Abstract
We elucidated the effect of spring water on the growth of Egeria densa Planch., a widespread submerged macrophyte in Japan. We observed the longitudinal distributions of physical (water temperature, particle diameter of the bed sediment, sediment layer thickness, etc.), chemical [pH, dissolved oxygen (DO), dissolved inorganic carbon (DIC), phosphate (PO4-P), total nitrogen (TN), and total phosphorus (TP) content of the sediment, etc.], and biological (species composition, biomass, and growth rate) factors related to E. densa in the Kurohashi River, a spring-fed stream flowing into the Lake Biwa. It was found that E. densa growth rate from summer to autumn was negatively correlated to pH and DO, which implies that the low pH spring water increases the growth rate of the species. The growth rate was also positively correlated to the free carbon dioxide (CO2) concentration (r = 0.67, p = 0.02). These results indicate that the low pH spring water increases E. densa growth rate by affecting free CO2 concentration in water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spring water provides stable-temperature habitats and supplies nutrients and inorganic ions (Hayashi and Rosenberry 2002). Likewise, it is an important component of many regional ecosystems (MOEJ 2010). However, many spring waters, especially in developed nations (e.g., Japan), are affected by ecological problems, such as reduced water quantity, drying up, and pollution, etc., due to rapid and intense urbanization and changes in land use (Eisen and Anderson 1979; Al-Kharabsheh 1999; MOEJ 2010). Therefore, conservation and restoration of spring water has become one of the environmental protection strategies (MOEJ 2010).
Several studies report that the input of spring water into streams and ponds determines the distribution of submerged macrophytes (Rosenberry et al. 2000; Kadono 2004; Okubo and Oike 2008; Demars and Trémolières 2009). However, literature on the relationship between spring water and growth of submerged macrophytes is very scarce (Lodge et al. 1989). Thus, it remains unclear what factors of spring water influence the growth of submerged macrophytes, and understanding this factor contributes to promoting conservation and restoration of spring water. The growth of submerged macrophytes is influenced by many factors, such as nutrients, flow velocities, and bed sediments. Previous works report that the biomass of submerged macrophytes is correlated to bed-sediment nutrients and is affected by bed-sediment texture, hydraulics (Asaeda et al. 2004; Hung et al. 2007; Haga et al. 2006), and nutrients in the water, especially ammonium and phosphate (Feijoó et al. 2002; Mony et al. 2007). Other factors that influence the growth of submerged macrophytes are light conditions (Barko et al. 1986; Maberly and Madsen 2002) and dissolved inorganic carbon (DIC) (Madsen and Maberly 1991; Rattray et al. 1991; Madsen and Sand-Jensen 1994; Maberly and Madsen 2002; Vadstrup and Madsen 2006).
One of the important characteristics of spring water is that it is rich in free CO2 due to respiration of organisms and lack of CO2 consumers in the ground and thereby is acidified (Hanya and Ogura 1995). It is known that lower pH shifts the equilibrium balance of DIC, free CO2, bicarbonate (HCO3 −), and carbonate (CO3 2−) toward the free CO2 dominant regime in water (Allan and Castillo 2007). Thus, spring water, which is generally acidified due to high concentration of free CO2, may promote growth of submerged macrophytes.
The Kurohashi River, a spring-fed stream flowing into the Lake Biwa, Japan, contains extremely high biomass of Egeria densa Planch. compared with other species, and its biomass distribution is not homogeneous. We hypothesize that the heterogeneity of biomass distribution of E. densa in the Kurohashi River is attributable to the input of spring water, which changes pH values and the free CO2 concentration in stream water. This study, therefore, aimed to explore the effect of spring water on the growth of submerged macrophyte E. densa.
Methods
Study site
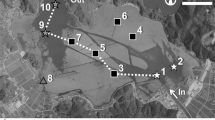
The study site is located in an upstream reach of the Kurohashi River (35°06′57.0 N, 136°07′11.2E–35°07′04.2 N, 136°06′51.1E), a spring-fed stream that extends for 5.5 km. Its catchment area is 6.9 km2 and the average river bed slope is approximately 1/1,000. The study site stretch is 0.8 km long, with a stream width ranging from 1 to 2 m. The water depths are 0.05–0.20 m and 0.10–0.70 m at times of no and peak vegetation biomass, respectively. The flow velocity is 0.1–0.7 ms−1 during times of no vegetation and between 0.1 and 0.4 ms−1 during peak vegetative biomass. These ranges of water depth and flow velocity allow the submerged macrophytes to grow (Otaki and Ishido 1980). The river bed is composed of stones with gravel in the upstream reach, whereas the mid- to downstream reach is basically clay or partially concrete. As shown in Fig. 1, there is a spring-fed pond in an upstream area, which feeds stream water. The upstream reach also contains several places where spring water gushes from the river bed. The main stream in the study site has seven tributaries without vegetation, one of which supplies spring water to the midstream reach.
The Kurohashi River flows through paddy fields and is used for agricultural purposes as an irrigation channel. Though the stream banks are mostly adjacent to paddy fields, some parts are bordered by riparian forests of bamboos, broad-leaved trees, and cedars in the upstream reach (Fig. 1). The stream bed, as well as submerged macrophytes, is dredged annually to maintain the flow condition of the channel. The most recent dredging was carried out on 5 July 2009. After their removal, however, macrophytes regrow rapidly (Fig. 1).
In situ water measurement
Field observations were done along the main channel and its tributaries. Plant, sediment, and water samples were collected from the main channels, whereas physical and chemical features of the water were measured in observation points of the tributaries channels. In the main channel, sampling points were located approximately every 50 m in summer (June to July) of 2009 (St. 1–18, Fig. 1). Four additional sampling points (St. 4′, 5′, 12′, 14′, Fig. 1) were set in the autumn (September to October) of 2009. Water parameters were recorded from one observation point at each tributary (St. A–G, Fig. 1). At each observation, pH, water temperature, dissolved oxygen (DO), and electric conductivity (EC) was measured by the Onsite Aqua Sensor Integrated System (OYO Corporation) at different times: morning, midday, and afternoon. Averaged values of three were used for analyses.
Sampling of macrophytes, water, and bed sediment
Macrophytes, water, and bed-sediment samples were collected from each sampling point in the main channel (St. 2–17). Macrophytes and sediment cores were sampled from 30 × 30-cm quadrats in summer on 20 June 2009 and in autumn on 20 September 2009. Sediment-layer thickness was measured at all sampling points. In addition, it was measured in winter on 6 December 2009 and in spring on 9 May 2010. Sediment-layer thickness is defined as the depth between the bed-sediment surface and the underlying concrete river bed or clay or stone layers. When the river bed was composed of stones, because it was difficult to recognize the boundary between the bed sediment and the river bed, sediment-layer thickness was considered as >5 cm.
Chemical analysis
Macrophytes, water, and bed-sediment samples were stored in cool boxes and immediately brought to the laboratory. In the laboratory, macrophytes and bed-sediment samples were dried at 80 °C using a convection oven, NDO700 (TOKYO RIKAKIKAI Co., Ltd.) for >3 days until no further weight change was detected. Bed sediment fractions of different particle sizes were measured based on the protocol of the American Society for Testing and Materials (ASTM D422-63, 2007). Total nitrogen content (TN) of the bed sediment was analyzed by the Vario Micro Cube (Elementar Co., Ltd.) and total phosphorus content (TP) of the bed sediment was determined with the ascorbic acid method postdigestion using the perchloric acid method (APHA 2005). Biomass of macrophyte samples was measured for each species. Dissolved inorganic nitrogen (DIN) (NH4-N, NO2-N, NO3-N) and phosphate (PO4-P) of water were analyzed by an automatic analyzer, TRAACS800 (BL TEC K.K.). Ammonium concentrations were determined by the phenate method and nitrite concentrations by the sulfanilamide-NED dihydrochloride colorimetric method (APHA 2005). To analyze nitrate concentrations, nitrate was reduced to nitrite in the presence of cadmium treated with copper sulfate (cadmium reduction method), whereupon nitrite concentrations were determined by the aforementioned colorimetric method (APHA 2005) and phosphate by the ascorbic acid method (APHA 2005). Total dissolved carbon (TDC) and dissolved inorganic carbon (DIC) were determined by the Total Organic Carbon Analyzer (Shimazu Co., Ltd.). Dissolved organic carbon (DOC) was calculated by the difference between TDC and DIC. As carbon is a key factor for photosynthesis, free CO2, HCO3 −, and CO3 2− concentrations were determined via DIC concentration multiplied by the carbon fraction at a particular pH, which was derived from the following chemical equilibriums as specified by Stumm and Morgan (1996) (Eqs. 1, 2)
Data analyses
Growth rate
Growth rates (GR) of submerged macrophyte E. densa from summer to autumn (5 July to 20 September 2009; 77 days) were calculated with the measured biomass by the Eq. 3.
where GR stands for growth rate, ΔBiomass is the net production after dredging on 5 July 2009 till the sampling day on 20 September 2009. ΔDays is the difference between dredging and sampling dates.
Habitat categorizing
To analyze the characteristic of the macrophyte habitat, data obtained were initially analyzed by principal component analysis (PCA; EXCEL Multivariate Analysis ver. 5, Esumi Co., Ltd.). Variables used for PCA were pH; water temperature; DO, EC, DIC, DOC, DIN, PO4-P of water; sediment particle size (d50); sediment TN and TP contents; sediment-layer thickness; and land-use type of adjacent area. Land use type was used as a dummy variable and was coded 1 and 0 for riparian forests and paddy fields, respectively. To elucidate the effect of spring water on E. densa growth rate, conditions in vegetation stands should be similar except for variables related to the spring water. The upstream reach of the main channel (St. 2–6, Fig. 1) is adjacent to riparian forests, whereas the remaining reaches (St. 7–17, Fig. 1) are adjacent to paddy fields. As a result of PCA, E. densa stands were considered to be categorized by TP content and particle diameter (d50) of the bed sediment, which is influenced by land-use type. Thus, differences in the average of those variables between reaches were tested with Mann–Whitney’s U test (Excel Statistics 2008, SSRI Co., Ltd.). Regression analyses between E. densa growth rate and sediment-layer thickness and between growth rate and free CO2, HCO3 −, and sum of free CO2 and HCO3 − concentrations were carried out (EXCEL Multivariate Analysis ver. 5, Esumi Co., Ltd.). Furthermore, to check whether E. densa growth rate is influenced by other physical and chemical factors, Spearman rank correlation coefficients between them were also determined (Excel Statistics 2008, SSRI Co., Ltd.).
Results
Biomass, growth rate, and pH distributions
The most dominant species in the study site is E. densa. Other species in the site are Elodea nuttalliii St. John, Potamogeton crispus L., and some Chlorophyceae species (Fig. 2). The biomass of E. densa in summer was higher at the midstream reach (St. 6–15, except for St. 9–11), with an average value of 0.799–1.351 kg DW m−2, in comparison with upstream and downstream reaches (St. 2–5 and 16–17), where the average biomass record was 0.052–0.272 kg DW m−2. The reach from St. 9–11 has a concrete river bed, and biomass ranged widely from 0.047 to 1.241 kg DW m−2, which was proportional to sediment-layer thickness (r = 0.71, p = 0.01) (Fig. 3).
The growth rate at the upstream, midstream, and downstream reaches were 0.2–5.1, 2.3–9.4, and 4.7–7.2 g DW m−2 day−1, respectively. In autumn, pH values of the channel decreased from St. 1–5 (7.37 ± 0.43 to 6.10 ± 0.06), maintained through St. 6–11 (6.10 ± 0.05 to 6.36 ± 0.07), and then again increased gradually from St. 12–18 (6.48 ± 0.08 to 6.90 ± 0.20). Heterogeneity of the pH distribution was more notable in autumn, and the biomass of E. densa at corresponding reaches seemed to follow the pH distribution pattern. The pH of the spring-fed pond (St. A) in autumn was 6.05 ± 0.04 and of the spring water supplied from the tributary to the midstream reach (St. C) 6.30 ± 0.02 (Figs. 2, 3).
DO and free CO2 concentrations of spring water
Spring water contains lower DO but higher free CO2 concentrations than the air-equilibrium concentrations. On the one hand, DO concentrations of the spring-fed pond (St. A) and spring water gushing from the river bed (St. C) were 6.88 ± 1.71 mg L−1 (20.4 ± 1.8 °C) and 4.18 ± 0.34 mg L−1 (18.1 ± 1.4 °C), respectively. The air-equilibrium values were 8.84 and 9.18 mg L−1 at 20 and 18 °C, respectively, as reported by Truesdale et al. (1955). On the other hand, free CO2 concentrations of St. A and St. C were 9.90 and 7.10 at mg L−1, respectively. The air-equilibrium values for CO2 were 0.60 mg L−1 and 0.64 mg L−1 at 20 and 18 °C, respectively (Weiss 1974).
Principal components derived from principal component analysis
Eigenvectors of the first three principal components (PCs) derived from PCA are shown in Table 1. PC1 represents the ratio of spring water to water from other sources because PC1 has a strong negative correlation with EC and a strong positive correlation with DOC and PO4-P. It indicates that EC values of spring water are higher but poor in DOC and PO4-P concentrations. PC1 also has positive correlation with water temperature, indicating that PC1 is related to spring water.
As shown in Fig. 3a, PC1 score variation over two seasons exceeds that recorded among observation points in the same season. It implies that the balance between the amount of spring water and that from surroundings, such as paddy fields, changed seasonally, and the ratio of spring water to all stream water in autumn was relatively greater than in summer. This might be attributable to no paddy field irrigation in autumn. PC2 represents the component associated with stream water acidity, as it has a strong positive correlation to pH. It has a strong negative correlation with TP content of bed sediment, indicating that PC2 also represents bed-sediment characteristics, especially the chemical aspects. As shown in Fig. 3a, sampling points indicating relatively lower pH of water (St. A3–13) in autumn, when the ratio of spring water to stream water was high, were located closer to springs, as shown in Fig. 1. This implies that stream pH was affected by spring water more strongly in autumn than in summer. PC3 mainly represents land-use type of adjacent areas: riparian forests or paddy fields. As PC3 has strong negative correlation to land-use type, higher PC scores of PC3 indicate paddy fields, whereas lower scores indicate riparian forests. PC3 also has negative correlation with particle diameter of bed sediment and sediment-layer thickness. These two variables correlate because a thin bed consisting of 1-cm-thick fine sand (d50 = 0.41 mm) (ASTM 422-63, 2007) accumulated on the concrete river bed at St. 9–11. Therefore, PC3 also represents bed-sediment characteristic, especially of physical aspects. Furthermore, PC3 has negative correlation to both particle diameter of the bed sediment and land-use type. Positive correlation to TP content of the bed sediment is due to inflow of relatively fine soils with high TP contents from paddy fields (PC3 at St. 7–17 are higher than at others, Fig. 1). The inverse correlation between pH and land-use type is due to the fact that the upstream reach adjacent to riparian forests includes spring water gushing from the river bed (Table 1; Fig. 4).
Sample score plots in principal components (PCs) (a plots in PC1–PC2, b plots in PC2–PC3). Numbers show sampling points: S prefix indicates sampling in summer (June); A prefix indicates sampling in autumn (September). Black and white filled circles are vegetation stands dominated by Egeria densa and by other macrophytes, respectively
Comparison of bed-sediment characteristic between reaches adjacent to different land-use types
TP content of bed sediment in both summer and autumn in the reach adjacent to paddy fields was significantly higher than that in the reach adjacent to riparian forests (p < 0.05) (Table 2). Particle diameter of bed sediment in the reach adjacent to paddy fields in autumn, on the other hand, was significantly smaller than that in the reach adjacent to riparian forests (p < 0.05) (Table 2). This implies that particle diameter of bed sediment in the reach adjacent to riparian forests changed from coarse to fine with the sedimentation process (Table 2).
Relationship between free CO2 and growth rate
Though the sum of free CO2 and HCO3 − concentrations were similar among E. densa vegetation stands, growth rates of those stands differed significantly (Fig. 5b). E. densa growth rate, however, was correlated to free CO2 concentration (r = 0.67, p = 0.02). This indicates that E. densa growth rate is influenced by free CO2 concentration (Fig. 5a).
Table 3 illustrates the Spearman rank correlation coefficients of growth rate to variables and shows that pH and DO had negative correlations and DOC had positive correlation with growth rate. Negative correlations of pH and DO with growth rate imply that E. densa growth rate increases with acidification of water (Table 3).
Discussion
Egeria densa growth rate
From summer to autumn (July to September), E. densa growth rate in the midstream reach was relatively higher (0.069–0.282 kg DW m−2 month−1) than in other reaches. These growth rates are comparable with that reported by Haramoto and Ikusima (1988) (0.102–0.415 kg DW m−2 month−1) for Lake Kasumigaura of eastern Japan between March and July. Slightly lower values in our study might be due to the difference in growing period and the interruption to growth during the developmental period of E. densa caused by dredging in July (Haramoto and Ikusima 1988). Roberts et al. (1999) reported an E. densa biomass range from 0.400 to 3.000 kg DW m−2 in summer in the Hawkesbury–Nepean River in Australia. Yarrow et al. (2009) reviewed E. densa biomass production and concluded that it can range from 0.800 to 1.000 kg DW m−2 during its rapid growth period. The growth rates in our study are in agreement with other studies of E. densa.
Free CO2 as a factor influencing growth
E. densa growth rate positively correlates to free CO2 concentration and negatively to that of HCO3 − in water. The change in pH shifts the equilibrium balance of free CO2 and HCO3 − toward either one. In the study site, the sum of free CO2 and HCO3 − concentrations among vegetation stands were similar. Therefore, as either form of DIC concentration becomes higher, the other one correspondingly becomes lower. This implies that if one DIC form has a positive correlation to the growth rate, the other automatically has a negative correlation. Approximately 50% of aquatic plants, including E. densa, can use HCO3 − for photosynthesis (Yarrow et al. 2009). However, HCO3 − can affect the photosynthesis of aquatic plants only to a limited extent (Vadstrup and Madsen 2006), as HCO3 − assimilation requires more energy (Jones et al. 1993). Therefore, CO2 must be far more important for E. densa growth rate, and it can thus be concluded that the growth rate is affected by free CO2 concentration. Thus, HCO3 − concentration has a negative correlation to the growth rate. This trend showing the relationship between the growth rate and HCO3 − concentration may be applicable given sufficient free CO2 concentration is present in the system. If the water has a free CO2 deficit, the relationship may become positive with HCO3 − (Sand-Jensen and Gordon 1986). Consequently, free CO2 influences E. densa growth rate and may be one of the key factors for E. densa. Several studies report that macrophyte growth at lower pH is greater than that at higher pH with constant DIC levels (Sand-Jensen 1983; Titus et al. 1990; Titus 1992). This indicates that free CO2 concentration determines macrophyte growth. Furthermore, the fact that CO2 diffuses more slowly in water than in air (Lara et al. 2002) promotes the potential for free CO2 to be the key factor affecting the growth of submerged macrophytes. Regression analysis between E. densa growth rate and free CO2 concentration indicates that these two components are nearly proportional (Fig. 5). E. densa has a Kranz-less C4-acid metabolism (Lara et al. 2002). In general, terrestrial C4 plants have a CO2 compensation point of 0–5 ppm in the gas phase. However, prediction of the same for submerged macrophytes is difficult, as the value varies from 150 ppm to nearly zero (Bowes and Salvucci 1989). It is also known that the CO2 compensation point of E. densa changes with temperature (Lara and Andreo 2005). The regression line, of which y intercept is almost zero, indicates that E. densa can use even very low CO2 concentrations for its growth.
Effect of spring water on Egeria densa growth
The features of spring water in the study site are rich in free CO2 concentration and poor in PO4-P concentration. The values of free CO2 concentration are 11–16.5 times greater than air-equilibrium concentration, and several streams contain more than ten times greater free CO2 than atmospheric partial pressure (Madsen and Maberly 1991; Rebsdorf et al. 1991). Lower PO4-P concentration compared with stream water is due to adsorption by soil particles (Pinoke and Urban 1985; Sakadevan and Bavor 1998). It is considered that spring water increases E. densa growth rate in two ways: One is that spring water itself is generally rich in free CO2 and therefore directly promotes photosynthesis rate. The other is that if it is acidified, such as when it is affected by carbon acid, it shifts the equilibrium balance of free CO2 and HCO3 − toward a free CO2-dominant regime in water. This change in equilibrium consequently increases free CO2 concentration in vegetation stands. Titus et al. (1990) assumed the vigorous macrophyte growth at high DIC levels in low pH lakes on the basis of experimental results. This hypothesis supports the latter idea.
In conclusion, we observed that with a supply of spring water, submerged macrophytes were growing faster in other nearby streams, whereas in the absence of spring water, no colonization was observed. Although a detailed investigation is required, within the scope of the study reported here, it can be concluded that low pH spring water promotes the growth of submerged macrophytes.
References
Al-Kharabsheh AA (1999) Influence of urbanization on water quality at Wadi Kufranja basin (Jordan). J Arid Environ 43:79–89
Allan DJ, Castillo MM (2007) Streamwater chemistry. In: Dordrecht AA (ed) Stream ecology. Springer, Netherlands, pp 57–74
American Public Health Association (2005) Standard methods for the examination of water and wastewater centennial. APHA, Washington, DC
American Standard Test Method (2007) particle-size analysis of soils (D422-63)
Asaeda T, Hung TN, Manatunge J, Fujino T (2004) The effects of flowing water and organic matter on the spatial distribution of submersed macrophytes. J Freshw Ecol 19(3):401–405
Barko JW, Adams MS, Clesceri NL (1986) Environmental factors and their consideration in the management of submerged aquatic vegetation: a review. J Aquat Plant Manag 24:1–10
Bowes G, Salvucci ME (1989) Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquat Bot 34:233–266
Demars BOL, Trémolières M (2009) Aquatic macrophytes as bioindicators of carbon dioxide in groundwater fed rivers. Sci Total Environ 407:4752–4763
Eisen C, Anderson MP (1979) The effect of urbanization on ground-water quality—a case study. Ground Water 17(5):456–462
Feijoó C, Garcia ME, Momo F, Toja J (2002) Nutrient absorption by the submerged macrophyte Egeria densa Planch.: effect of ammonium and phosphorus availability in the water column on growth and nutrient uptake. Limnetica 21(1–2):93–104
Haga H, Ohtsuka T, Matsuda M, Ashiya M (2006) Spatial distribution of biomass and species composition in submerged macrophytes in the southern basin of Lake Biwa in summer of 2002. Jpn J Limnol 67:69–79 (in Japanese)
Hanya T, Ogura N (1995) Water quality examination. Maruzen Co., Ltd., Tokyo (in Japanese)
Haramoto T, Ikusima I (1988) Life cycle of Egeria densa Planch., an aquatic plant naturalized in Japan. Aquat Bot 30:389–403
Hayashi M, Rosenberry DO (2002) Effects of ground water exchange on the hydrology and ecology of surface water. Ground Water 40(3):309–316
Hung NT, Asaeda T, Manatunge J (2007) Modeling interactions of submerged plant biomass and environmental factors in a stream using structural equation modeling. Hydrobiologia 583:183–193
Jones JI, Eaton JW, Hardwick K (1993) Physiological plasticity in Elodea nuttallii(Planch.) St.John. J Aquat Plant Manag 31:88–94
Kadono Y (2004) Alien aquatic plants naturalized in Japan: history and present status. Global Environ Res 8(2):163–169
Lara MV, Andreo CS (2005) Photosynthesis in nontypical C4 species. In: Pessarakli M (ed) Handbook of photosynthesis. Taylor & Francis, Tucson, pp 391–421
Lara MV, Casati P, Andreo CS (2002) CO2-concentration mechanisms in Egeria densa, a submerged aquatic plant. Physiol Plant 115:487–495
Lodge DM, Krabbenhoft DP, Striegl RG (1989) A positive relationship between groundwater velocity and submerged macrophyte biomass in Sparkling Lake, Wisconsin. Limnol Oceanogr 34:235–239
Maberly SC, Madsen TV (2002) Freshwater angiosperm carbon concentration mechanisms: processes and patterns. Funct Plant Biol 29:393–405
Madsen TV, Maberly SC (1991) Diurnal variation in light and carbon limitation of photosynthesis by two species of submerged freshwater macrophyte with a differential ability to use bicarbonate. Freshw Biol 26:175–187
Madsen TV, Sand-Jensen K (1994) The interactive effects of light and inorganic carbon on aquatic plant growth. Plant Cell Environ 17:955–962
Ministry of Environment Japan (2010) Guideline for conservation and restoration of spring water (in Japanese)
Mony C, Koshnick TJ, Haller WT, Muller S (2007) Competition between two invasive Hydrocharitaceae (Hydrilla verticillata (L.f.) (Royle) and Egeria densa (Plnch)) as influenced by sediment fertility and season. Aquat Bot 86:236–242
Okubo K, Oike S (2008) Relationship between the aquatic plant distributions and environmental conditions in irrigation canals in Kamiina district, Nagano prefecture. Landsc Study 71(5):549–552 (in Japanese)
Otaki S, Ishido T (1980) Illustrated Japanese water plants. Hokuryukan, Tokyo (in Japanese)
Pinoke HB, Urban JB (1985) Effect of agricultural land use on ground-water quality in a small Pennsylvania watershed. Ground Water 23(1):68–80
Rattray MR, Howard-Williams C, Brown MAJ (1991) The photosynthesis and growth rate responses of two freshwater angiosperms in lakes of different trophic status: responses to light and dissolved inorganic carbon. Freshw Biol 25:399–407
Rebsdorf A, Thyssen N, Erlandsen M (1991) Regional and temporal variation in pH, alkalinity and carbon dioxide in Danish streams, related to soil type and land use. Freshw Biol 25:419–435
Roberts DE, Church AG, Cummins SP (1999) Invasion of Egeria into the Hawkesbury-Nepean River. Aust J Aquat Plant Manag 37:31–34
Rosenberry DO, Striegl RG, Hudson DC (2000) Plants as indicators of focused ground water discharge to a northern Minnesota lake. Ground Water 38(2):296–303
Sakadevan K, Bavor HJ (1998) Phosphate adsorption characteristics of soils, slags and zeolite to be used as substrates in constructed wetland systems. Wat Res 32(2):393–399
Sand-Jensen K (1983) Photosynthetic carbon sources of stream macrophytes. J Exp Bot 34(139):198–210
Sand-Jensen K, Gordon DM (1986) Variable HCO3 − affinity of Elodea canadaensis Michaux in response to different HCO3 − and CO2 concentrations during growth. Oecologia 70:426–432
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. A Willy-Interscience Publication, New York
Titus JE (1992) Submerged macrophyte growth at low pH II. CO2 × sediment interactions. Oecologia 92:391–398
Titus JE, Feldman RS, Grisé D (1990) Submersed macrophyte growth at low pH I. CO2 enrichment effects with fertile sediment. Oecologia 84:307–313
Truesdale GA, Downing AL, Lowden GF (1955) The solubility of oxygen in pure water and sea water. Appl Chem 6:53–62
Vadstrup M, Madsen TV (2006) Growth limitation of submerged aquatic macrophytes by inorganic carbon. Freshw Biol 34(3):411–4199
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2:203–215
Yarrow M, Marin VH, Finlayson M, Tironi A (2009) The ecology of Egeria densa planchon(Liliosida: Alismatales): a wetland ecosystem engineer? Revista Chilena de Historia Natural 82:299–313
Acknowledgments
Special thanks to Dr. R. Shinohara of the Graduate School of Frontier Sciences, University of Tokyo, and Dr. Md. H. Rashid of Bangladesh Agricultural University, for their kind support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Takahashi, K., Asaeda, T. The effect of spring water on the growth of a submerged macrophyte Egeria densa . Landscape Ecol Eng 10, 99–107 (2014). https://doi.org/10.1007/s11355-012-0191-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-012-0191-6