Abstract
Purpose
Obstructive sleep apnea (OSA) results in systemic intermittent hypoxia. By one model, hypoxic stress signaling in OSA patients alters the levels of inflammatory soluble cytokines TNF and IL6, damages the blood brain barrier, and activates microglial targeting of neuronal cell death to increase the risk of neurodegenerative disorders and other diseases. However, it is not yet clear if OSA significantly alters the levels of the soluble isoforms of TNF receptors TNFR1 and TNFR2 and IL6 receptor (IL6R) and co-receptor gp130, which have the potential to modulate TNF and IL6 signaling.
Methods
Picogram per milliliter levels of the soluble isoforms of these four cytokine receptors were estimated in OSA patients, in OSA patients receiving airways therapy, and in healthy control subjects. Triplicate samples were examined using Bio-Plex fluorescent bead microfluidic technology. The statistical significance of cytokine data was estimated using the nonparametric Wilcoxon rank-sum test. The clustering of these high-dimensional data was visualized using t-distributed stochastic neighbor embedding (t-SNE).
Results
OSA patients had significant twofold to sevenfold reductions in the soluble serum isoforms of all four cytokine receptors, gp130, IL6R, TNFR1, and TNFR2, as compared with control individuals (p = 1.8 × 10−13 to 4 × 10−8). Relative to untreated OSA patients, airways therapy of OSA patients had significantly higher levels of gp130 (p = 2.8 × 10−13), IL6R (p = 1.1 × 10−9), TNFR1 (p = 2.5 × 10−10), and TNFR2 (p = 5.7 × 10−9), levels indistinguishable from controls (p = 0.29 to 0.95). The data for most airway-treated patients clustered with healthy controls, but the data for a few airway-treated patients clustered with apneic patients.
Conclusions
Patients with OSA have aberrantly low levels of four soluble cytokine receptors associated with neurodegenerative disease, gp130, IL6R, TNFR1, and TNFR2. Most OSA patients receiving airways therapy have receptor levels indistinguishable from healthy controls, suggesting a chronic intermittent hypoxia may be one of the factors contributing to low receptor levels in untreated OSA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder associated with numerous adverse health effects. OSA patients may display any one or several symptoms including fragmented sleep, snoring, excessive daytime sleepiness, fatigue, high blood pressure, irritability, depression, memory loss, and loss of concentration. Fragmented sleep patterns, abnormally long pauses in breathing, or abnormally low levels of breathing during sleep result in poor oxygenation of the blood and chronic intermittent tissue hypoxia. Hypoxia leads to tissue inflammation, which appears to be one major cause of the diseases associated with OSA and OSA’s increased mortality risk. These health problems develop over months and years and include cardiovascular disease, metabolic syndrome, kidney disease, autoimmune diseases, and the focus of this study, neurodegenerative disease (ND). OSA has been associated with the increased risk and severity of the symptoms of Alzheimer’s disease [1, 2], amyotrophic lateral sclerosis [3], Parkinson’s disease [4, 5], multiple sclerosis [6, 7], schizophrenia [8, 9], depression disorders [10, 11], and cognitive dysfunction [12, 13]. Continuous positive airways pressure therapy (CPAP) is the mainstay of OSA treatment because it improves oxygenation, reduces inflammation, and CPAP is shown to reverse many symptoms and risks associated with OSA, including ND [5, 14,15,16,17,18,19,20,21]. Dental airway devices produce a similar treatment effect and have gained some acceptance as an effective alternative to CPAP [22, 23]. The success of CPAP treatment further supports a possible role for hypoxia-induced inflammation in ND risk. Unfortunately, a significant portion of patients find nightly CPAP therapy intolerable and reported non-compliance rates are high, ranging from 10 to 40% [24,25,26,27,28]. Hence, there is a pressing need for pharmaceutical treatments that might amend CPAP.
Brain inflammation is common to most NDs. A cytokine-centric model of neurodegeneration predicts that strong peripheral inflammation results in increased levels of inflammatory leukocytes and cytokines infiltrating the central nervous system (CNS), where they initiate neuroinflammation and neurodegeneration [29, 30]. Inflammatory monocytes and macrophages and cytokines in the brain activate microglial cells. Activated microglia secrete their own factors and some of the same cytokines that direct neuronal death and increase in the permeability of the blood brain barrier (BBB) to amplify the problem [30]. Elevated levels of two pro-inflammatory cytokines, in particular, tumor necrosis factor alpha (TNF) and interleukin 6 (IL6), are observed in patients with Alzheimer’s disease [31], amyotrophic lateral sclerosis [32, 33], multiple sclerosis [34], Parkinson’s disease [35,36,37], depression disorders [38, 39], and acute patients with schizophrenia or bipolar disorder [40, 41]. Elevated expressions of both TNF [42, 43] and IL6 [44, 45] are strongly associated with microglial activation, neuroinflammation, and neurodegeneration. Hence, their signaling appears central to many NDs.
The molecular mechanisms by which OSA increases the risk of various NDs and behaviorial symptoms such as irritability, depression, memory loss, and loss of concentration are poorly understood. Untreated OSA patients all suffer from chronic intermittent hypoxia. Hypoxia-induced oxidative stress signaling is known to alter the otherwise balanced levels of a number of pro-inflammatory and anti-inflammatory cytokines [46]. Elevated levels of TNF and IL6 are observed in cultured cells treated with mild hypoxia [47, 48], in the carotid body of mice treated with chronic hypoxia [49], in the ischemic rodent brain [50], and in most patients with OSA. In a majority of studies examining TNF levels, OSA patients have shown 1.2- to 2.5-fold higher serum levels [51] relative to control subjects. In some, but not all studies, OSA patients also show modest increases in IL6 [52,53,54,55,56,57,58]. CPAP treatment of OSA patients was most often, but not always, associated with normal levels of these cytokines similar to the levels in control subjects [52, 59,60,61,62,63,64,65,66,67,68,69]. The normal levels of TNF and IL6 observed in most OSA patients receiving airways therapy and the generally protective role of airways therapy to patient health strongly support the idea that chronic intermittent hypoxia induces inflammatory cytokines that are causal to neuroinflammation and ND.
The goal of this study was to identify other neuroinflammatory cytokines in serum whose expression levels were altered in OSA patients and but normal with CPAP treatment, focusing on TNF receptors, TNFR1 and TNFR2, and the IL6 receptor IL6R and its co-receptor gp130. To date, neither large increases nor large decreases in the expression of the soluble isoforms of these cytokine receptors have been associated with OSA. IL6 receptor complexes may be composed of soluble IL6, the membrane-bound receptor isoform or soluble isoform of IL6R, and the membrane-bound receptor or soluble isoform of co-receptor gp130 [70,71,72,73,74,75,76,77]. IL6 receptor complexes are central to inflammatory signaling, neurodegeneration, and ND [70,71,72,73,74,75,76,77]. The soluble, non-membrane-bound, isoforms of gp130 and IL6R both modulate inflammatory IL6/IL6R/gp130 membrane signaling and both are capable of attenuating IL6 signaling [78,79,80]. Altered serum levels of the soluble isoforms of two TNF receptors, tumor necrosis factor receptor TNFR1 (TNFRSF1A) and TNFR2 (TNFRSF1B), are linked to inflammatory signaling, neuronal cell death, and regeneration, and altered in patients with ND [29, 32, 81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97]. The soluble, non-membrane-bound, isoforms of these cytokine receptors can significantly antagonize signaling by IL6 and TNF, respectively [98,99,100]. Changes in the expression of these cytokine receptors have the potential to increase or decrease neuroinflammatory signaling by IL-6 and TNF, and hence, contribute to ND risk. The serum levels of the soluble isoforms of gp130, IL6R, TNFR1, and TNFR2 were significantly lower in OSA patients relative to control subjects, but OSA patients receiving airways therapy had levels indistinguishable from controls.
Materials and methods
Study subjects
A total of 46 study subjects were enrolled in the study following informed consent, including nineteen subjects with untreated OSA (i.e., not on airways therapy) and nineteen treated OSA individuals, who were diagnosed by polysomnography using the Apnea Hypopnea Index (AHI) (Table 1) [101, 102]. The airway-treated OSA subjects recorded using primary CPAP, except for one patient who used a dental airways device, and both devices had been employed for more than 6 months [22, 23]. Eight control individuals were recruited that had undergone polysomnography and did not have sleep disordered breathing. Subject characteristics (age, gender), anthropometrics (weight, weight, BMI), history of CVD, medication history, AHI, SaO2, and ESS (Epworth Sleepiness Scale) as well as fasting cholesterol, glucose, and HS-CRP were evaluated in all subjects [54, 103,104,105] are shown in Table 1. Treated and untreated OSA patients are well matched for nearly all parameters, while the control group was considerably younger and leaner, and presumably represented an optimal cytokine levels for comparison.
Powering the study
The null hypothesis being tested was that OSA patients and airway-treated OSA patients would express the same levels of soluble cytokine receptors. The alternate hypothesis being tested was that OSA patients would express higher or lower levels of soluble cytokine receptors relative to the airway-treated OSA patients [106]. These hypotheses were tested using Bio-Plex system to assay cytokine receptor levels, which provides greater sensitivity, a wider dynamic range, greater effect sizes, and more statistical significance for each assay than conventional cytokine receptor immunoassays used in most previous studies. In sample size planning, the potential for large effect sizes for the differences in the levels of the four cytokines examined and study costs were considered [107, 108]. Preliminary data showed large effect sizes for cytokine receptor levels with high levels of statistical significance (see statistical analysis). Hence, it was concluded that modest OSA and airway-treated OSA patient sample sizes would be sufficient to power a statistically significant preliminary study that would avoid both type I (false positive) and type II (false negative) errors [108] as recommended by the Federal Food and Drug Administration’s study guidelines for estimating minimum appropriate human subject sample size [109].
Cytokine receptor assay
The levels of soluble inflammatory cytokine receptors in serum were examined using multiplex kits that quantify biomarkers of human inflammation (Bio-Rad #171AL001M). The 96-well plates were assayed using a Bio-Rad Bio-Plex instrument. The Bio-Plex system has the advantage that hundreds of beads estimate each cytokine level in each well, which improves the statistical accuracy of each individual well estimate. All the serum samples, standards, and assay controls were prepared as per the manufacturer’s instructions (Bio-Rad Bulletin 10044281). As recommended, each serum sample was diluted fourfold. Fifty microliters of this dilution was run in triplicate for the 8 control, 19 untreated OSA patients, and 19 airways therapy–treated OSA patients instead of duplicate samples recommended by the manufacturer, in order to better estimate experimental errors for each cytokine measurement. The picogram per milliliter output data for each serum cytokine receptor level was normalized to the concentration of standards, run as eight times fourfold dilutions of each cytokine, and run in duplicate on each plate. The quantitative nature of these assays over the expected concentration ranges estimated for serum samples was confirmed by comparing the fluorescence output of quadruplicate standard samples run in eight steps of a fourfold dilution series. The standard error of the lowest concentration standards used to estimate concentration was less than 15% and much less than that for higher concentrations.
Management of data sets and statistical analysis
The data output from separate Bio-Plex plates were combined to make a single Excel data file, with one sheet for each cytokine receptor and separate sheets for biometric data (see Supplemental Data File 1). At this point, the data were moved into R v3.5.1 for further statistical analysis. The data for individual human subjects were visualized using Boxplot. After applying the Kolmogorov–Smirnov test, it was obvious that the airway-treated patient data for each the cytokine were not normally distributed (p value < 0.05) and generally fell into two groups of values. The Kolmogorov–Smirnov test is a nonparametric goodness-of-fit test and could be used to determine whether an underlying probability distribution differs from a hypothesized distribution. Therefore, the nonparametric Wilcoxon rank-sum test was used to estimate p values for the significance of pairwise differences in cytokine receptor levels among OSA patients, airway-treated OSA patients, and controls. To visualize the high-dimensional data for the levels of all four cytokine receptors among all patients and controls in a two-dimensional map, the nonparametric t-SNE visualization method [110] was applied using the Rtsne statistical package available online [111].
Results
Serum cytokine receptor levels
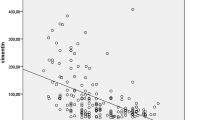
Soluble serum cytokine receptor levels in airway-treated OSA patients were compared with untreated OSA patients and with control subjects. The picogram per milliliter (pg/mL) protein expression levels of the soluble isoforms of four inflammatory cytokine receptors known to be involved in ND, but not yet linked to OSA, were significantly lower in the serum of OSA patients relative to the levels for both the control individuals and the OSA patients receiving airways therapy. These data on the levels of soluble Gp130 (IL6ST), IL6R (IL6Ra, IL6R), TNF-R1 (TNFRSF1A), and TNF-R2 (TNFRSF1B) are shown in Fig. 1 and summarized in Table 2.
The levels of four cytokine receptors involved in neurodegenerative disease risk are low in OSA patients, but their levels in airway-treated OSA patients are indistinguishable from those in control subjects. The serum picogram per milliliter (pg/mL) levels of the soluble isoforms of a gp130, b IL6R, c TNFR1, and c TNFR2 for the eight control individuals, nineteen airway-treated apneic patients, and nineteen apneic patients not receiving airways therapy are summarized in box blots. The boxed area encloses the second and third quartile and is bounded by median pg/mL value indicated by a black line. The lower and upper whiskered ranges indicate the first quartile-1.5*IQR (interquartile range) and the third quintile +1.5*IQR, respectively. Each of the three independent Bio-Plex estimates of a cytokine level for each patient is represented by separate data points. Potential outlying data among airways treated patients are encircled by a red dotted line
The pg/mL concentrations of soluble gp130 and IL6R are shown as Box plot comparisons among the OSA patient population, OSA patients receiving airways therapy, and control individuals in Fig. 1a and b, respectively. It was observed that the median serum level of gp130 in OSA patients was 2.3-fold lower than that of the control subjects ((p = 2.9 × 10−13). OSA patients receiving airways treatment had a median level of gp130 that was much higher than that in untreated patients (p = 2.8 × 10−13), a median level indistinguishable from that observed in the younger control group (p = 0.87, Fig. 1a, Table 2). Similarly, the median serum level of soluble IL6R in OSA patients was 2.1-fold lower than in the serum of control subjects (p = 1.3 × 10−12). OSA patients receiving airways therapy had a median level of soluble IL6R that was 2.8-fold higher than that observed in untreated patients (p = 1.1 × 10−9), a level that was indistinguishable from that of the control group (p = 0.95, Fig. 1b).
The median pg/mL serum levels of soluble TNFR1 and TNFR2 were 3.4-fold and 6.8-fold lower in OSA patients relative to control subjects, p = 4.1 × 10−8 and 1.8 × 10−13, respectively (Fig. 1c and c, Table 2). The median serum level of TNFR1 was 3.4-fold higher in airway-treated patients than untreated apneic patients (p = 2.5 × 10−10, Fig. 1c) and was statistically indistinguishable from the median level in controls (p = 0.29). The median level of TNFR2 was 7.9-fold higher in airway-treated patients than untreated apneic patients (p = 5.7 × 10−9, Fig. 1c) and was also indistinguishable from the median level in controls (p = 0.58).
In short, OSA patients had aberrantly and significantly low pg/mL levels of all four soluble cytokine receptors linked to ND relative to younger healthy control individuals. By contrast, OSA patients receiving airways therapy had cytokine receptor levels that were impossible to differentiate from those observed for control subjects. However, some of the data for five airway-treated patients (patient numbers 12, 26, 34, 47, and 70) were widely distributed and were more similar to levels in untreated OSA patients (see areas encircled by red dotted lines, Fig. 1). To better visualize the potentially similar response among most airway-treated individual patients independent of the direction or magnitude of change in the level of all four cytokines, the t-distributed stochastic neighbor embedding (t-SNE) method was applied. This machine-learning 2D visualization strategy has the capacity of capturing the local structure of these high-dimensional data and revealing the presence of data clusters as a global structure [110]. Figure 2 shows that all the patient and control subject cytokine receptor data group in two clusters. Cluster 1 represents the soluble cytokine receptor data for all nine control individuals (red data points in Fig. 2) and fourteen of the nineteen airway-treated patients (green data points in cluster 1). Cluster 2 represents the cytokine receptor data for all nineteen of the untreated OSA patients (blue data points in cluster 2) and five of the patients classified as airway treated (i.e., green data points within cluster 2). The cytokine receptor levels for patient numbers 12, 26, 34, 47, and 70 (numbered data points in Fig. 2) clearly distinguished themselves, because the combined levels of all four cytokines remain more similar to the levels in untreated OSA patients (again the encircled data points in Fig. 1).
t-SNE clustered the cytokine receptor data for all OSA and airway-treated OSA patients and control individuals into two groups. Cluster 1 represents the dimensional distribution and grouping of data for four cytokines for all the control individuals, fifteen of the nineteen airway-treated OSA patients, and one untreated OSA patient. Cluster 2 represents the dimensional distribution of cytokine data among all but one of the untreated OSA patients and five of the airway-treated OSA patients. Each patient is represented by three separate data points. The individual patient numbers for the airway-treated OSA patient data are indicated in each cluster
The OSA patients, airway-treated patients, control subjects, and the outlying airway-treated OSA patient group did not appear to distinguish themselves based on a cursory analysis of biometric data such as age, BMI, gender, or particular chronic medications. A further analysis was made of the biostatistical, laboratory, and sleep data for potential causes for the variation in cytokine levels among all patients, controls, and particularly these five outlying airway-treated patients. Biometric, laboratory, and sleep data were collected at the time of serum collection for controls and for the untreated OSA patients and these data were collected months before serum collection at the time of their diagnosis of OSA for airway-treated patients. A linear regression analysis was performed, plotting the pg/mL level of each cytokine against age, BMI, heart rate, Apnea Hypopnea Index (AHI), and oxygen as SaO2 low %, ESS, and the laboratory data from duplicate serum samples including glucose levels, total cholesterol, HDL, LDL, and CRP. None of the biometric, laboratory, or sleep variables accounted for more than 40% of the variance in any of the cytokine levels among any of the any of the OSA patients, airway-treated OSA patients, control subjects or outliers, or all 49 subjects taken together. It was surprising that none of the sleep-related measures obtained correlated with cytokine levels in OSA. Although intermittent hypoxia is the hallmark of OSA, the findings in this study suggest that other mechanisms may be important in altering levels of GP130, IL6Ra, TNF-R1, and TNF-R2. One explanation for the nonparametric data for the small number outlying patients would be that their lack of complete adherence to airways treatment was misreported as adherence. The airways treated OSA patient using the dental airways device was not among the outliers, but had cytokine receptor levels similar to healthy controls.
Discussion
We are working under a model in which chronic oxidative stress signaling in OSA patients alters the levels of soluble inflammatory cytokines in serum to initiate an inflammatory cascade that passes the blood brain barrier and increases the risk of ND. Making the presumption that cytokine levels respond to airways therapy, then aberrant levels in OSA patients may be directly or indirectly linked to oxidative stress signaling. The soluble, non-membrane-bound, isoforms of the membrane receptors gp130, IL6R, TNFR1, and TNFR2 all have been shown to regulate inflammatory signaling by their membrane-bound isoforms and by their cognate cytokines [78, 100]. Cytokines can penetrate the blood brain barrier particularly under circumstances of neuroinflammation [75]. If the significant reductions observed in the levels of all four soluble cytokine receptors in the serum of OSA patients pass the blood brain barrier, this should produce the mis-regulation of TNF and IL6 signaling in the brain. While this discussion is focused on the activities of the four cytokine receptors in ND, these receptors also have reported roles in autoimmune and cardiovascular diseases.
IL6R and gp130
Altered levels of the membrane and/or soluble isoforms of gp130 and IL6R have been reported in the serum, cerebral fluid, and/or brain of patients with Alzheimer disease [112,113,114], amyotrophic lateral sclerosis [70], multiple sclerosis [71], and schizophrenia and bipolar disorder [72, 115]. A few single nucleotide polymorphisms affecting IL6R expression levels or protein activity also correlate with ND [70, 116,117,118]. Hence, it is reasonable to consider that the altered levels of soluble IL6R and gp130 observed in OSA patients are risk factors of ND and may even contribute causally to ND risk. Pro-and anti-inflammatory signaling by IL6 involving IL6R and gp130 is divided into two pathways. In the classical signaling pathway, IL6 binds to a classical receptor complex composed of the membrane isoforms of IL6R and gp130. Their inflammatory signaling is thought to contribute primarily to beneficial anti-inflammatory activities and tissue regeneration. In the trans-signaling pathway, both soluble and membrane isoforms of both cytokines are involved, and hence, their soluble isoforms have a greater potential to regulate trans-signaling. The trans-signaling pathway is thought to produce most of the harmful pro-inflammatory signaling leading to cell death directed by IL6 [78]. While the two signaling pathways were elucidated in non-neuronal systems and cell types [78], these are the best models for us to consider, while examining the roles of soluble IL6R and gp130 in neurodegeneration and ND.
Significant reductions in the levels of both soluble pg130 and soluble IL6R were observed in OSA patients relative to controls and relative to most airway-treated patients. Most airway-treated OSA patients in this study had serum levels of both cytokines that were essentially the same as controls. Because soluble gp130 is directly involved in attenuating harmful inflammatory trans-signaling by binding both the soluble and membrane isoforms of IL6R, the dramatic reductions observed in gp130 in OSA patients are likely to be harmful, increasing neuroinflammation, neurodegeneration, and ND risk [78]. However, it is harder to predict the consequences of reduced levels of soluble IL6R on ND, because soluble IL6R bound to IL6 binds with the membrane isoform of gp130 to enhance trans-signaling and IL6R binds to soluble gp130 with the potential to attenuate trans-signaling [78]. Previous studies had shown that the levels of the membrane isoforms of gp130 and IL6R were elevated by acute hypoxia in rodent models [119, 120], but the level of soluble IL6R was not altered in teenage OSA patients [58]. Finally, the data presented herein may be the first showing significant reductions in the levels of soluble gp130 and IL6R in the serum of older OSA patients and the normal levels of these cytokine receptors in patients receiving airways therapy.
There are immunotherapeutic approaches emerging to counter the reduction in gp130 and/or IL6R levels for OSA patients with symptoms of ND as a supplement to CPAP. Prenissl et al. [121] have shown that the monoclonal antibody tocilizumab, which specifically binds to the membrane receptor isoform of IL6R, increases the production of soluble IL6R twofold in peripheral tissues. Clinical studies have shown that tocilizumab significantly reduces symptoms of inflammatory disease, and it has been approved for the treatment of rheumatoid arthritis [122]. There is an ongoing clinical trial to treat depressed patients with tocilizumab [123]. However, the treatment of schizophrenic patients with tocilizumab did not improve patient behavior [124]. Assuming that reductions in the soluble isoforms of these receptors increase inflammatory signaling, then reducing IL6 activity is another alternative. Schuett et al. [125] showed treatment with modest concentrations of a fusion between the soluble isoform of gp130 and the IgG1 heavy chain constant region (sgp130Fc) dramatically reduced IL6 inflammatory signaling, presumably by substituting for soluble gp130 in trans-signaling. It has been proposed that sgp130Fc may be an effective therapeutic to treat depression [80, 126]. Unfortunately, inhibitors of IL6 itself, such as the IL6-specific monoclonal siltuximab, have not been particularly effective at inhibiting the symptoms of inflammatory disease [127], presumably because IL6’s activities are so pleiotropic.
TNFR1 and TNFR2
The membrane and processed soluble isoforms of TNFR1 and TNFR2 both bind TNF [128, 129]. Altered expressions of one or both isoforms of these proteins are associated with Alzheimer’s disease [89,90,91], amyotrophic lateral sclerosis [32], depression [29, 86,87,88], Parkinson’s disease [85], and schizophrenia [93,94,95,96]. Both isoforms of TNFR1 and TNFR2 are involved in inflammatory signaling, but they generally signal via different transduction pathways and with nearly opposing outcomes [129]. TNFR1 predominantly promotes inflammation and neuronal cell death [83], while TNFR2 plays more neuroprotective roles in promoting cell survival and tissue regeneration [89]. The soluble isoforms of both are found in serum, and increasing their levels can antagonize or promote receptor signaling [98,99,100]. Hence, altering the ratio or levels of their co-expression can shift the balance between cellular survival, regeneration, and apoptosis. The expression of both receptors increases when cultured cells are treated with acute intermittent hypoxia [130], but in vitro studies examining the impact of chronic hypoxia have not been reported.
Previous studies yielded marginal or conflicting results concerning the levels of the soluble isoforms of TNF receptors in OSA patients, with TNFR1 levels reported as 2.1-fold higher than the levels control subjects [131] or only 1.1-fold higher [132] or indistinguishable from controls [58, 133]. An earlier report showed that airways therapy of OSA patients decreased plasma levels of soluble TNFR1 marginally 1.2-fold relative to untreated OSA patients [134]. Finally, one study showed that OSA patients with AHI scores higher than 10 had 1.5-fold higher levels of soluble TNFR1 and TNFR2, relative to patients with an AHI lower than 10 [135]. Hence, both the direction and small magnitude of change in these studies of OSA patients are inconsistent with the data collected herein. Although, deficiencies in soluble TNFR2 have been associated with inflammatory autoimmune diseases [136].
By contrast to most previous studies, herein, statistically significant several-fold lower levels of soluble TNFR1 and TNFR2 were observed in untreated OSA patients relative to control subjects. Cytokine receptor levels were dramatically higher in airway-treated OSA patients and were essentially the same as controls. These results strongly suggest that the serum levels of the soluble isoforms of both cytokines are positively regulated by blood oxygen levels. Consistent with these data, chronically increased TNF expression has been associated with reduced levels of TNFR1 and TNFR2 in the brain [97, 137]. Perhaps the chronic intermittent hypoxia experienced by OSA patients creates a chronic increase in TNF, with the potential to lower soluble TNFR1 and TNFR2 levels.
It is worth considering why such large, highly statistically significant changes were observed herein in the levels of TNFR1 and TNFR2 as compared with the small 1.2- to 1.5-fold changes observed in other studies of OSA patients relative to controls or to the airway-treated OSA patients. The fluorescent bead immunocapture microfluidic technology applied to this patient population gives highly dynamic results based on hundreds of fluorescent beads for each measurement of pg/mL cytokine levels, instead of using less dynamic colorimetric assay in a few wells of a microtiter plate provided by ELISA kits [131] such as reported in most earlier studies. Fluorescent bead immunocapture microfluidics is more sensitive or at least as sensitive as and statistically reproducible as ELISA microtiter plate measurements of cytokines used in these earlier studies [138, 139] and fluorescence bead assays have a higher dynamic range. The standard errors among the three independent replicate assays performed for each cytokine were very small, even for the lowest concentrations observed in untreated OSA patients (see Materials and methods). It appears that fluorescent bead capture technology provided an advantage, when assaying the dynamic changes observed in cytokine levels among apneic patients and airway-treated apneic patients and control subjects.
One reasonable interpretation of these results would be that the aberrantly low levels of soluble TNFR1 and TNFR2 in OSA patients might no longer provide appropriate attenuation of TNF inflammatory signaling, thus increasing the risk of ND. A few therapeutic treatments have been shown to specifically block membrane TNFR1 signaling or to increase TNFR2 activity [97]. ATROSAB is a humanized mouse monoclonal to TNFR1 that covers the TNF binding epitope and neutralizes TNFR1’s membrane signaling activity [140]. ATROSAB prevents neuronal cell death in a mouse model of cognitive impairment and ND [141]. Whereas in the same model, simultaneously blocking both TNFR1 and TNFR2 proved ineffective. ATROSAB also proved to be an effective treatment for multiple sclerosis in a mouse disease model [142]. Perhaps therapeutic treatment of OSA patients with ATROSAB could aid OSA patients with symptoms of ND, as a supplement to CPAP. Finally, the cholesterol-lowering small molecule drug, lovastatin, selectively increases TNFR2 expression [143] and prevents cognitive deficits in mice [144].
Conclusions
The serum levels of the soluble isoforms of four membrane receptors, gp130, IL6R, TNFR1, and TNFR2, with roles in attenuating inflammatory signaling and neuronal cell death, and hence, risk factors for ND, were examined. The pg/mL protein levels of all four were expressed at aberrantly low levels in OSA patients. The majority of airway-treated OSA patients had levels for all four soluble cytokine receptors equivalent to those observed in healthy control individuals. In short, airways treatment was strongly correlated with normal cytokine receptor levels in most of the airway-treated OSA patients. This correlation with airways treatment suggests that chronic intermittent hypoxia may be among the factors contributing to the aberrantly low expression of TNF and IL6 receptors in untreated OSA patients. Several other OSA-associated abnormalities such as sleep fragmentation and daytime sleepiness may also play a role in altering the expression of cytokines and cytokine receptors. Furthermore, any conclusions drawn from this study are partially compromised by the surprising lack of a correlation between cytokine receptor levels and CRP levels, SaO2 low percent, and perhaps the relatively young age of our control subjects. Increasing the serum levels of these cytokines in OSA patients may be a beneficial supplement to airways therapy.
Data availability
See Supplementary Data File 1.
Abbreviations
- ND:
-
Neurodegenerative disease
- OSA:
-
Obstructive sleep apnea syndrome
- CPAP:
-
Continuous positive airways pressure
- t-SNE:
-
t-distributed stochastic neighbor embedding
- gp130 (IL6ST):
-
Interleukin 6 signal transducer, membrane glycoprotein 130
- IL6R (IL6R):
-
IL-6R, IL6Ra, interleukin 6 receptor
- TNFR1 (TNFRSF1A), TNF-R1:
-
Tumor necrosis factor receptor superfamily member 1A
- TNFR2 (TNFRSF1B) TNF-R2:
-
Tumor necrosis factor receptor superfamily member 1B
- TNF (TNF):
-
TNF-α, tumor necrosis factor ligand superfamily member 2
- IL6 (IL6):
-
IL-6, interleukin 6, B cell stimulatory factor 2
References
Daulatzai MA (2013) Death by a thousand cuts in Alzheimer’s disease: hypoxia--the prodrome. Neurotox Res 24(2):216–243
Abrams B (2005) Add Alzheimer’s to the list of sleep apnea consequences. Med Hypotheses 65(6):1201–1202
Quaranta VN, Carratu P, Damiani MF, Dragonieri S, Capozzolo A, Cassano A et al (2017) The prognostic role of obstructive sleep apnea at the onset of amyotrophic lateral sclerosis. Neurodegener Dis 17(1):14–21
Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD (2015) A 5-year follow-up study on the relationship between obstructive sleep apnea and Parkinson disease. J Clin Sleep Med 11(12):1403–1408
Neikrug AB, Liu L, Avanzino JA, Maglione JE, Natarajan L, Bradley L, Maugeri A, Corey-Bloom J, Palmer BW, Loredo JS, Ancoli-Israel S (2014) Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 37(1):177–185
Abdel Salam OA, Ghonimi NAM, Ismail MH (2019) Risk of obstructive sleep apnea in multiple sclerosis: frequency, clinical and radiological correlates. Mult Scler Relat Disord 28:184–188
Dias RA, Hardin KA, Rose H, Agius MA, Apperson ML, Brass SD (2012) Sleepiness, fatigue, and risk of obstructive sleep apnea using the STOP-BANG questionnaire in multiple sclerosis: a pilot study. Sleep Breath 16(4):1255–1265
Stubbs B, Vancampfort D, Veronese N, Solmi M, Gaughran F, Manu P, Rosenbaum S, de Hert M, Fornaro M (2016) The prevalence and predictors of obstructive sleep apnea in major depressive disorder, bipolar disorder and schizophrenia: a systematic review and meta-analysis. J Affect Disord 197:259–267
Myles H, Myles N, Antic NA, Adams R, Chandratilleke M, Liu D, Mercer J, Vakulin A, Vincent A, Wittert G, Galletly C (2016) Obstructive sleep apnea and schizophrenia: a systematic review to inform clinical practice. Schizophr Res 170(1):222–225
Pamidi S, Knutson KL, Ghods F, Mokhlesi B (2011) Depressive symptoms and obesity as predictors of sleepiness and quality of life in patients with REM-related obstructive sleep apnea: cross-sectional analysis of a large clinical population. Sleep Med 12(9):827–831
Deldin PJ, Phillips LK, Thomas RJ (2006) A preliminary study of sleep-disordered breathing in major depressive disorder. Sleep Med 7(2):131–139
Feng J, Wu Q, Zhang D, Chen BY (2012) Hippocampal impairments are associated with intermittent hypoxia of obstructive sleep apnea. Chin Med J 125(4):696–701
Haensel A, Bardwell WA, Mills PJ, Loredo JS, Ancoli-Israel S, Morgan EE, Heaton RK, Dimsdale JE (2009) Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep Breath 13(1):35–41
Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S (2009) Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 5(4):305–309
Hiraoka T, Yamada N (2013) Treatment of psychiatric symptoms in schizophrenia spectrum disorders with comorbid sleep apnea syndrome: a case report. Seishin Shinkeigaku Zasshi = Psychiatr Neurol Jpn 115(2):139–146
Boufidis S, Kosmidis MH, Bozikas VP, Daskalopoulou-Vlahoyianni E, Pitsavas S, Karavatos A (2003) Treatment outcome of obstructive sleep apnea syndrome in a patient with schizophrenia: case report. Int J Psychiatry Med 33(3):305–310
Kaminska M, Mery VP, Lafontaine AL, Robinson A, Benedetti A, Gros P, Kimoff RJ (2018) Change in cognition and other non-motor symptoms with obstructive sleep apnea treatment in Parkinson disease. J Clin Sleep Med 14(5):819–828
Kallweit U, Baumann CR, Harzheim M, Hidalgo H, Pohlau D, Bassetti CL et al (2013) Fatigue and sleep-disordered breathing in multiple sclerosis: a clinically relevant association? Mult Scler Int 2013:286581
Haddock N, Wells ME (2018) The association between treated and untreated obstructive sleep apnea and depression. Neurodiagn J 58(1):30–39
Gupta MA, Simpson FC, Lyons DC (2016) The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: a systematic review and meta-analysis. Sleep Med Rev 28:55–68
Rezaeitalab F, Moharrari F, Saberi S, Asadpour H, Rezaeetalab F (2014) The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci 19(3):205–210
Kostrzewa-Janicka J, Sliwinski P, Wojda M, Rolski D, Mierzwinska-Nastalska E (2017) Mandibular advancement appliance for obstructive sleep apnea treatment. Adv Exp Med Biol 944:63–71
Cantore S, Ballini A, Farronato D, Malcangi G, Dipalma G, Assandri F, Garagiola U, Inchingolo F, de Vito D, Cirulli N (2016) Evaluation of an oral appliance in patients with mild to moderate obstructive sleep apnea syndrome intolerant to continuous positive airway pressure use: preliminary results. Int J Immunopathol Pharmacol 29(2):267–273
Collard P, Pieters T, Aubert G, Delguste P, Rodenstein DO (1997) Compliance with nasal CPAP in obstructive sleep apnea patients. Sleep Med Rev 1(1):33–44
Ghosh D, Allgar V, Elliott MW (2013) Identifying poor compliance with CPAP in obstructive sleep apnoea: a simple prediction equation using data after a two week trial. Respir Med 107(6):936–942
Krieger J (1992) Long-term compliance with nasal continuous positive airway pressure (CPAP) in obstructive sleep apnea patients and nonapneic snorers. Sleep. 15(6 Suppl):S42–S46
Samson P, Casey KR, Knepler J, Panos RJ (2012) Clinical characteristics, comorbidities, and response to treatment of veterans with obstructive sleep apnea, Cincinnati Veterans Affairs Medical Center, 2005-2007. Prev Chronic Dis 9:E46
Vozoris NT (2012) Sleep apnea-plus: prevalence, risk factors, and association with cardiovascular diseases using United States population-level data. Sleep Med 13(6):637–644
Brunoni AR, Machado-Vieira R, Sampaio-Junior B, Vieira EL, Valiengo L, Bensenor IM et al (2015) Plasma levels of soluble TNF receptors 1 and 2 after tDCS and sertraline treatment in major depression: results from the SELECT-TDCS trial. J Affect Disord 185:209–213
Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (review). Mol Med Rep 13(4):3391–3396
Rubio-Perez JM, Morillas-Ruiz JM (2012) A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012:756357
Tortarolo M, Lo Coco D, Veglianese P, Vallarola A, Giordana MT, Marcon G et al (2017) Amyotrophic lateral sclerosis, a multisystem pathology: insights into the role of TNFalpha. Mediat Inflamm 2017:2985051
Hu Y, Cao C, Qin XY, Yu Y, Yuan J, Zhao Y, Cheng Y (2017) Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci Rep 7(1):9094
Sedighian M, Djafarian K, Dabiri S, Abdollahi M, Shab-Bidar S (2019) Effect of omega-3 supplementation on expanded disability status scale and inflammatory cytokines in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. CNS Neurol Disord Drug Targets 18:523–529
Chen X, Hu Y, Cao Z, Liu Q, Cheng Y (2018) Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front Immunol 9:2122
McCoy MK, Ruhn KA, Blesch A, Tansey MG (2011) TNF: a key neuroinflammatory mediator of neurotoxicity and neurodegeneration in models of Parkinson’s disease. Adv Exp Med Biol 691:539–540
Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M (1999) IL-1 beta, IL-2, IL-6 and TNF-alpha production by peripheral blood mononuclear cells from patients with Parkinson’s disease. Biomed Pharmacother 53(3):141–145
Kim YK, Na KS, Myint AM, Leonard BE (2016) The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 64:277–284
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67(5):446–457
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21(12):1696–1709
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70(7):663–671
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics. 7(4):354–365
Eskes C, Juillerat-Jeanneret L, Leuba G, Honegger P, Monnet-Tschudi F (2003) Involvement of microglia-neuron interactions in the tumor necrosis factor-alpha release, microglial activation, and neurodegeneration induced by trimethyltin. J Neurosci Res 71(4):583–590
Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH (1997) Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A 94(4):1500–1505
Matsumoto J, Dohgu S, Takata F, Machida T, Bolukbasi Hatip FF, Hatip-Al-Khatib I et al (2018) TNF-alpha-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IkappaB-NFkappaB and JAK-STAT3 pathways. Brain Res 1692:34–44
Vakil M, Park S, Broder A (2018) The complex associations between obstructive sleep apnea and auto-immune disorders: a review. Med Hypotheses 110:138–143
Lewis A, Elks PM (2019) Hypoxia induces macrophage tnfa expression via cyclooxygenase and prostaglandin E2 in vivo. Front Immunol 10:2321
Smith SM, Friedle SA, Watters JJ (2013) Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One 8(12):e81584
Lam SY, Tipoe GL, Liong EC, Fung ML (2008) Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem Cell Biol 130(3):549–559
Wang LW, Chang YC, Chen SJ, Tseng CH, Tu YF, Liao NS, Huang CC, Ho CJ (2014) TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain. J Neuroinflammation 11:215
Kheirandish-Gozal L, Gozal D (2019) Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci 20(3)
De Santis S, Cambi J, Tatti P, Bellussi L, Passali D (2015) Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in obstructive sleep apnea syndrome (OSAS) patients. Pol Otolaryngol 69(2):1–8
Shi ZM, Li TP, Xian LW, Li ZG (2011) Role of inflammation factors in impaired glucose tolerance and sleep apnea/hypopnea syndrome in pregnant women. Nan Fang Yi Ke Da Xue Xue Bao 31(8):1357–1359
Qian X, Yin T, Li T, Kang C, Guo R, Sun B, Liu C (2012) High levels of inflammation and insulin resistance in obstructive sleep apnea patients with hypertension. Inflammation. 35(4):1507–1511
Vicente E, Marin JM, Carrizo SJ, Osuna CS, Gonzalez R, Marin-Oto M et al (2016) Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J 48(4):1108–1117
Kim J, Lee CH, Park CS, Kim BG, Kim SW, Cho JH (2010) Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg 136(9):896–899
Kurt OK, Tosun M, Talay F (2013) Serum cardiotrophin-1 and IL-6 levels in patients with obstructive sleep apnea syndrome. Inflammation. 36(6):1344–1347
Gaines J, Vgontzas AN, Fernandez-Mendoza J, Calhoun SL, He F, Liao D, Sawyer MD, Bixler EO (2016) Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am J Physiol Endocrinol Metab 311(5):E851–E8E8
Hegglin A, Schoch OD, Korte W, Hahn K, Hurny C, Munzer T (2012) Eight months of continuous positive airway pressure (CPAP) decrease tumor necrosis factor alpha (TNFA) in men with obstructive sleep apnea syndrome. Sleep Breath 16(2):405–412
Jiang YQ, Xue JS, Xu J, Zhou ZX, Ji YL (2017) Efficacy of continuous positive airway pressure treatment in treating obstructive sleep apnea hypopnea syndrome associated with carotid arteriosclerosis. Exp Ther Med 14(6):6176–6182
Jin F, Liu J, Zhang X, Cai W, Zhang Y, Zhang W, Yang J, Lu G, Zhang X (2017) Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome. Mol Med Rep 16(5):6334–6339
Yoshikawa M, Yamauchi M, Fujita Y, Koyama N, Fukuoka A, Tamaki S, Yamamoto Y, Tomoda K, Kimura H (2014) The impact of obstructive sleep apnea and nasal CPAP on circulating adiponectin levels. Lung. 192(2):289–295
Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N (2012) Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol 35(4):231–236
Steiropoulos P, Kotsianidis I, Nena E, Tsara V, Gounari E, Hatzizisi O, Kyriazis G, Christaki P, Froudarakis M, Bouros D (2009) Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep. 32(4):537–543
Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R (2008) Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. 134(4):686–692
Ming H, Tian A, Liu B, Hu Y, Liu C, Chen R et al (2019) Inflammatory cytokines tumor necrosis factor-alpha, interleukin-8 and sleep monitoring in patients with obstructive sleep apnea syndrome. Exp Ther Med 17(3):1766–1770
Unuvar Dogan F, Yosunkaya S, Kuzu Okur H, Can U (2014) Relationships between obstructive sleep apnea syndrome, continuous positive airway pressure treatment, and inflammatory cytokines. Sleep Disord 2014:1–6
Karamanli H, Ozol D, Ugur KS, Yildirim Z, Armutcu F, Bozkurt B et al (2014) Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath 18(2):251–256
Can M, Uygur F, Tanriverdi H, Acikgoz B, Alper B, Guven B (2016) Effect of continuous positive airway pressure (CPAP) therapy on IL-23 in patients with obstructive sleep apnea. Immunol Res 64(5–6):1179–1184
Wosiski-Kuhn M, Robinson M, Strupe J, Arounleut P, Martin M, Caress J, Cartwright M, Bowser R, Cudkowicz M, Langefeld C, Hawkins GA, Milligan C (2019) IL6 receptor(358)Ala variant and trans-signaling are disease modifiers in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 6(6):e631
Senecal V, Deblois G, Beauseigle D, Schneider R, Brandenburg J, Newcombe J et al (2016) Production of IL-27 in multiple sclerosis lesions by astrocytes and myeloid cells: modulation of local immune responses. Glia. 64(4):553–569
Lobentanzer S, Hanin G, Klein J, Soreq H (2019) Integrative transcriptomics reveals sexually dimorphic control of the cholinergic/neurokine Interface in schizophrenia and bipolar disorder. Cell Rep 29(3):764–777 e5
Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP (2004) Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem 279(19):19936–19947
Rosell DR, Nacher J, Akama KT, McEwen BS (2003) Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 122(2):329–348
Na KS, Jung HY, Kim YK (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 48:277–286
Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN (2002) Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem 83(4):754–763
Sriram K, Miller DB, O’Callaghan JP (2006) Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem 96(3):706–718
Morieri ML, Passaro A, Zuliani G (2017) Interleukin-6 “trans-signaling” and ischemic vascular disease: the important role of soluble gp130. Mediat Inflamm 2017:1396398
Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8(9):1254–1266
Rothaug M, Becker-Pauly C, Rose-John S (2016) The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta 1863(6 Pt A):1218–1227
Reuss R, Pohle S, Retzlaff K, Hemberger J, Oschmann P (2009) Interferon beta-1a induces tumor necrosis factor receptor 1 but decreases tumor necrosis factor receptor 2 leukocyte mRNA levels in relapsing-remitting multiple sclerosis. Neuroimmunomodulation. 16(3):171–176
Hofman FM, Hinton DR, Johnson K, Merrill JE (1989) Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med 170(2):607–612
Brambilla L, Guidotti G, Martorana F, Iyer AM, Aronica E, Valori CF, Rossi D (2016) Disruption of the astrocytic TNFR1-GDNF axis accelerates motor neuron degeneration and disease progression in amyotrophic lateral sclerosis. Hum Mol Genet 25(14):3080–3095
Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G (2011) Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest Ophthalmol Vis Sci 52(11):8442–8454
Scalzo P, Kummer A, Cardoso F, Teixeira AL (2009) Increased serum levels of soluble tumor necrosis factor-alpha receptor-1 in patients with Parkinson’s disease. J Neuroimmunol 216(1–2):122–125
Bobinska K, Galecka E, Szemraj J, Galecki P, Talarowska M (2017) Is there a link between TNF gene expression and cognitive deficits in depression? Acta Biochim Pol 64(1):65–73
Teixeira AL, de Sousa RT, Zanetti MV, Brunoni AR, Busatto GF, Zarate CA Jr et al (2015) Increased plasma levels of soluble TNF receptors 1 and 2 in bipolar depression and impact of lithium treatment. Hum Psychopharmacol 30(1):52–56
Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, Gedrich K, Kloiber S, Lucae S, Ising M, Uhr M, Holsboer F, Pollmächer T (2008) Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. 23(6):421–429
Orti-Casan N, Wu Y, Naude PJW, De Deyn PP, Zuhorn IS, Eisel ULM (2019) Targeting TNFR2 as a novel therapeutic strategy for Alzheimer’s disease. Front Neurosci 13:49
Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonca VA, Gattaz WF et al (2010) Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis 22(4):1305–1311
Cheng X, Yang L, He P, Li R, Shen Y (2010) Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer’s disease and non-demented patients. J Alzheimers Dis 19(2):621–630
Culpan D, Cornish A, Love S, Kehoe PG, Wilcock GK (2007) Protein and gene expression of tumour necrosis factor receptors I and II and their promoter gene polymorphisms in Alzheimer’s disease. Exp Gerontol 42(6):538–544
Hoseth EZ, Ueland T, Dieset I, Birnbaum R, Shin JH, Kleinman JE, Hyde TM, Mørch RH, Hope S, Lekva T, Abraityte AJ, Michelsen AE, Melle I, Westlye LT, Ueland T, Djurovic S, Aukrust P, Weinberger DR, Andreassen OA (2017) A study of TNF pathway activation in schizophrenia and bipolar disorder in plasma and brain tissue. Schizophr Bull 43(4):881–890
Turhan L, Batmaz S, Kocbiyik S, Soygur AH (2016) The role of tumour necrosis factor alpha and soluble tumour necrosis factor alpha receptors in the symptomatology of schizophrenia. Nord J Psychiatry 70(5):342–350
Noto C, Gadelha A, Belangero SI, Spindola LM, Rocha NP, de Miranda AS, Teixeira AL, Cardoso Smith MA, de Jesus Mari J, Bressan RA, Brietzke E (2013) Circulating levels of sTNFR1 as a marker of severe clinical course in schizophrenia. J Psychiatr Res 47(4):467–471
Coelho FM, Reis HJ, Nicolato R, Romano-Silva MA, Teixeira MM, Bauer ME, Teixeira AL (2008) Increased serum levels of inflammatory markers in chronic institutionalized patients with schizophrenia. Neuroimmunomodulation. 15(2):140–144
Dong Y, Dekens DW, De Deyn PP, Naudé PJW, Eisel ULM (2015) Targeting of tumor necrosis factor alpha receptors as a therapeutic strategy for neurodegenerative disorders. Antibodies 4:369–408
Waetzig GH, Rosenstiel P, Arlt A, Till A, Brautigam K, Schafer H et al (2005) Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-beta1. FASEB J 19(1):91–93
Neirynck N, Glorieux G, Schepers E, Verbeke F, Vanholder R (2015) Soluble tumor necrosis factor receptor 1 and 2 predict outcomes in advanced chronic kidney disease: a prospective cohort study. PLoS One 10(3):e0122073
Fischer R, Kontermann R, Maier O (2015) Targeting sTNF/TNFR1 signaling as a new therapeutic strategy. Antibodies. 4:48–70
Watanabe T, Kumano-Go T, Suganuma N, Shigedo Y, Motonishi M, Honda H, Kyotani K, Uruha S, Terashima K, Teshima Y, Takeda M, Sugita Y (2000) The relationship between esophageal pressure and apnea hypopnea index in obstructive sleep apnea-hypopnea syndrome. Sleep Res Online 3(4):169–172
Watanabe T, Mikami A, Shigedo Y, Motonishi M, Honda H, Kyotani K, Uruha S, Terashima K, Teshima Y, Sugita Y, Takeda M (2000) Esophageal pressure and apnea hypopnea index in sleep-disordered breathing. Psychiatry Clin Neurosci 54(3):338–339
Epstein LJ, Strollo PJ Jr, Donegan RB, Delmar J, Hendrix C, Westbrook PR (1995) Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep. 18(5):368–376
Barkin JS, Krieger B, Blinder M, Bosch-Blinder L, Goldberg RI, Phillips RS (1989) Oxygen desaturation and changes in breathing pattern in patients undergoing colonoscopy and gastroscopy. Gastrointest Endosc 35(6):526–530
Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V et al (2002) Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 105(21):2462–2464
Sakpal TV (2010) Sample size estimation in clinical trial. Perspect Clin Res 1(2):67–69
Guo J-H (2012) Optimal sample size planning for the Wilcoxon–Mann–Whitney and van Elteren tests under cost constraints. J Appl Stat 39(10):2153–2164
Happ M, Bathke AC, Brunner E (2019) Optimal sample size planning for the Wilcoxon-Mann-Whitney test. Stat Med 38(3):363–375
Gray G (2018) Adaptive designs for medical device clinical studies: guidance for Industry and Food and Drug Administration Staff.1-49
van der Maaten L, Hinton G (2008) Visualizing data using t-SNE. JMLR 9:2579–2605
Krijthe J. Rtsne: T-Distributed Stochastic Neighbor Embedding Using a Barnes-Hut Implementation. Available from: https://github.com/jkrijthe/Rtsne
Lopez Gonzalez I, Garcia-Esparcia P, Llorens F, Ferrer I (2016) Genetic and Transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int J Mol Sci 17(2):206
Hampel H, Haslinger A, Scheloske M, Padberg F, Fischer P, Unger J, Teipel SJ, Neumann M, Rosenberg C, Oshida R, Hulette C, Pongratz D, Ewers M, Kretzschmar HA, Möller HJ (2005) Pattern of interleukin-6 receptor complex immunoreactivity between cortical regions of rapid autopsy normal and Alzheimer’s disease brain. Eur Arch Psychiatry Clin Neurosci 255(4):269–278
Marz P, Heese K, Hock C, Golombowski S, Muller-Spahn F, Rose-John S et al (1997) Interleukin-6 (IL-6) and soluble forms of IL-6 receptors are not altered in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett 239(1):29–32
Sundaresh A, Oliveira J, Chinnadurai RK, Rajkumar RP, Hani L, Krishnamoorthy R, Leboyer M, Negi VS, Tamouza R (2019) IL6/IL6R genetic diversity and plasma IL6 levels in bipolar disorder: an indo-French study. Heliyon. 5(1):e01124
Maldonado-Montoro M, Canadas-Garre M, Gonzalez-Utrilla A, Angel C-HM (2018) Influence of IL6R gene polymorphisms in the effectiveness to treatment with tocilizumab in rheumatoid arthritis. Pharmacogenomics J 18(1):167–172
Khandaker GM, Zammit S, Burgess S, Lewis G, Jones PB (2018) Association between a functional interleukin 6 receptor genetic variant and risk of depression and psychosis in a population-based birth cohort. Brain Behav Immun 69:264–272
Draganov M, Arranz MJ, Salazar J, de Diego-Adelino J, Gallego-Fabrega C, Jubero M et al (2019) Association study of polymorphisms within inflammatory genes and methylation status in treatment response in major depression. Eur Psychiatry 60:7–13
Tamura Y, Phan C, Tu L, Le Hiress M, Thuillet R, Jutant EM et al (2018) Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J Clin Invest 128(5):1956–1970
Lam SY, Liu Y, Ng KM, Lau CF, Liong EC, Tipoe GL, Fung ML (2012) Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem Cell Biol 137(3):303–317
Prenissl N, Lokau J, Rose-John S, Haybaeck J, Garbers C (2019) Therapeutic blockade of the interleukin-6 receptor (IL-6R) allows sIL-6R generation by proteolytic cleavage. Cytokine. 114:1–5
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295
Khandaker GM, Oltean BP, Kaser M, Dibben CRM, Ramana R, Jadon DR, Dantzer R, Coles AJ, Lewis G, Jones PB (2018) Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJ Open 8(9):e025333
Girgis RR, Ciarleglio A, Choo T, Haynes G, Bathon JM, Cremers S, Kantrowitz JT, Lieberman JA, Brown AS (2018) A randomized, double-blind, placebo-controlled clinical trial of Tocilizumab, an Interleukin-6 receptor antibody, for residual symptoms in schizophrenia. Neuropsychopharmacology. 43(6):1317–1323
Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJF, Schieffer B, Grote K (2012) Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol 32(2):281–290
Maes M, Anderson G, Kubera M, Berk M (2014) Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin Ther Targets 18(5):495–512
Rossi JF, Lu ZY, Jourdan M, Klein B (2015) Interleukin-6 as a therapeutic target. Clin Cancer Res 21(6):1248–1257
Turner MD, Chaudhry A, Nedjai B (2012) Tumour necrosis factor receptor trafficking dysfunction opens the TRAPS door to pro-inflammatory cytokine secretion. Biosci Rep 32(2):105–112
Faustman DL, Davis M (2013) TNF receptor 2 and disease: autoimmunity and regenerative medicine. Front Immunol 4:478
Dyugovskaya L, Polyakov A, Ginsberg D, Lavie P, Lavie L (2011) Molecular pathways of spontaneous and TNF-{alpha}-mediated neutrophil apoptosis under intermittent hypoxia. Am J Respir Cell Mol Biol 45(1):154–162
Hanikoglu F, Huseyinoglu N, Ozben S, Cort A, Ozdem S, Ozben T (2015) Increased plasma soluble tumor necrosis factor receptor-1 and myeloperoxidase activity in patients with obstructive sleep apnea syndrome. Int J Neurosci 125(9):655–662
Gaines J, Vgontzas AN, Fernandez-Mendoza J, Kritikou I, Basta M, Bixler EO (2015) Gender differences in the association of sleep apnea and inflammation. Brain Behav Immun 47:211–217
Kritikou I, Basta M, Vgontzas AN, Pejovic S, Liao D, Tsaoussoglou M, Bixler EO, Stefanakis Z, Chrousos GP (2014) Sleep apnoea, sleepiness, inflammation and insulin resistance in middle-aged males and females. Eur Respir J 43(1):145–155
Arias MA, Garcia-Rio F, Alonso-Fernandez A, Hernanz A, Hidalgo R, Martinez-Mateo V, Bartolome S, Rodriguez-Padial L (2008) CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J 32(4):1009–1015
Kunz AB, Kraus J, Young P, Reuss R, Wipfler P, Oschmann P, Blaes F, Dziewas R (2012) Biomarkers of inflammation and endothelial dysfunction in stroke with and without sleep apnea. Cerebrovasc Dis 33(5):453–460
Yang S, Wang J, Brand DD, Zheng SG (2018) Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol 9:784
Ding YH, Mrizek M, Lai Q, Wu Y, Reyes R Jr, Li J et al (2006) Exercise preconditioning reduces brain damage and inhibits TNF-alpha receptor expression after hypoxia/reoxygenation: an in vivo and in vitro study. Curr Neurovasc Res 3(4):263–271
Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE (2001) Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 45(1):27–36
Moncunill G, Campo JJ, Dobano C (2014) Quantification of multiple cytokines and chemokines using cytometric bead arrays. Methods Mol Biol 1172:65–86
Richter F, Liebig T, Guenzi E, Herrmann A, Scheurich P, Pfizenmaier K, Kontermann RE (2013) Antagonistic TNF receptor one-specific antibody (ATROSAB): receptor binding and in vitro bioactivity. PLoS One 8(8):e72156
Dong Y, Fischer R, Naude PJ, Maier O, Nyakas C, Duffey M et al (2016) Essential protective role of tumor necrosis factor receptor 2 in neurodegeneration. Proc Natl Acad Sci U S A 113(43):12304–12309
Williams SK, Fairless R, Maier O, Liermann PC, Pichi K, Fischer R, Eisel ULM, Kontermann R, Herrmann A, Weksler B, Romero N, Couraud PO, Pfizenmaier K, Diem R (2018) Anti-TNFR1 targeting in humanized mice ameliorates disease in a model of multiple sclerosis. Sci Rep 8(1):13628
Nubel T, Schmitt S, Kaina B, Fritz G (2005) Lovastatin stimulates p75 TNF receptor (TNFR2) expression in primary human endothelial cells. Int J Mol Med 16(6):1139–1145
Dolga AM, Granic I, Nijholt IM, Nyakas C, van der Zee EA, Luiten PG et al (2009) Pretreatment with lovastatin prevents N-methyl-D-aspartate-induced neurodegeneration in the magnocellular nucleus basalis and behavioral dysfunction. J Alzheimers Dis 17(2):327–336
Acknowledgments
The authors would like to thank David Hall of the University of Georgia and Nick Pervolarakis of UC Irvine for their help with the statistical data and to Julie Nelson for her help running the Bio-Plex Instrument at UGA’s Cytometry Shared Resource Laboratory.
Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This project, RBM, SA, and BGP, were financially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and the University of Georgia’s Clinical and Translational Research Unit. YW and PM were supported by U.S. NIH grants R01 GM113242 and R01 GM122080 and U.S. National Science Foundation grants DMS-1903226 and DMS-1925066.
Author information
Authors and Affiliations
Contributions
YW managed the data sets in Excel and R and prepared all the figures in R in collaboration with RBM. RBM focused the project on neuroinflammatory cytokines, managed the technical aspects of the project and wrote the manuscript with generous input from the team. SA prepared all the serum samples and ran the Bio-Plex assays. PM directed YW in the statistical analysis of the data. BGP conceived of and initiated the study of OSA and airway-treated OSA patients, defined the patient recruitment criteria, and recruited and consented all the patients.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Yes.
Consent for publication
No consent for publication is needed.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 179 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Meagher, R.B., Ambati, S. et al. Patients with obstructive sleep apnea have suppressed levels of soluble cytokine receptors involved in neurodegenerative disease, but normal levels with airways therapy. Sleep Breath 25, 1641–1653 (2021). https://doi.org/10.1007/s11325-020-02205-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-020-02205-y