTA) to assess the blood hematology (Toghyani et al. 2010), while the second part was centrifuged at 3500 rpm/15 min and used to all biochemical analyses. Serum immunoglobulin fractions (IgM and IgG) were assessed according to Kincade et al. (1970). The phagocytic activity (PA) was calculated according to Hafez et al. (2020). The phagocytic index (PI) was evaluated according to Hafez et al. (2020). Moreover, the hemagglutination inhibition test was utilized to define the humoral antibody titer versus the NDV. Serum lysozyme activity was established through the turbidimetry method described by He et al. (2007). Serum oxidative/antioxidant index was carried out by kits produced by Biodiagnostic, according to Motor et al. (2014).
Lymphoid organ weight and some carcass traits
Eights chicks from each treatment (4 replicates) were selected, slaughtered to full bleeding, and weighed to calculate the immune organs’ relative weight.
Nutrient retention
At 5 weeks of age, a digestibility trial was done using 28 males (four cocks per treatment), and each was housed in an individual metabolic cage, which allowed for complete separation and collection of excreta and assessing each dietary treatment. Chemical analyses for nutrients were performed according to the Association of Official Agricultural Chemists (2007). The fecal nitrogen was determined following the procedure outlined in Jacobson et al. (1960) and Jakobsen et al. (2003). Digestibility was determined by accurately measuring feed intake and fecal output. Also, the digestibility coefficient of nutrients was determined.
Histopathological evaluation
Shortly after slaughter, small specimens from the thymus, spleen, kidneys, liver, and bursa of control and treated chicks were obtained. For fixation, the obtained samples were cleaned and soaked in 10% neutral buffered formalin liquid for 48 h. The paraffin-embedding technique was used to prepare the fixed samples (Saad et al. 2021; Sato et al. 1986, Wright and Manos 1990). Several 4 mm in thick sections were cut and dyed with hematoxylin and eosin on a regular basis. A qualified pathologist (AFK) conducted the blinded assessment and picture capture. A specific digital camera (Leica EC3; Leica, Germany) linked to a microscope was used to take representative micrographs (Leica DM500).
Ultrastructural evaluations of muscle tissues
Limited specimens of breast muscle were collected immediately after slaughter. Specimens were cut into tiny parts (~ 1 mm3) and immediately fixed in 0.1 M phosphate-buffered saline (PBS) for at least 3 h in 3% glutaraldehyde solution C5H8O2 (Merck, Darmstadt, Germany) (pH 7). After two buffer switches, fixed samples were moved to a 1% osmium tetroxide (OsO4) solution (Electron Microscope Science, Sigma-Aldrich) for 1 h in 0.1 M PBS (pH = 6.9). After that, the samples were rewashed in 0.1 M PBS for 5 min, dehydrated in increasing ethanol concentrations, and impregnated with Epon embedding resin. The samples were embedded for 48 h at 60 °C and then blocked. For light microscopy, semithin parts were prepared and stained with 1% simple toluidine blue. After that, the ultrathin parts (50–80 nm) were cut from the chosen areas and put on copper grids (200 mesh). Finally, segment comparing was carried out with uranyl acetate dihydrate (2%) and lead citrate. Tissues were investigated and photographed using a JEM-1220 transmission electron microscope (TEM; JEOL, Tokyo, Japan).
Gene expression
A real-time polymerase chain reaction was used to evaluate the precise expression of inflammatory genes (IL-B1 and TNF-α) in muscle tissues (Dosoky et al. 2021; Saad et al. 2021). TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA) and NanoDrop for quantification were utilized for RNA extraction from ~ 100 mg muscle. A260 or A260/A280 RNA samples were used to synthesize DNA with a cDNA synthesis package (Fermentas, Waltham, MA, USA). Table 2 shows the primers and housekeeping gene.
Statistical analysis
To analyze the effects of ZnO NP levels on the dependent variables, SPSS version 16 (SPSS, Inc., Chicago, IL, USA) was used based on one-way ANOVA. Data are represented as means. Significant variation intergroup means were obtained (Duncan 1955). Statistical significance was accepted at P ≤ 0.05.
Results
Characterization ZnO NPs
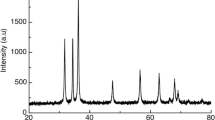
The morphology, particle size structure, and distribution of ZnO NPs were evaluated using TEM. The instrument was adjusted within an acceleration voltage of 120 kV. The findings are outlined in Fig. 1A. As it is evident, the formed particles are nearly spherical with a comparatively narrow size distribution, as observed in Fig. 1A. The major sizes were in the range of 6.53 nm. In addition, there was a plausible portion of ~ 26% with the total sizes of ~ 12.3 nm, which might be attributed to the tendency of the formed particles to aggregate to larger sizes. The polycrystalline nature of ZnO NPs is detected by the resultant selected area electron diffraction (Fig. 1B). Once electron diffraction is conceded on a limited number of crystals, the exterior of the discrete points in the circle pattern affirmed that most ZnO NPs are more or less single-crystalline materials and are predominately along with ZnO NP direction, as ordinarily originated for the ZnO crystal lattice. The X-ray diffraction (XRD) study of the generated ZnO-based NPs is depicted in Fig. 1C. The broad XRD signals designate that the generated ZnO-based powder is composed of nanoscale particles. The diffraction XRD signals at 31.79°, 34.40°, 36.21°, 44.51°, 47.68°, 56.75°, 62.95°, 68.15°, and 69.07° have been reported previously and indexed as a hexagonal wurtzite structure of ZnO-based NPs with lattice constants a = b = 0.325 nm and c = 0.519 nm. The XRD spectra proved that the produced powder of ZnO-based NPs is pure because no characteristic XRD signals were detected other than the ZnO signals.
Growth performance parameters
The effects of ZnO NP supplementation on the growth performance of broilers are shown in Table 3. Results indicated that the average live BW of broilers did not differ significantly (P > 0.05) between treatment groups. Treatment with the 5 ppm ZnO NPs/kg diet recorded the highest LBW at 21 and 35 days. At 35 days, the BW was 1940.90 g at the 5 ppm/kg level and improved by 21.60 g compared to the control (Table 3). BWG increased by 873.02 g for the age of 1 to 21 days under the 5 ppm/kg level and by 1898 g for the period of 21 to 35 days. Results revealed no significant impacts for FI and FCR among the treated and control birds. Furthermore, the FCR had the lowest nonsignificant values (P > 0.05) compared to the control birds, for example, 1.410 g in the control birds and under a level of 5 ppm/kg for 1 to 35 days. In the same trend, growth rate (%) and PI were found (Table 3).
Biochemical and hematological parameters
Among hematological and immunological variables, WBC and RBC counts, PCV, lymphocytes (%), heterophils (%), lymphocytes/heterophils ratio, and monocytes were significantly (P ≤ 0.05) affected by ZnO NP supplementation. The group given the 10 ppm ZnO NPs/kg diet had the highest RBC counts compared to the other treated groups. However, Hb concentration, basophils (%), and eosinophils (%) were not significantly influenced by different treatments (Table 4).
Immunological parameters
Data in Table 4 showed that significant differences (P ≤ 0.05) between groups were obtained for PA, PI, IgM, and IgG, where the best value was recorded in the groups given 5 and 10 ppm ZnO NPs/kg diet, followed by the group given 20 ppm ZnO NPs/kg diet. However, antibody titers against the NDA, lysosomes, and interleukin (IL)-6 were not significantly affected by different treatments.
Biochemical parameters
Data obtained on serum biochemical estimates in chicks as affected by ZnO NPs are shown in Table 5. Results showed that serum albumin, total protein, globulin, and albumin/globulin ratio were not significantly different in all groups. Results also showed that serum ALP was insignificantly (P ≤ 0.05) enhanced in all treated groups, except the group given the 20 ppm ZnO NPs/kg diet. In contrast, serum AST was significantly (P ≤ 0.05) increased in the groups given the 40 and 80 mg ZnO NPs/kg diet compared to the control.
ALT was significantly (P ≤ 0.05) decreased by different treatments, and the lowest value was obtained in the group given the 40 ppm ZnO NPs/kg diet. Serum total lipid and cholesterol concentrations were insignificantly (P ≤ 0.05) decreased due to the addition of different levels of ZnO NPs compared with control. However, triglyceride and HDL concentrations were significantly (P ≤ 0.05) decreased. In addition, LDL levels were significantly (P ≤ 0.05) improved compared to the control. Serum uric acid showed a nonsignificant difference between the control and treated groups. However, creatinine was significantly (P ≤ 0.05) increased in groups given the 40 and 80 ppm ZnO NPs/kg diet. The lowest creatinine and uric acid concentrations were recorded in groups given the 5 and 10 ppm ZnO NPs/kg diet, respectively.
Oxidative parameters
Serum CAT was significantly (P ≤ 0.05) decreased in groups the given 40 and 80 ppm ZnO NPs/kg diet compared to the group control. TAC showed a significant reduction (P ≤ 0.01) in broilers given the 60 and 80 ppm ZnO NPs/kg diet. Additionally, serum lipid peroxide (MDA) concentrations were significantly (P ≤ 0.01) increased in groups given the 40, 60, and 80 ppm ZnO NPs/kg diet (Table 5).
Organ weight and digestibility
Table 5 shows a significant (P ≤ 0.05) increase in serum Zn, calcium, and inorganic phosphorous concentrations observed with increased ZnO NP levels in the diet. The carcass relative weights, proventriculus gland, liver, gizzard, spleen, pancreas, intestine, cecum, bursa, and thymus were not significantly (P ≤ 0.05) affected by ZnO NP supplementation. However, the relative weights of the gizzard, spleen, pancreas, and thymus were significantly (P ≤ 0.001 or 0.01) affected by ZnO NP treatments (Table 6). Data regarding the effects of including different ZnO NP levels in broiler diets on the digestibility coefficients of nutrients are shown in Table 7. Different supplementations had nonsignificant effects on the digestibility of nutrients (P ≤ 0.05).
Histopathological evaluations
Liver
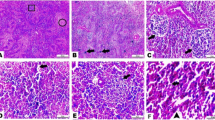
The histopathological analysis of control chickens showed normal hepatic tissue with the natural structure of hepatic lobules, portal areas, and central veins and no specific lesions (Fig. 2A). In the meantime, chickens given 5 mg (Fig. 2B) and 10 mg (Fig. 2C) ZnO NPs had infrequent moderate centrilobular hydropic vacuolization. The pathological analysis of liver tissues from chickens given 20, 40, and 60 mg ZnO NPs revealed multifocal aggregation of mononuclear cells (Fig. 2D), portal obstruction and thickening of portal areas with infiltrated mononuclear cells (Fig. 2E), and fibroplasia (Fig. 2F). Furthermore, in chicks given 80 mg ZnO NPs, there was pronounced thickening of periportal fibrous tissues with newly developed bile ductile, multifocal areas of coagulative necrosis, and diffuse vacuolization of hepatic lobules (Fig. 2G and H).
Representative photomicrographs from the liver of chicken treated with several levels of ZnO NPs for 5 weeks: H&E staining. The control group (A), broilers treated with 5 ppm of ZnO NPs (B), broilers treated with 10 ppm of ZnO NPs (C), broilers treated with 20 ppm of ZnO NPs (D), broilers treated with 40 ppm of ZnO NPs (E), broilers treated with 60 ppm of ZnO NPs (F), and broilers treated with 80 ppm of ZnO NPs (G, H) showing normal hepatic tissue (A), infrequent mild centrilobular hydropic vacuolization (arrows) (B, C), multifocal aggregation of mononuclear cells (arrow) and thickening of portal areas (yellow star) (D), fibroplasia (arrow) (E, F), pronounced thickening of the periportal fibrous tissues (star), newly developed bile ductules, and papillary hyperplasia (arrows) (G), and diffuse vacuolization of hepatic lobules (arrow) (H). ZnO NP, zinc oxide nanoparticle; H&E, hematoxylin and eosin. Scale bars = 100 μm (× 10) for photomicrographs (A–G) and 50 μm (× 40) for photomicrographs (H)
Kidney
Histopathological analysis of kidney tissues indicated that the kidneys of control chickens and those given 5 and 10 mg ZnO NPs had nearly average histological structures of the glomerulus, renal epithelium, and renal tubules (Fig. 3A–C). In contrast, renal tissues from chicks given 20 mg ZnO NPs demonstrated moderate vacuolization and degeneration of the renal epithelium (Fig. 3D). Furthermore, kidneys from chickens supplemented with 40, 60, and 80 mg ZnO NPs demonstrated significant infiltration of interstitial mononuclear inflammatory cells (Fig. 3E) and significant vacuolization and degeneration of the renal epithelium (Fig. 3F and G).
Representative photomicrographs from the kidney of chicken treated with several levels of ZnO NPs for 5 weeks: H&E staining. The control group (A), broilers treated with 5 ppm of ZnO NPs (B), broilers treated with 10 ppm of ZnO NPs (C), broilers treated with 20 ppm of ZnO NPs (D), broilers treated with 40 ppm of ZnO NPs (E), broilers treated with 60 ppm of ZnO NPs (F), and broilers treated with 80 ppm of ZnO NPs (G) showing normal histologic structures of renal tissue (A–C), moderate vacuolization and degeneration of the renal epithelium (arrow) (D), significant infiltration of interstitial mononuclear inflammatory cells (star) (E), and marked vacuolization and degeneration of renal epithelium (black arrow) together with atrophied glomerulus (yellow arrow) (G). ZnO NP, zinc oxide nanoparticle; H&E, hematoxylin and eosin. Scale bars = 50 μm (× 40)
Bursa of Fabricius
Control and treated chickens (5 mg) showed normal histological architecture of bursa in normal size and follicles number, normal intensity of medullary and cortical lymphocytic populations, and distinct corticomedullary junction (Fig. 4A and B). However, chickens given 10 or 20 mg ZnO NPs showed reduced number and size of follicles, reduced medullary cell populations, and widened interfollicular space with edema (Fig. 4C and D). However, tissue analysis of chickens given 40, 60, and 80 mg ZnO NPs showed atrophy of most bursa follicles, with atrophic follicles comprising a single cystic structure containing tissue debris (Fig. 4E). Furthermore, interfollicular edema and inflammatory filtrates were abundant in almost all parts (Fig. 4F and G).
Representative photomicrographs from the bursa of broilers treated with different concentrations of ZnO NPs for 35 days: H&E staining. The control group (A), broilers treated with 5 ppm of ZnO NPs (B), broilers treated with 10 ppm of ZnO NPs (C), broilers treated with 20 ppm of ZnO NPs (D), broilers treated with 40 ppm of ZnO NPs (E), broilers treated with 60 ppm of ZnO NPs (F), and broilers treated with 80 ppm of ZnO NPs (G) showing normal histological archicture of bursa (A, B), reduced number and size of follicles, reduced medullary cell populations, and widened interfollicular space with edema (arrows) (C, D), atrophy of the majority of the bursa follicles, with the atrophied follicles comprising single cystic structure containing tissue debris (arrow) (E), atrophied follicle (yellow star), interfollicular edema (black star), inflammatory filtrates (arrow) (F), interfollicular edema (black star), and focal coagulative necrosis of follicle (arrow) ZnO NPs, zinc nanoparticle; H&E, hematoxylin and eosin. Scale bars = 100 μm (× 10)
Spleen
Splenic tissues from the control group revealed the normal histological structure of lymphoid follicles and white and red pulps (Fig. 5A). In addition, chickens given the smaller doses of ZnO NPs (5, 10, and 20 mg) showed multifocal lymphoid depletion and reduction of lymphoid follicle size (Fig. 5B and C). However, chickens given 40–80 mg ZnO NPs demonstrated a complete absence of lymphoid follicles (Fig. 5D), with marked depletion and necrosis of the entire white pulps (Fig. 5E and F).
Representative photomicrographs from the spleen of broilers treated with different concentrations of ZnO NPs for 35 days: H&E staining. The control group (A), broilers treated with 5 ppm of ZnO NPs (B), broilers treated with 10 ppm of ZnO NPs (C), broilers treated with 20 ppm of ZnO NPs (D), broilers treated with 40 ppm of ZnO NPs (E), broilers treated with 60 ppm of ZnO NPs (F), and broilers treated with 80 ppm of ZnO NPs (G) showing normal histological structure of lymphoid follicles, white and red spleen pulps (A), multifocal lymphoid depletion and reduction of lymphoid follicles size (arrows) (B-D), complete absence of lymphoid follicles (arrow) (E), marked depletion and necrosis of the entire white pulps (F, G). ZnO NPs, zinc nanoparticle; H&E, hematoxylin and eosin. Scale bars = 100 μm (× 10)
Thymus
Examination of thymus tissues from control and treated chickens (5 and 10 mg) revealed normal architecture and intensity of cortical and medullary thymocytes with prominent corticomedullary junctions (Fig. 6A–C). However, chicks given 20 mg ZnO NPs showed a marked reduction of cortical and medullary thymocytes (Fig. 6D). However, thymus tissues from chicken given 40, 60, and 80 mg ZnO NPs showed a severe reduction of medullary and cortical basophilic thymocytes and accumulation of hemosiderin-laden macrophages (Fig. 6E), large infarct area within medullary tissues (Fig. 6F), and perifollicular edema with severe congestion and hemorrhage (Fig. 6G).
Representative photomicrographs from the thymus of broilers treated with different concentrations of ZnO NPs for 35 days: H&E staining. The control group (A), broilers treated with 5 ppm of ZnO NPs (B), broilers treated with 10 ppm of ZnO NPs (C), broilers treated with 20 ppm of ZnO NPs (D), broilers treated with 40 ppm of ZnO NPs (E), broilers treated with 60 ppm of ZnO NPs (F), and broilers treated with 80 ppm of ZnO NPs (G) showing normal architecture and intensity of cortical and medullary thymocytes (A–C), marked reduction of cortical and medullary thymocytes (stars) (D), severe reduction of medullary and cortical basophilic thymocytes and accumulation of hemosiderin-laden macrophages (arrow) (E), large infarct area within medullary tissues (arrow) (F), and perifollicular edema with severe congestion (stars) and hemorrhage (G). ZnO NPs, zinc nanoparticle; H&E, hematoxylin and eosin
IL-1β and TNF-α mRNA expression in muscle tissues
IL-1β mRNA expression was investigated (Fig. 7A). The degree of IL-1β expression in muscle tissues was significantly higher (P > 0.05) in chickens given 20, 40, 60, and 80 mg ZnO NPs than the other treated and control birds in a dose-dependent manner. In contrast, a nonsignificant difference (P ≤ 0.05) was detected in chicken given 5 and 10 mg ZnO NPs compared to the control group. In addition, compared to the control group, TNF-α mRNA expression in muscle tissues of chickens given ZnO NPs at all dose levels revealed a significantly (P ≤ 0.05) dose-dependent upregulation (Fig. 7B).
Assessment of ultrastructural morphology of pectoral muscles of chickens
Compared to the control group, TEM was used to validate the existence or absence of ZnO NPs and localize their presence in the pectoral muscles of chickens. The nucleus, nuclear envelope, and spherical or ovoid-shaped mitochondria with well-developed cristae, filaments, and Z-bands were present in control muscle cells. In contrast, treated muscles had an uneven nucleus and irregular nuclear envelope, fragmented nuclear chromatin, and aggregation of ZnO NP deposits within the nuclear chromatin (Fig. 8A). Degenerated fibers, minor cytoplasmic vacuolization, fractured mitochondrial cristae, and aggregation of ZnO NPs were also found within the mitochondrial cristae and lysosome internal membranes (Fig. 8B and C).
Showing the ultrastructure morphology of muscle tissue from Zn NP-treated broilers. A An irregular nucleus with disintegrated nuclear chromatin and aggregations of Zn NPs deposits within the nuclear chromatin. B Numerous areas of degenerated fibers and mild cytoplasmic vacuolization (arrows). C Fragmented mitochondrial cristae and aggregations of Zn NP deposits
Discussion
This study showed nonsignificant effects for live weight, gain, growth rate percentage, PI, feed consumption, and FCR between treated and control birds. Similarly, in Asheer et al. (2018), no significant differences were observed among 0.0, 25%, 50%, 75%, and 100% of ZnO NP treatments on the final live body weight, cumulative feed intake, and cumulative FCR at the sixth week of broiler age. However, these results indicated a numerical increase in growth performance parameters and agreed with those in El-Katcha et al. (2017b), which showed that 60, 30, and 45 ppm ZnO NPs/kg in broilers diet improved the growth performance and feed efficiency parameters. In contrast with our data, the authors in Hafez et al. (2017) have stated that a diet containing 40 and/or 80 mg/kg ZnO NPs is a significant Zn source for chicken, with beneficial effects on performance. Additionally, diets with ZnO at 20 or 40 mg/kg (Fathi et al. 2016) or 60 mg/kg (Hussan et al. 2022; Pathak et al. 2016) improved the growth performance of broiler chicks and reduced mortality due to ascites. Moreover, researchers (Ibrahim et al. 2017) have found that ZnO NPs improved Zn retention, enzyme antioxidant activity, and metabolism of broiler chickens, resulting in better performance. In contrast, in Mohammadi et al. (2015), it has been revealed that ZnO NP sulfate (80 mg/kg diet) decreased BWG of broilers from 1 to 42 days. The conflicting results of different studies could be related to the differences in the physical and chemical features of the different sources of Zn. Many studies revealed that the improvement in broiler growth performance parameters when ZnO NP supplementation is added in broiler diet might be due to zinc polysaccharide uptake related to organic minerals, which are a good vehicle to supply broilers with more minerals without increasing dietary mineral levels (Abdallah et al. 2009). Moreover, another reason is that the zinc nanoparticle size has a faster diffusion through GIT membrane, resulting in higher uptake of zinc nanoparticles in the gastrointestinal tract. The differences between this study and other research papers may be attributed to differences in concentration levels, breed, and environment and management procedures.
Among the hematological and immunological variables, WBC and RBC counts, PCV, lymphocytes (%), heterophils (%), lymphocytes/heterophils ratio, and monocytes were significantly (P ≤ 0.05) influenced by ZnO NP supplementation. However, Hb concentration, basophils (%), and eosinophils (%) were not significantly detected in the different treatments. These results indicated that the use of ZnO NPs has no detrimental effect on the hematological parameters of broilers. On the contrary, in Salama et al. (2003), Zn at higher concentrations tends to inhibit copper and iron absorption that are required for WBC and RBC maturation and proliferation; therefore, further investigation concerning blood parameters on different ZnO NP is warranted. In partial agreement with these findings, in Abed and Ezzat (2021), nonsignificant differences in PCV and Hb concentrations in the blood were found when adding ZnO NPs to broilers feed at 21 and 42 days.
In this study, the antibody titer against the NDV showed a nonsignificant difference between the control and treated groups. Similarly, in Abed and Ezzat (2021) and Khalifa et al. (2021), no significant differences among different groups given nano- and ZnO NPs for NDV disease were found (P ≤ 0.05). In contrast, the authors in Khajarern et al. (2002) have indicated that high levels of Zn supplementation (75 vs. 175 mg/kg) have led to a high antibody titer for NDV disease and IB. In Kim et al. (2014), differently sized and charged ZnO NPs would cause in vitro and in vivo immunotoxicity, which is considered a naturally immunosuppressant. On the other hand, researchers in Sahoo et al. (2014) assumed that dietary ZnO NPs might have elicited a better immune response even at lower physiological limits.
This study revealed a significant reduction in IgM and IgG in broilers given high levels of ZnO NPs (60 and 80 ppm/kg diet). In partial agreement with this finding, the authors in Hafez et al. (2020) have shown that birds fed diets supplemented with ZnO NPs had a significant increase (P ≤ 0.05) in IgY, total lymphocyte counts, and macrophages compared with the control. However, this highly significant response was observed in birds given the 5 ppm ZnO NPs/kg diet rather than the 80 ppm ZnO NPs/kg diet. On the contrary, the authors in Sahoo et al. (2014) found that when 15 ppm organic zinc and 0.06 ppm nano-zinc were added to the basal diet, the antibody titer and immune organ response were increased, thus improving the immune status of the birds. Another study (Kidd et al. 1996) discussed the immunity-enhancing role of Zn as it can increase the thymocytes and peripheral T cells count and enhance the interferon and interleukin-2 production. Therefore, increasing the bioavailability of zinc, as in the ZnO NPs, might promote immune responses via induction of extra thymulin activity, which subsequently enhances the maturation of T lymphocytes and the activation of B lymphocytes by T helper cells (Abedini et al. 2018).
In this study, significant increases in PA and PI were observed in groups fed ZnO NPs, with a higher significance in the group given the 20 ppm ZnO NPs/kg diet than that given the 80 ppm ZnO NPs/kg diet compared to the control. These results agree with those in Sahoo et al. (2014), which indicated that 15 ppm Zn in organic form and 0.06 ppm Zn NPs increased the antibody titer of the birds. In contrast, Zn from an inorganic source had not improved the birds’ immunity status (Abedini et al. 2018, Moghaddam and Jahanian 2009, Sahoo et al. 2014). These results were consistent with those in El-Katcha et al. (2017b), which showed an enhancement in the PI of broilers fed with 45 ppm ZnO NPs. These findings were also parallel to those in Chand et al. (2021), Sahoo et al. (2014), and Swain et al. (2015), which indicated that compared to control, Zn NPs seem to be more bioavailable even at lesser levels, IL-2 was significantly secreted, and cell-mediated immune response was better (Prasad et al. 2002). The increase in immune response parameters due to Zn NP uptake may be attributed to the enhanced maturation of T lymphocyte and activation of B lymphocytes by T helper cells (Hudson et al. 2004). Moreover, the authors in O'Dell (1992) concluded that the immune system is dependent on the functions of cellular metabolism. Zinc is ubiquitous in cellular metabolism and functions both structurally and catalytically in metalloenzymes. However, this description did not show matches with our histopathologic results of immune organs, where dose-dependent lymphoid depletion was discovered in ZnO NP-treated chicks. Further confirmatory studies are needed in this point to uncover the possible causes of this difference.
In this study, a significant dose-dependent increase in triglycerides and nonsignificant changes in LDL and cholesterol levels were reported in treated groups in equivalence to the control group. These obtained data were in partial agreement with those in Abed and Ezzat (2021) and Hussan et al. (2022), where no significant effect was found among the treatments in total cholesterol at 21 and 42 days of age and triglyceride concentration at 21 days of age, whereas triglyceride concentration was significantly affected at 42 days of age. In addition, in Zaghari et al. (2013), an increase in triglyceride was reported when birds were fed with Zn, in turn affecting lipid metabolism (Aksu et al. 2010). In contrast with these findings, in Ahmadi et al. (2013), a reduction in triglycerides and LDL was noticed when 60 and 90 mg/kg ZnO NPs were added to birds’ diets. Additionally, in Zaghari et al. (2013), a reduction in triglyceride and an increase in LDL in serum were observed in birds fed a diet supplemented with 100 mg ZnO. In agreement with these findings, other studies Sarvari et al. (2015) and Hussan et al. (2022) and Radi et al. (2021) showed that ZnO NP supplementation did not influence cholesterol and serum protein concentrations at 42 days of age.
Oxidative stress is primarily uncovered via the modulation of the antioxidant enzyme and the downregulation of the nonenzymatic antioxidants. In this study, TAC and CAT were significantly (P ≤ 0.05) reduced with increased ZnO NP dose in the diet compared with the control. Furthermore, MDA concentrations were significantly (P ≤ 0.01) increased by increasing ZnO NPs in the diets. Contrary to these results, in Alam et al. (2018) and Zhao et al. (2014), CAT activity was increased and maintained by 20 ppm of ZnO NPs compared to 60 ppm ZnO (Eskandani et al. 2021; Zhao et al. 2014). Furthermore, in Fathi et al. (2016), 20 mg/kg ZnO NPs significantly reduced MDA compared to the control. Other authors have discussed their results regarding the upregulation of Nrf2, which may be a major mechanism controlling Zn antioxidant action (Abdel-Daim et al. 2019; Cortese et al. 2008; Eskandani et al. 2021) or the competition of Zn with copper and iron for binding to the cell membrane and decreasing the free radicals production (Tate et al. 1999). The differences between this study and previous research may be due to differences in dose, breed, and environment and management procedures.
In a more recent study (Bartlett and Smith 2003), blood zinc levels were not affected by zinc quantity in the diet of birds subjected to heat stress. Additionally, similar results were stated (Hussan et al. 2022; Sarvari et al. 2015) regarding broiler growth and various levels of supplemented Zn. Similarly, in Feng et al. (2010a), no positive effect on immune organ weights was found. On the contrary, in Khah et al. (2015), significantly (P ≤ 0.05) enhanced dressing percentage and carcass and breast weight with ZnO NPs were reported. Moreover, in Sahoo et al. (2014), an increase in immune organ weights with ZnO NPs in broilers was observed.
In our study, the histopathologic examination of different tissues supports the chemical, immune-related, and oxidative findings, where there was dose-dependent damage in the liver, kidney, spleen, bursa, and thymus, which may be correlated to the dose-dependent oxidative damage described above. Limited studies are available regarding the detailed description of histopathologic changes associated with Zn (Wight et al. 1986) or ZnO NPs (El-Katcha et al. 2017a; Radi et al. 2021). At the same time, no previous studies are available regarding the description of such pathologic lesions in lymphoid organs. In the present study, the predominant pathological lesions could also be attributed to their solubility, increasing intracellular Zn2 + (Saman et al. 2013). In previous studies, the authors reported nanoparticle-related inflammatory reactions in different tissues, particularly the lymph nodes. Moreover, in Watson et al. (2015), the inhibition of Kupffer cell phagosomal motility by ZnO NPs with consequent hepatic damage was reported. In addition, the ultrastructure examination of muscles tissues in this study revealed several dose-dependent lesions such as the irregular nucleus, disintegrated nuclear chromatin, numerous areas of degenerated fibers and mild cytoplasmic vacuolization, and fragmented mitochondrial cristae. These lesions could be attributed to oxidative stress and the upregulations in gene expression of IL1α and TNF-α. This correlated increment in ROS production and inflammatory cytokines can diminish the mitochondrial function within cells (Hussain et al. 2005; Khalifa et al. 2021; Xia et al. 2006), leading to moderate to severe damage in the internal morphology of different organs.
Conclusion
Based on the obtained findings, ZnO NPs could be practically used in broiler diet at doses of 5 and 10 ppm/kg diet as an alternative of high-dose zinc oxide as these doses showed the most favorable effects and less toxic effects on the immune status of birds, followed by 20 ppm. Moreover, the histopathological examination of internal organs revealed dose-dependent morphological and structural changes for the kidney, liver, and lymphoid organs (bursa, spleen, and thymus). Consequently, more than 10 ppm ZnO NPs/kg diet is not recommended as this might induce harmful effects on the immune status and histologic structure of immune organs. Further studies are needed to properly detect the possible toxic effects and mechanism/s of ZnO NPs.
Data availability
The data used to support the findings of this study are included within the article, and the coding of the data is available from the corresponding author upon reasonable request.
References
Abdallah A, El-Husseiny O, Abdel-Latif K (2009) Influence of some dietary organic mineral supplementations on broiler performance. Int J Poult Sci 8:291–298
Abdel-Daim MM, Eissa IA, Abdeen A, Abdel-Latif HM, Ismail M, Dawood MA, Hassan AM (2019) Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ Toxicol Pharmacol 69:44–50. https://doi.org/10.1016/j.etap.2019.03.016
Abdel-Daim MM, Abdeen A, Jalouli M, Abdelkader A, Megahed A, Alkahtane A, Almeer R, Alhoshani NM, Al-Johani NS, Alkahtani S (2021) Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci Total Environ 768:144781. https://doi.org/10.1016/j.scitotenv.2020.144781
Abdel-Latif MA, El-Hack A, Mohamed E, Swelum AA, Saadeldin IM, Elbestawy AR, Shewita RS, Ba-Awadh HA, Alowaimer AN, El-Hamid A (2018) Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals 8:184. https://doi.org/10.3390/ani8100184
Abdelsalam NR, Fouda MM, Abdel-Megeed A, Ajarem J, Allam AA, El-Naggar ME (2019) Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. Int J Biol Macromol 133:1008–1018. https://doi.org/10.1016/j.ijbiomac.2019.04.134
Abed FI, Ezzat HN (2021) The effect of adding different levels of nano and non-nano zinc oxide to the diet on physiological traits of some broilers. Plant Arch 21:875–881. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.135
Abedini M, Shariatmadari F, Torshizi MAK, Ahmadi H (2018) Effects of zinc oxide nanoparticles on performance, egg quality, tissue zinc content, bone parameters, and antioxidative status in laying hens. Biol Trace Elem Res 184:259–267. https://doi.org/10.1007/s12011-017-1180-2
Aggrey SE (2004) Modelling the effect of nutritional status on pre-asymptotic and relative growth rates in a random-bred chicken population. J Anim Breed Genet 121:260–268. https://doi.org/10.1111/j.1439-0388.2004.00462.x
Ahmadi F, Ebrahimnezhad Y, Sis NM, Ghiasi J (2013) The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. Int J Biosci 3:23–29. https://doi.org/10.12692/ijb/3.7.23-29
Aksu DS, Aksu T, Ozsoy B, Baytok E (2010) The effects of replacing inorganic with a lower level of organically complexed minerals (Cu, Zn and Mn) in broiler diets on lipid peroxidation and antioxidant defense systems. Asian-Australas J Anim Sci 23:1066–1072. https://doi.org/10.5713/ajas.2010.90534
Alam R, Fawzi EM, Alkhalf MI, Alansari WS, Aleya L, Abdel-Daim MM (2018) Anti-inflammatory, immunomodulatory, and antioxidant activities of allicin, norfloxacin, or their combination against Pasteurella multocida infection in male New Zealand rabbits. Oxid Med Cell Long 2018:1780956. https://doi.org/10.1155/2018/1780956
Ali S, Masood S, Zaneb H, Faseeth-ur-Rehman H, Masood S, Khan M, Tahir SK, Rehman H (2017) Supplementation of zinc oxide nanoparticles has beneficial effects on intestinal morphology in broiler chicken. Pak Vet J 37:335–339
Applegate TJ, Angel R (2014) Nutrient requirements of poultry publication: history and need for an update. J Appl Poul Res 23:567–575. https://doi.org/10.3382/japr.2014-00980
Asheer M, Manwar S, Gole M, Sirsat S, Wade M, Khose K, Ali SS (2018) Effect of dietary nano zinc oxide supplementation on performance and zinc bioavailability in broilers. Indian J 53:70–75. https://doi.org/10.5958/0974-8180.2018.00004.1
Association of Official Agricultural Chemists H, W (2007): Official methods of analysis. Association of Official Agricultural Chemists 222
Aydin A, Bahr C, Viazzi S, Exadaktylos V, Buyse J, Berckmans D (2014) A novel method to automatically measure the feed intake of broiler chickens by sound technology. Comput Electron Agric 101:17–23. https://doi.org/10.1016/j.compag.2013.11.012
Bartlett J, Smith M (2003) Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci 82:1580–1588. https://doi.org/10.1093/ps/82.10.1580
Bhattacharya T, Maishu SP, Akter R, Rahman M, Akhtar MF, Saleem A, Bin-Jumah M, Kamel M, Abdel-Latif MA, Abdel-Daim MM (2021) A review on natural sources derived protein nanoparticles as anticancer agents. Curr Top Med Chem 21(13):1014–1026. https://doi.org/10.2174/1568026621666210412151700
Biria A, Navidshad B, Mirzaei Aghjehgheshlag F, Nikbin S (2020) The effect of in ovo supplementation of nano zinc oxide particles on hatchability and post-hatch immune system of broiler chicken. Iran J Appl Anim Sci 10:547–553
Bratz K, Gölz G, Riedel C, Janczyk P, Nöckler K, Alter T (2013) Inhibitory effect of high-dosage zinc oxide dietary supplementation on Campylobacter coli excretion in weaned piglets. J Appl Microbiol 115:1194–1202. https://doi.org/10.1111/jam.12307
Broom L, Miller H, Kerr K, Toplis P (2002): Removal of both zinc oxide and avilamycin from the post-weaning diet has a detrimental effect on pig performance through to slaughter, Proceedings of the British Society of Animal Science. Cambridge University Press, pp. 24–24. https://doi.org/10.1017/S1752756200006803
Chand N, Ali P, Alhidary IA, Abdelrahman MA, Albadani H, Khan MA, Seidavi A, Laudadio V, Tufarelli V, Khan RU (2021) Protective effect of grape (Vitis vinifera) seed powder and zinc-glycine complex on growth traits and gut health of broilers following Eimeria tenella challenge. Antibiotics 10:186
Chand NZKRU, Shah M, Naz S, Tinelli A (2020) Zinc source modulates zootechnical characteristics, intestinal features, humoral response and paraoxonase (PON1) activity in broilers. Tropical Anim Health Prod 52:511–515
Cortese MM, Suschek CV, Wetzel W, Kröncke K-D, Kolb-Bachofen V (2008) Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radical Biol Med 44:2002–2012. https://doi.org/10.1016/j.freeradbiomed.2008.02.013
Council NR (1994) Nutrient requirements of poultry. National Academies Press
Dawood MA, Koshio S, Zaineldin AI, Van Doan H, Ahmed HA, Elsabagh M, Abdel-Daim MM (2019a) An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: growth, tissue bioaccumulation, and antioxidative responses. Environ Sci Pollut Res 26:30876–30884. https://doi.org/10.1007/s11356-019-06223-6
Dawood MA, Koshio S, Zaineldin AI, Van Doan H, Moustafa EM, Abdel-Daim MM, Esteban MA, Hassaan MS (2019b) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45:219–230. https://doi.org/10.1007/s10695-018-0556-3
De Grande A, Leleu S, Delezie E, Rapp C, De Smet S, Goossens E, Haesebrouck F, Van Immerseel F, Ducatelle R (2020) Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult Sci 99:441–453. https://doi.org/10.3382/ps/pez525
Desai MA, Vyas AN, Saratale GD, Sartale SD (2019) Zinc oxide superstructures: recent synthesis approaches and application for hydrogen production via photoelectrochemical water splitting. Int J Hydrogen Energy 44:2091–2127. https://doi.org/10.1016/j.ijhydene.2018.08.042
Dosoky WM, Fouda MM, Alwan AB, Abdelsalam NR, Taha AE, Ghareeb RY, El-Aassar M, Khafaga AF (2021) Dietary supplementation of silver-silica nanoparticles promotes histological, immunological, ultrastructural, and performance parameters of broiler chickens. Sci Rep 11:1–15. https://doi.org/10.1038/s41598-021-83753-5
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42. https://doi.org/10.2307/3001478
El-Haliem A, Haiam S, Attia FA, Saber H, Hermes I (2020) Impacts of zinc oxide nano-particles supplementation in broiler diets on growth performance, some carcass characteristics and immune organs. Egypt J Nutr Feeds 23:113–122
El-Katcha M, Soltan MA, El-Badry M (2017a) Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alex J Vet Sci 55:129–145. https://doi.org/10.5455/ajvs.266925
El-Katcha M, Soltan MA, El-Badry M (2017b) Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alex J Vet Sci 55
El-Seedi HR, El-Shabasy RM, Khalifa SA, Saeed A, Shah A, Shah R, Iftikhar FJ, Abdel-Daim MM, Omri A, Hajrahand NH (2019) Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv 9:24539–24559. https://doi.org/10.1039/C9RA02225B
Eskandani M, Janmohammadi H, Mirghelenj S, Ebrahimi M, Kalanaky S (2021) Effects of zinc nanoparticles on growth performance, carcass characteristics, immunity, and meat quality of broiler chickens. Iran J Appl Anim Sci 11:135–146
Fathi M, Haydari M, Tanha T (2016) Effects of zinc oxide nanoparticles on antioxidant status, serum enzymes activities, biochemical parameters and performance in broiler chickens. J Livestock Sci Technol 4:7–13
Feng J, Ma W, Niu H, Wu X, Wang Y (2010a) Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol Trace Elem Res 133:203–211
Feng J, Ma WQ, Niu HH, Wu XM, Wang Y, Feng J (2010b) Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol Trace Elem Res 133:203–211. https://doi.org/10.1007/s12011-009-8431-9
Fouda MM, Abdelsalam NR, El-Naggar ME, Zaitoun AF, Salim BM, Bin-Jumah M, Allam AA, Abo-Marzoka SA, Kandil EE (2020) Impact of high throughput green synthesized silver nanoparticles on agronomic traits of onion. Int J Biol Macromol 149:1304–1317
Fouda MM, Dosoky WM, Radwan NS, Abdelsalam NR, Taha AE, Khafaga AF (2021) Oral administration of silver nanoparticles–adorned starch as a growth promotor in poultry: immunological and histopathological study. Int J Biol Macromol 187:830–839. https://doi.org/10.1016/j.ijbiomac.2021.07.157
Gangadoo S, Stanley D, Hughes RJ, Moore RJ, Chapman J (2016) Nanoparticles in feed: progress and prospects in poultry research. Trends Food Sci Technol 58:115–126. https://doi.org/10.1016/j.tifs.2016.10.013
Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI (2007) Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect 115:403–409. https://doi.org/10.1289/ehp.8497
Hafez A, Hegazi S, Bakr A, Shishtawy H (2017) Effect of zinc oxide nanoparticles on growth performance and absorptive capacity of the intestinal villi in broiler chickens. Life Sci J 14:67–72. https://doi.org/10.7537/marslsj141117.18
Hafez A, Nassef E, Fahmy M, Elsabagh M, Bakr A, Hegazi E (2020) Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ Sci Pollut Res 27:19108–19114. https://doi.org/10.1007/s11356-019-04344-6
He X, Yang X, Guo Y (2007) Effects of different dietary oil sources on immune function in cyclophosphamide immunosuppressed chickens. Anim Feed Sci Technol 139:186–200. https://doi.org/10.1016/j.anifeedsci.2007.01.009
Herrera J, Saldaña B, Guzmán P, Cámara L, Mateos GG (2017) Influence of particle size of the main cereal of the diet on egg production, gastrointestinal tract traits, and body measurements of brown laying hens1 1Financial support was provided by the Ministerio de Economía y Competitividad (Project AGL 2014–56139). Poult Sci 96:440–448. https://doi.org/10.3382/ps/pew256
Hudson BP, Dozier WA, Wilson JL, Sander JE, Ward TL (2004) Reproductive performance and immune status of caged broiler breeder hens provided diets supplemented with either inorganic or organic sources of zinc from hatching to 65 wk of age. J Appl Poultry Res 13:349–359. https://doi.org/10.1093/japr/13.2.349
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ (2005) In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in Vitro 19:975–983
Hussan F, Krishna D, Preetam VC, Reddy PB, Gurram S (2022) Dietary supplementation of nano zinc oxide on performance, carcass, serum and meat quality parameters of commercial broilers. Biol Trace Elem Res 200:384–353. https://doi.org/10.1007/s12011-021-02635-z
Ibrahim D, Ali HA, El-Mandrawy SA (2017) Effects of different zinc sources on performance, bio distribution of minerals and expression of genes related to metabolism of broiler chickens. Zagazig Vet J 45:292–304. https://doi.org/10.21608/zvjz.2017.7954
Ibrahim KA, Abdelgaid HA, El-Desouky MA, Fahmi AA, Abdel-Daim MM (2021) Linseed ameliorates renal apoptosis in rat fetuses induced by single or combined exposure to diesel nanoparticles or fenitrothion by inhibiting transcriptional activation of p21/p53 and caspase-3/9 through pro-oxidant stimulus. Environ Toxicol 36:958–974. https://doi.org/10.1002/tox.23097
Ishak NM, Kamarudin S, Timmiati S (2019) Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Mater Res Expr 6:112004
Jacobson ED, Chodos RB, Faloon WW (1960) An experimental malabsorption syndrome induced by neomycin. Am J Med 28:524–533. https://doi.org/10.1016/0002-9343(60)90146-7
Jadhao G, Sawai D, Kedare G (2020) Nutritional ways to reduce heat stress in broilers. J Entomol Zool Stud 8:1872–1877
Jakobsen J, Pedersen AN, Ovesen L (2003) Para-aminobenzoic acid (PABA) used as a marker for completeness of 24 hour urine: effects of age and dosage scheduling. Eur J Clin Nutr 57:138–142. https://doi.org/10.1038/sj.ejcn.1601505
Jang A, Liu X-D, Shin M-H, Lee B-D, Lee S-K, Lee J-H, Jo C (2008) Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult Sci 87:2382–2389. https://doi.org/10.3382/ps.2007-00506
Kalantar M, Saki A, Zamani P, Aliarabi H (2011) Effect of drinking thyme essence on performance, energy and protein efficiency and economical indices of broiler chickens
Kandeil MA, Mohammed ET, Hashem KS, Aleya L, Abdel-Daim MM (2019) Moringa seed extract alleviates titanium oxide nanoparticles (TiO 2-NPs)-induced cerebral oxidative damage, and increases cerebral mitochondrial viability. Environ Sci Pollut Res 1–16. https://doi.org/10.1007/s11356-019-05514-2
Khah MM, Ahmadi F, Amanlou H (2015) Influence of dietary different levels of zinc oxide nano particles on the yield and quality carcass of broiler chickens during starter stage. Indian J Anim Sci 85:287–290
Khajarern J, Ratanasethakul C, Kharajarern S, Ward T, Fakler T, Johnson A (2002) Effect of zinc and manganese amino acid complexes (AvailaZ/M) on broiler breeder production and immunity. Poult Sci 81:40
Khalifa OA, Al Wakeel RA, Hemeda SA, Abdel-Daim MM, Albadrani GM, El Askary A, Fadl SE, Elgendey F (2021) The impact of vitamin E and/or selenium dietary supplementation on growth parameters and expression levels of the growth-related genes in broilers. BMC Vet Res 17:1–10. https://doi.org/10.1186/s12917-021-02963-1
Kidd M, Ferket P, Qureshi M (1996) Zinc metabolism with special reference to its role in immunity. Worlds Poult Sci J 52:309–324
Kim J-H, Kim C-S, Ignacio RMC, Kim D-H, Sajo MEJ, Maeng EH, Qi X-F, Park S-E, Kim Y-R, Kim M-K (2014) Immunotoxicity of silicon dioxide nanoparticles with different sizes and electrostatic charge. Int J Nanomed 9:183
Kincade PW, Lawton AR, Bockman DE, Cooper MD (1970) Suppression of immunoglobulin G synthesis as a result of antibody-mediated suppression of immunoglobulin M synthesis in chickens. Proc Natl Acad Sci 67:1918–1925. https://doi.org/10.1073/pnas.67.4.1918
Lara LJ, Rostagno MH (2013) Impact of heat stress on poultry production. Animals 3:356–369
Li X, Piao X, Kim S, Liu P, Wang L, Shen Y, Jung S, Lee H (2007) Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult Sci 86:1107–1114. https://doi.org/10.1093/ps/86.6.1107
Marcu A, Vacaru-Opriş I, Dumitrescu G, Ciochina LP, Marcu A, Nicula M, Peţ I, Dronca D, Kelciov B (2013) The influence of the genotype on economic efficiency of broiler chickens growth. Sci Papers Anim Sci Biotechnol 46:339–346
Miller RJ, Lenihan HS, Muller EB, Tseng N, Hanna SK, Keller AA (2010) Impacts of metal oxide nanoparticles on marine phytoplankton. Environ Sci Technol 44:7329–7334. https://doi.org/10.1021/es100247x
Moghaddam HN, Jahanian R (2009) Immunological responses of broiler chicks can be modulated by dietary supplementation of zinc-methionine in place of inorganic zinc sources. Asian Australas J Anim Sci 22:396–403
Mohammadi V, Ghazanfari S, Mohammadi-Sangcheshmeh A, Nazaran M (2015) Comparative effects of zinc-nano complexes, zinc-sulphate and zinc-methionine on performance in broiler chickens. Br Poult Sci 56:486–493. https://doi.org/10.1080/00071668.2015.1064093
Mohammed E, Safwat G (2013) Assessment of the ameliorative role of selenium nanoparticles on the oxidative stress of acetaminophen in some tissues of male albino rats. Beni-Suef Univ J Basic Appl Sci 2:80–85. https://doi.org/10.1016/j.bjbas.2013.01.003
Mohammed ET, Hashem KS, Abdelazem AZ, Foda FA (2020) Prospective protective effect of ellagic acid as a SIRT1 activator in iron oxide nanoparticle-induced renal damage in rats. Biol Trace Elem Res 1–12. https://doi.org/10.1007/s12011-020-02034-w
Mohammed ET, Safwat GM (2020) Grape seed proanthocyanidin extract mitigates titanium dioxide nanoparticle (TiO 2-NPs)–induced hepatotoxicity through TLR-4/NF-κB signaling pathway. Biol Trace Elem Res 196:579–589. https://doi.org/10.1007/s12011-019-01955-5
Motor S, Ozturk S, Ozcan O, Gurpinar AB, Can Y, Yuksel R, Yenin JZ, Seraslan G, Ozturk OH (2014) Evaluation of total antioxidant status, total oxidant status and oxidative stress index in patients with alopecia areata. Int J Clin Exp Med 7:1089
Naz S, Idris M, Khalique MA, Zia-Ur-Rahman U, Alhidary IA, Abdelrahman MM, Khan RU, Chand N, Farooq U, Ahmad S (2016) The activity and use of zinc in poultry diets. World’s Poult Sci J 72(159–167):167. https://doi.org/10.1017/S0043933915002755
O’Dell BL (1992) Zinc plays both structural and catalytic roles in metalloproteins. Nutr Rev 50:48–50. https://doi.org/10.1111/j.1753-4887.1992.tb02513.x
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839. https://doi.org/10.1289/ehp.7339
Pathak S, Reddy K, Prasoon S (2016) Effect of dietary supplementation of inorganic, organic and nano zinc on serum cholesterol, SGOT and SGPT levels of dual purpose chicken. J Indian Vet Assoc Kerala (JIVA) 14:19–22
Prasad AS, Bao B, Beck FW, Sarkar FH (2002) Zinc enhances the expression of interleukin-2 and interleukin-2 receptors in HUT-78 cells by way of NF-κB activation. J Lab Clin Med 140:272–289. https://doi.org/10.1067/mlc.2002.127908
Radi AM, Azeem NMA, EL-Shaymaa E-N (2021) Comparative effects of zinc oxide and zinc oxide nanoparticle as feed additives on growth, feed choice test, tissue residues, and histopathological changes in broiler chickens. Environ Sci Pollut Res 28:5158–5167. https://doi.org/10.1007/s11356-020-09888-6
Ramiah SK, Awad EA, Mookiah S, Idrus Z (2019) Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult Sci 98:3828–3838
Rao SR, Prakash B, Raju M, Panda A, Kumari R, Reddy EPK (2016) Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biol Trace Elem Res 172:511–520. https://doi.org/10.1007/s12011-015-0587-x
Rossi P, Rutz F, Anciuti M, Rech J, Zauk N (2007) Influence of graded levels of organic zinc on growth performance and carcass traits of broilers. J Appl Poultry Res 16:219–225. https://doi.org/10.1093/japr/16.2.219
Saad AH, Ahmed MS, Aboubakr M, Ghoneim HA, Abdel-Daim MM, Albadrani GM, Arafat N, Fadl SE, Abdo W (2021) Impact of dietary or drinking water Ruminococcus sp. supplementation and/or heat stress on growth, histopathology, and bursal gene expression of broilers. Front Vet Sci 8:663577. https://doi.org/10.3389/fvets.2021.663577
Saeed M, Naveed M, Leskovec J, Kakar I, Ullah K, Ahmad F, Sharif M, Javaid A, Rauf M, Abd El-Hack ME (2020) Using guduchi (Tinospora cordifolia) as an eco-friendly feed supplement in human and poultry nutrition. Poult Sci 99:801–811. https://doi.org/10.1016/j.psj.2019.10.051
Sahin K, Kucuk O (2003) Heat stress and dietary vitamin supplementation of poultry diets, Nutrition Abstracts and Reviews. Series B, Livestock Feeds and Feeding. CAB International
Sahin K, Sahin N, Kucuk O (2003) Effects of chromium, and ascorbic acid supplementation on growth, carcass traits, serum metabolites, and antioxidant status of broiler chickens reared at a high ambient temperature (32 C). Nutr Res 23:225–238. https://doi.org/10.1016/S0271-5317(02)00513-4
Sahoo A, Swain R, Mishra SK (2014) Effect of inorganic, organic and nano zinc supplemented diets on bioavailability and immunity status of broilers. Int J Adv Res 2:828–837
Salama AAK, Caja G, Albanell E, Such X, Casals R, Plaixats J (2003) Effects of dietary supplements of zinc-methionine on milk production, udder health and zinc metabolism in dairy goats. J Dairy Res 70:9–17
Salim HM, Jo C, Lee BD (2008) Zinc in broiler feeding and nutrition. Avian Biology Research 1:5–18. https://doi.org/10.3184/175815508x334578
Samak DH, El-Sayed YS, Shaheen HM, Ali H, Onoda A, Abdel-Daim MM, Umezawa M (2018) In-ovo exposed carbon black nanoparticles altered mRNA gene transcripts of antioxidants, proinflammatory and apoptotic pathways in the brain of chicken embryos. Chem Biol Interact 295:133–139. https://doi.org/10.1016/j.cbi.2018.02.031
Saman S, Moradhaseli S, Shokouhian A, Ghorbani M (2013) Histopathological effects of ZnO nanoparticles on liver and heart tissues in wistar rats. Adv Biores 4:83–88
Sarvari B, Seyedi A, Shahryar H, Sarikhan M, Ghavidel S (2015) Effects of dietary zinc oxide and a blend of organic acids on broiler live performance, carcass traits, and serum parameters. Braz J Poult Sci 17:39–45. https://doi.org/10.1590/1516-635X
Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y (1986) The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol 125:431
Schmidt CW (2009) Nanotechnology-related environment, health, and safety research: examining the national strategy. Natl Instit Environ Health Sci 1–4. https://doi.org/10.1289/ehp.117-a158
Shah M, Zaneb H, Masood S, Khan RU, Mobashar M, Khan I, Din S, Khan MS, Rehman HU, Tinelli A (2020) Single or combined applications of zinc and multi-strain probiotic on intestinal histomorphology of broilers under cyclic heat stress. Probiotics Antimicrob Proteins 12:473–480. https://doi.org/10.1007/s12602-019-09561-6
Sinha R, Karan R, Sinha A, Khare S (2011) Interaction and nanotoxic effect of ZnO and Ag nanoparticles on mesophilic and halophilic bacterial cells. Biores Technol 102:1516–1520. https://doi.org/10.1016/j.biortech.2010.07.117
Sizova E, Miroshnikov S, Lebedev S, Usha B, Shabunin S (2020) Use of nanoscale metals in poultry diet as a mineral feed additive. Anim Nutr 6:185–191. https://doi.org/10.1016/j.aninu.2019.11.007
Sundaresan NR, Anish D, Sastry KVH, Saxena VK, Nagarajan K, Subramani J, Leo MDM, Shit N, Mohan J, Saxena M, Ahmed KA (2008) High doses of dietary zinc induce cytokines, chemokines, and apoptosis in reproductive tissues during regression. Cell Tissue Res 332:543–554. https://doi.org/10.1007/s00441-008-0599-3
Swain PS, Rajendran D, Rao SBN, Dominic G (2015) Preparation and effects of nano mineral particle feeding in livestock: a review. Vet World 8:888–891. https://doi.org/10.14202/vetworld.2015.888-891
Swain PS, Rao SB, Rajendran D, Dominic G, Selvaraju S (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Anim Nutr 2:134–141. https://doi.org/10.1016/j.aninu.2016.06.003
Tate DJ, Miceli MV, Newsome DA (1999) Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radical Biol Med 26:704–713. https://doi.org/10.1016/S0891-5849(98)00253-6
Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Mohammadrezaei M (2010) Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita). Livest Sci 129:173–178. https://doi.org/10.1016/j.livsci.2010.01.021
Wang H-J, Growcock AC, Tang T-h, O’Hara J, Huang Y-w, Aronstam RS (2010) Zinc oxide nanoparticle disruption of store-operated calcium entry in a muscarinic receptor signaling pathway. Toxicol in Vitro 24:1953–1961. https://doi.org/10.1016/j.tiv.2010.08.005
Watson CY, Molina RM, Louzada A, Murdaugh KM, Donaghey TC, Brain JD (2015) Effects of zinc oxide nanoparticles on Kupffer cell phagosomal motility, bacterial clearance, and liver function. Int J Nanomed 10:4173–4184
Wight P, Dewar W, Saunderson C (1986) Zinc toxicity in the fowl: ultrastructural pathology and relationship to selenium, lead and copper. Avian Pathol 15:23–38. https://doi.org/10.1080/03079458608436263
Wright DK, Manos MM (1990) Sample preparation from paraffin-embedded tissues. PCR Protocols 19:153–159
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Zaghari M, Sedaghat V, Shivazad M (2013) Effect of vitamin E on reproductive performance of heavy broiler breeder hens. J Appl Poult Res 22:808–813. https://doi.org/10.3382/japr.2012-00718
Zahin N, Anwar R, Tewari D, Kabir MT, Sajid A, Mathew B, Uddin MS, Aleya L, Abdel-Daim MM (2020) Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environ Sci Pollut Res 27:19151–19168. https://doi.org/10.1007/s11356-019-05211-0
Zhang T, Liu J, Zhang J, Zhang N, Yang X, Qu H, Xi L, Han J (2018) Effects of dietary zinc levels on the growth performance, organ zinc content, and zinc retention in broiler chickens. Braz J Poult Sci 20:127–132. https://doi.org/10.1590/1806-9061-2017-0604
Zhao C-Y, Tan S-X, Xiao X-Y, Qiu X-S, Pan J-Q, Tang Z-X (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160:361–367. https://doi.org/10.1007/s12011-014-0052-2
Acknowledgements
The authors gratefully acknowledge Alexandria University for the support and to Prof. Dr. rer. nat. Moustafa M.G. Fouda, National Research Center, for the preparing of ZnO NP material.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors contributed to the present study as follows: data curation, Waleed M. Dosoky and Soliman M. Zahran; formal analysis, Aya A. Al-Banna and Waleed M. Dosoky; resources, Soha A. Farag, Nader R. Abdelsalam, and Asmaa F. Khafaga; software, Asmaa F. Khafaga; visualization, Waleed M. Dosoky, Soha A. Farag, Aya A. Al-Banna, Nader R. Abdelsalam, and Asmaa F. Khafaga; writing — original draft, Waleed M. Dosoky, Nader R. Abdelsalam, and Asmaa F. Khafaga; and writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complied with relevant institutional, national, and international guidelines and legislation under the approval of ethical standards of scientific research № AU: 14/19/12/19/01/06 from Alexandria University, 2019.
Consent for publication
All the authors agree for consent for publication, and the current article does not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dosoky, W.M., Al-Banna, A.A., Zahran, S.M. et al. Zinc oxide nanoparticles induce dose-dependent toxicosis in broiler chickens reared in summer season. Environ Sci Pollut Res 29, 54088–54107 (2022). https://doi.org/10.1007/s11356-022-19156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19156-4