Abstract
A growing number of studies have demonstrated the importance of ATPe-signalling via P2 receptors as an important component of the inflammatory response to infection. More recent studies have shown that ATPe can also have a direct effect on infection by intracellular pathogens, by modulating membrane trafficking in cells that contain vacuoles that harbour intracellular pathogens, such as mycobacteria and chlamydiae. A conserved mechanism appears to be involved in controlling infection by both of these pathogens, as a role for phospholipase D in inducing fusion between lysosomes and the vacuoles has been demonstrated. Other P2-dependent mechanisms are most likely operative in the cases of pathogens, such as Leishmania, which survive in an acidic phagolysosomal-like compartment. ATPe may function as a ‘danger signal–that alerts the immune system to the presence of intracellular pathogens that damage the host cell, while different intracellular pathogens have evolved enzymes or other mechanisms to inhibit ATPe-mediated signalling, which should, thus, be viewed as virulence factors for these pathogens.
Similar content being viewed by others
Introduction
Intracellular pathogens invade, survive and replicate in mammalian cells, modulating host-cell membrane trafficking and cytoskeletal dynamics in order to establish persistent infection in the mammalian host [1–3]. Macrophages are a frequent target of microbial infections, and they respond to microbial invasion by producing factors such as reactive nitrogen and oxygen intermediates (RNIs and ROIs) that have strong microbicidal activity [4]. But many pathogens have evolved different strategies for avoiding destruction by the macrophage. Some intracellular pathogens, such as Mycobacteria, inhabit a compartment whose endocytic maturation is delayed, while Chlamydiae survive within a membrane-bound vacuole that avoids fusion with lysosomes and maintains a neutral pH [5, 6]. Unlike Mycobacteria and Chlamydia, the protozoan parasite Leishmania thrives in a parasitophorous vacuole that has an acidic pH and high hydrolytic activity, and Trypanosoma cruzi actively induces its uptake into lysosome-like host-cell vacuoles, from which, the parasite rapidly escapes into the cytosol [7–10].
At the end of their infection cycle, each of these intracellular parasites is released from the host cell, triggering macrophage death and inducing local inflammation, accompanied by the possible release of ATP, as shown for macrophages infected with Mycobacterium tuberculosis [11]. As extracellular ATP (ATPe) can be used by neighbouring macrophages as ammunition to inhibit infection (described below), many intracellular pathogens such as Mycobacterium bovis BCG, Vibrio cholerae, M. tuberculosis, T. cruzi and Leishmania also secrete or express on their outer surface enzymes that degrade or synthesise nucleotides [12–15]; and microbial enzymes that consume or produce ATP are considered as virulence factors for M. tuberculosis, Leishmania amazonensis and T. cruzi [14, 16–18].
Macrophages activate microbicidal pathways and contribute to inflammation after the ligation of purinergic P2 receptors by ATPe. This review will, therefore, describe some of the P2-dependent mechanisms used by macrophages to eliminate infection by intracellular bacteria and protozoan parasites, and, whenever possible, will correlate these findings with host susceptibility and resistance to infection.
Effect of ATPe on macrophage infection by M. tuberculosis
The first evidence for an involvement of ATPe in the control of intracellular infections came from the laboratory of Kaplan, who demonstrated in 1994, that ATPe-mediated apoptosis in Mycobacterium tuberculosis-infected macrophages is associated with the inhibition of mycobacterial infection [19]. This early study also showed that ATPe-induced macrophage apoptosis, but not H2O2-induced necrosis, is associated with the killing of the intracellular mycobacteria. These findings were confirmed by Lammas et al., who proposed that the ATPe-induced elimination of BCG-infected human macrophages are mediated by the purinergic receptor, P2X7, through a mechanism independent of both RNIs and ROIs [20]. In comparison with other ligands that can trigger the lysis of macrophages, including complement-mediated cytolysis, Fas ligation and CD69 activation, only ATPe treatment could stimulate the death of both host macrophages and intracellular mycobacteria. Subsequently, several laboratories confirmed that the P2X7 receptor plays a role in limiting infection in murine and bovine macrophages infected with mycobacteria, and in human macrophages infected with BCG bacillus and virulent strains of M. tuberculosis [11, 21–23].
In addition to the P2X7 receptor, other P2 receptor subtypes, possibly P2Y, are apparently involved in ATPe-mediated bactericidal activity in macrophages [11, 21, 23]. Experiments using macrophages derived from P2X7-deficient mice revealed that ATPe stimulates the production of reactive species such as RNIs equally well in both wildtype and P2X7-deficient macrophages [11]. Moreover, it was found that ATPe induces bactericidal effects in the macrophages better than BzATP (the most potent known agonist for the P2X7 receptor), suggesting that P2X7 receptors are necessary, but not sufficient, for maximal ATPe-dependent killing of intracellular M. tuberculosis by human and bovine macrophages [21, 22]. Lammas et al. have further observed that the ATPe activity is potentiated by extracellular Zn2+ [20]. This effect was initially ascribed to the P2X7 receptor, but now, P2X7 activity is known to be blocked by extracellular Zn2+, while the activity of another purinergic receptor, P2X4, is potentiated by Zn2+ [24]. Since macrophages express functional P2X4 receptors [25] and inflammatory mediators can upregulate this receptor on macrophages [26], it is likely that both P2X7 and P2X4 are involved in the ATPe-induced killing of M. tuberculosis in macrophages.
More recent reports have also confirmed the predominant role of the P2X7 receptor in mycobacterial clearance, extending these results to show that loss-of-function polymorphisms in human P2X7 receptors lead not only to reduced ATPe-induced apoptosis, but also to impaired ATPe-induced killing of intracellular mycobacteria (BCG) by macrophages [27–29]. Nonetheless, more experiments will be required to elucidate the role that P2X7, possibly in conjunction with other P2 receptors, may play in the killing of intracellular mycobacteria in vivo, since P2X7-deficient mice control lung infection as well as wildtype mice after low-dose aerosol infection with virulent M. tuberculosis [30].
Cellular mechanisms of ATPe-induced mycobacterial killing
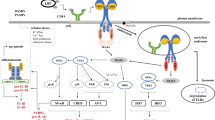
ATPe ligation of P2X7 on macrophages results in a variety of different cellular effects, including the activation of phospholipase D (PLD), maturation and release of interleukin-1β (IL-1β), generation of macrophage polykarions, modulation of lipopolysaccharide (LPS) induced macrophage activation through modulation of iNOS expression and NO production, and the induction of macrophage death by necrosis and/or apoptosis [31–40]. The original findings from the Kaplan laboratory suggested that the apoptosis of macrophages is necessary for ATPe-mediated killing of intracellular bacteria [19], but it was later established that the ATPe-induced killing of mycobacteria in human and mice macrophages can occur without macrophage death, through a pathway requiring PLD activation, the acidification of phagosomes and phagosome–lysosome fusion [21] (Fig. 1) [23, 41]. A more recent study showed that, when combined, two loss-of-function polymorphisms in human P2X7 receptors impair ATPe-mediated apoptosis, despite the normal killing of BCG bacillus [28], reinforcing the view that the apoptosis of macrophages is not necessary for the elimination of mycobacteria.
ATP released from infected cells undergoing necrosis or sites of inflammation can bind to the P2X7 receptor on neighbouring macrophages and other cells. Ligation of the P2X7 receptor initiates signalling through several pathways, which result in the activation of caspase-1, activation of phospholipase D (PLD) and, ultimately, cell death. Caspase-1 activation stimulates the inflammatory response by the cleavage of pro-IL-1β and secretion of the mature cytokine. PLD activation modifies membrane trafficking in the cell, which can induce fusion between lysosomes and vacuoles, harbouring intracellular pathogens such as mycobacteria and chlamydiae. The death of macrophages is partly necrotic, which may amplify inflammation even further, through the ligation of P2X7 receptors on other cells. Some extracellular ATP may also bind to other P2 receptors, which may inhibit infection by activating as-yet-uncharacterised pathways
In fact, it was recently shown [42] that cyclosporine A, an inhibitor of the mitochondrial permeability transition, increases the survival of human monocyte-derived macrophages infected with M. tuberculosis, restores P2X7 function and enhances antimycobacterial activity. Conversely, M. tuberculosis has developed mechanisms to evade P2X7-triggered mechanisms, since it can secrete a nucleoside diphosphate kinase that produces ATP and kills macrophages through a P2X7-dependent mechanism [14]. New experiments are needed to clarify the role played by ATPe in killing intracellular pathogens and inducing host-cell death.
Inhibition of chlamydial infection in macrophages
The Chlamydia species are obligate intracellular bacteria that infect mainly epithelial mucosa, where they survive within intracellular vacuoles that avoid fusion with host-cell lysosomes [6, 43]. Different strains of C. trachomatis are responsible for the infection of genital and ocular tissue in humans [44–48]. C. pneumoniae is a common cause of community-acquired pneumonia in humans and is associated with an increased risk for atherosclerosis [44, 49]. Both C. trachomatis and C. pneumoniae can invade epithelial cells and macrophages in vitro and in vivo [49, 50].
It was recently shown that ATPe inhibits the infection of macrophages by the murine species of C. trachomatis, C. muridarum, through a mechanism that required PLD activation and fusion between lysosomes and the Chlamydia vacuoles [51]. The effect of ATPe was dependent on the presence of the P2X7 receptor, since there was no PLD activation nor killing of chlamydiae in infected macrophages that had been isolated from P2X7-deficient mice. Although P2X7 ligation also led to macrophage death, the inhibition of PLD prevented chlamydial killing but had no effect on macrophage death, suggesting that PLD activation was directly responsible for the inhibition of chlamydial infection (Table 1). Moreover, fusion between lysosomes and chlamydial vacuoles preceded macrophage death, further strengthening the conclusion that the killing of chlamydiae is independent of host-cell death [51].
Conversely, as the activation of P2X7-dependent pathways is deleterious for Chlamydia, both directly and through the demise of the macrophage, the intracellular pathogen has also evolved a mechanism for protecting its host cell. Thus, the infection of macrophages with a related species, C. psittaci (also known as C. caviae), inhibits partially ATPe-mediated macrophage death [52]. While the molecular basis for host-cell protection remains to be investigated, chlamydial infection decreases partially the ability of ATPe to induce plasma–membrane permeabilisation and calcium fluxes [52]. Chlamydiae, therefore, resemble other pathogenic bacteria and protozoan parasites that attempt to protect themselves and the host cell by degrading nucleotides or hydrolysing ATP.
Effects of ATPe on leishmaniasis
Leishmaniasis is used to describe several diseases caused by the obligate intracellular protozoan parasite Leishmania, which infects mainly macrophages [53]. The diseases range from self-healing cutaneous lesions to visceral and potentially fatal disseminating infection. Leishmania infections are found in 80 countries, with a prevalence of 12 million human cases. The development of different clinical forms is associated with both the immunological status of the host and the parasite species [53]. The expression of the P2X7 receptor has recently been examined during Leishmania infection, revealing upregulation of the receptor during both in vivo and in vitro infection with L. amazonensis [54]. These changes were correlated with functional responses, as reflected by an increase in ATPe-mediated plasma–membrane permeabilisation and host-cell apoptosis ([54] and Chaves et al. (manuscript in preparation)) (Table 1). The increase in ATPe-induced membrane permeabilisation was also observed in spleen macrophages isolated from mice infected with L. donovani, suggesting that this may be a general phenomenon relevant for all Leishmania infections.
It has been proposed that intracellular infection by Leishmania donovani inhibits macrophage apoptosis induced by growth factor deprivation [55]. In contrast, there is an increase in the ATPe-mediated apoptosis of macrophages infected with Leishmania (our unpublished data), consistent with the increases in ATPe-mediated membrane permeabilisation. Thus, despite the production of ecto-ATPases by Leishmania [16], this strategy is not sufficient to protect the host cell against the infection-dependent upregulation in P2X7 expression.
We have observed that the presence of ATPe during L. amazonensis infection does not interfere with Leishmania invasion, but the ATPe treatment of macrophages that are already infected with L. amazonensi leads to a decrease in Leishmania survival (our unpublished observations). In addition, we observed that ATPe has no effect on the viability of extracellular Leishmania promastigotes. In fact, some nucleotides, such as UTP, stimulate the proliferation of promastigotes. It is also worthwhile noting that the more infective forms of L. amazonensis express more magnesium-dependent ecto-ATPase on their membranes than less virulent Leishmania, leading to the proposal that the ecto-ATPase should be viewed as a virulence factor of the parasite [16].
The cellular pathway allowing ATPe to decrease Leishmania infection remains to be determined, but must be different from the PLD activation observed during Chlamydia and Mycobacterium infection [41, 51], since both Chlamydia and Mycobacterium inhibit phagolysosome formation and acidification [4, 6, 8], while Leishmania survives well in acidic phagolysosomes [56]. Interestingly, Leishmania lipophosphoglycans, which promote parasite survival, act by perturbing MAPKinase signalling in macrophages to inhibit macrophage IL-1β [57]. This might be relevant for the involvement of P2 receptors in the escape mechanisms used by Leishmania, since several P2 receptors are connected to MAPKinase pathways [58].
The involvement of ATPe in Chagas–disease
Chagas–disease is caused by the facultative intracellular protozoan pathogen, T. cruzi. The disease is a chronic inflammatory condition characterised by cardiomyopathy and digestive disorders [59, 60]. T. cruzi infection affects over 17 million people in endemic areas of Latin America, leading to 45 thousand deaths per year [61]. The involvement of ATPe signalling was recently examined during the acute phase of T. cruzi infection. Thymus atrophy occurs during the acute phase of infection but the thymus recovers weight and cellularity during the chronic phase [62]. These alterations do not appear to be associated with stress or glucocorticoid release [63]. It has recently been observed that thymocytes are sensitive to ATPe-induced membrane permeabilisation and host-cell death only during the atrophy phase of infection, with CD4+/CD8+ double-positive thymocytes being the most sensitive subpopulation of thymocytes [64] (Table 1). Since the phenomenon of ATP-induced permeabilisation can be blocked by the P2X7 inhibitors, oxidised ATP and Mg2+, and the P2X7 agonist, BzATP, was more potent than ATP, we proposed that the increased sensitivity to ATPe may be responsible, at least in part, for the thymocyte clearance and thymic atrophy observed during the acute phase of Chagas’s disease [64].
It is, therefore, reasonable to suppose that ATPe plays an important role in the T. cruzi infection cycle. In this context, it is worth noting that the ecto-ATPases produced by these parasites have been associated with strain virulence [17].
P2X7 receptors and inflammation
The stimulation of P2X7 by ATPe leads to caspase-1 activation, cleavage of pro-IL-1β and the secretion of mature IL-1β [66] (Fig. 1). The P2X7 receptor is also upregulated in macrophages by inflammatory cytokines, such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), and lipopolysaccharide (LPS). LPS, a surface component of most Gram-negative bacteria, and IFN-γ act synergistically to upregulate P2X7 receptor function and P2X7 mRNA in the human monocyte cell line, THP-1, and human macrophages [34, 37]. In fact, P2X7 receptors contain several motifs that are homologous to motifs from other receptors known to be involved in protein–protein interactions and LPS binding [67]. The upregulation of P2X7 expression in monocytes by TNF-α, LPS and IFN-γ is consistent with the ability of these cytokines to act as inflammatory mediators, and these effects are markedly attenuated by coincubation with prostaglandin E2 (PGE2) or the membrane-permeable cAMP analogue, dibutyril cAMP [68]. It is tempting to speculate that the temporal sequence of macrophage exposure to pro-inflammatory activators and anti-inflammatory stimuli (such as PGE2) might regulate not only receptor expression, but also downstream signalling by the P2X7 receptors.
It is known that macrophages express adenosine receptors, which are expressed during the differentiation of monocytes to macrophages and may influence phagocytosis [69]. Moreover, the treatment of macrophages with IFN-γ upregulates expression of the adenosine receptor, A2B, and the activation of A2B receptors is involved with the deactivation of macrophages, possibly through an increase of cAMP [70]. Therefore, the extracellular nucleotides may be involved with activation and a feedback mechanism for macrophage deactivation, depending on the timing and type of nucleotide released during infection. In this context, \studies with P2X7-deficient mice have reinforced the view that P2X7 receptors are involved in inflammation. Thus, disruption of the P2X7 receptor gene is associated with less severe disease in an arthritis model [71] and studies of chronic inflammation and neuropathic pain [72]. These results led to the hypothesis that the P2X7 receptor, through the regulation of IL-1β production and secretion, plays a common, early role in the development of pain of neuropathic and inflammatory origin [71, 72]. Additionally, a recent study demonstrates that the inhibition of the P2X7 receptor attenuates fever and cytokine responses induced by LPS in rats [73]. Finally, the P2X7 receptor is present and its expression is modulated by inflammation in sites of chronic inflammation [74].
All of these findings reinforce the conclusion that P2X7-dependent signalling plays a significant role in host responses during various types of inflammatory disease. However, the involvement of P2X7 during disease is complicated by the presence of other P2 receptors, which may also contribute to either pro- or anti-inflammatory immune responses. Moreover, ATP can negatively regulate Toll-like receptor signalling, suppress LPS-induced MCP-1 and TNF-α, and augments IL-10 production in human monocytes [75]. Many infectious agents that survive in these immune effector cells may have evolved complex nucleotide-based strategies to evade the immune system. Dissection of these strategies may lead us to a better understanding of the role of nucleotide signalling in the immune response and to the development of new approaches to combat infectious diseases.
Concluding remarks
As large concentrations of ATP are present outside of the cell only when the cell is damaged or is part of an inflamed tissue (Fig. 1), it has been proposed that ATPe may function as a generic ‘danger signal,–which could alert the immune system to the presence of any type of intracellular pathogen that induces host-cell death [76–78]. Different intracellular pathogens, such as bacteria and protozoan parasites, express ecto-ATPases or other mechanisms to either inhibit or enhance ATPe-mediated death of their host cell, suggesting that ATPe may have been used by the host as an ancient danger signal, to which, intracellular pathogens have been exposed since the early evolution of the immune system. In this context, the ability of some intracellular pathogens to inhibit the ATPe-dependent response mediated by P2 receptors could be an example of adaptation of the pathogenic invaders to the immune response, and may help to explain why the most virulent pathogens express high ecto-ATPase levels on their surface [14–17]. Given the availability of animals that are deficient in P2X7 and other P2 receptors, further research in the future will, thus, need to address the relevance of P2-dependent immune mechanisms in controlling infections in whole organisms.
Abbreviations
- ATPe :
-
Extracellular ATP
- IL-1β:
-
Interleukin-1β
- IFN-γ:
-
Interferon-γ
- LPS:
-
Lipopolysaccharide
- PGE2 :
-
Prostaglandin E2
- PLD:
-
Phospholipase D
- ROI:
-
Reactive oxygen intermediates
- RNI:
-
Reactive nitrogen intermediates
- TNF-α:
-
Tumour necrosis factor-α
References
Knodler LA, Celli J, Finlay BB (2001) Pathogenic trickery: deception of host cell processes. Nat Rev Mol Cell Biol 2(8):578–88
Finlay BB, Falkow S (1989) Common themes in microbial pathogenicity. Microbiol Mol Biol Rev 53(2):210–30
Cossart P, Sansonetti PJ (2004) Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304(5668):242–48
Stafford JL, Neumann NF, Belosevic M (2002) Macrophage-mediated innate host defense against protozoan parasites. Crit Rev Microbiol 28(3):187–48
Scott CC, Botelho RJ, Grinstein S (2003) Phagosome maturation: a few bugs in the system. J Membr Biol 193(3):137–52
Wyrick PB (2000) Intracellular survival by Chlamydia. Cell Microbiol 2(4):275–82
Sacks D, Sher A (2002) Evasion of innate immunity by parasitic protozoa. Nat Immunol 3(11):1041–047
Denkers EY, Butcher BA (2005) Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol 21(1):35–1
Andrews NW (2002) Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol 158(3):389–94
Burleigh BA, Woolsey AM (2002) Cell signalling and Trypanosoma cruzi invasion. Cell Microbiol 4(11):701–11
Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom BR (1999) Purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol 163(2):558–61
Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM (2000) Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun 68(9):4930–937
Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM (1999) Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol 31(5):1333–343
Chopra P, Singh A, Koul A, Ramachandran S, Drlica K, Tyagi AK, Singh Y (2003) Cytotoxic activity of nucleoside diphosphate kinase secreted from Mycobacterium tuberculosis. Eur J Biochem 270(4):625–34
Bisaggio DF, Peres-Sampaio CE, Meyer-Fernandes JR, Souto-Padron T (2003) Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite-host cell interaction. Parasitol Res 91(4):273–82
Berredo-Pinho M, Peres-Sampaio CE, Chrispim PP, Belmont-Firpo R, Lemos AP, Martiny A, Vannier-Santos MA, Meyer-Fernandes JR (2001) A Mg-dependent ecto-ATPase in Leishmania amazonensis and its possible role in adenosine acquisition and virulence. Arch Biochem Biophys 391(1):16–4
Meyer-Fernandes JR, Saad-Nehme J, Peres-Sampaio CE, Belmont-Firpo R, Bisaggio DF, Do Couto LC, Fonseca De Souza AL, Lopes AH, Souto-Padron T (2004) A Mg-dependent ecto-ATPase is increased in the infective stages of Trypanosoma cruzi. Parasitol Res 93(1):41–0
Gounaris K, Selkirk ME (2005) Parasite nucleotide-metabolizing enzymes and host purinergic signalling. Trends Parasitol 21(1):17–1
Molloy A, Laochumroonvorapong P, Kaplan G (1994) Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med 180(4):1499–509
Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS (1997) ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7(3):433–44
Kusner DJ, Adams J (2000) ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol 164(1):379–88
Smith RA, Alvarez AJ, Estes DM (2001) The P2X7 purinergic receptor on bovine macrophages mediates mycobacterial death. Vet Immunol Immunopathol 78(3–):249–62
Stober CB, Lammas DA, Li CM, Kumararatne DS, Lightman SL, McArdle CA (2001) ATP-mediated killing of Mycobacterium bovis Bacille Calmette-Guerin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol 166(10):6276–286
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82(4):1013–067
Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Bisaggio RC, Mendes AN, Marks J, Burnstock G, Dunn PM (2005) Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol 69(4):641–55
Yeung D, Kharidia R, Brown SC, Gorecki DC (2004) Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol Dis 15(2):212–20
Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS (2003) A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol 171(10):5442–446
Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS (2005) A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 281(4):2079–086
Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ (2005) Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis 192(1):149–55
Myers AJ, Eilertson B, Fulton SA, Flynn JL, Canaday DH (2005) The purinergic P2X7 receptor is not required for control of pulmonary Mycobacterium tuberculosis infection. Infect Immun 73(5):3192–195
el-Moatassim C, Dubyak GR (1992) A novel pathway for the activation of phospholipase-D by P(2z) purinergic receptors in BAC1.2F5 macrophages. J Biol Chem 267(33):23664–3673
Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159(3):1451–458
Perregaux DG, Gabel CA (1998) Human monocyte stimulus-coupled IL-1beta posttranslational processing: modulation via monovalent cations. Am J Physiol 275(6 Pt 1):C1538–C1547
Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di Virgilio F (1995) The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest 95(3):1207–216
Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN, Collo G, Di Virgilio F (1997) Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol 138(3):697–06
Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ (1998) Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem 273(42):27170–7175
Humphreys BD, Dubyak GR (1996) Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol 157(12):5627–637
Humphreys BD, Rice J, Kertesy SB, Dubyak GR (2000) Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem 275(35):26792–6798
Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D (2005) P2X7 receptor: death or life? Purinergic Signalling 1(3):219–27
Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR (1998) Cytolytic P2X purinoceptors. Cell Death Differ 5(3):191–99
Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA (2001) ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome–lysosome fusion. J Immunol 167(6):3300–307
Gan H, He X, Duan L, Mirabile-Levens E, Kornfeld H, Remold HG (2005) Enhancement of antimycobacterial activity of macrophages by stabilization of inner mitochondrial membrane potential. J Infect Dis 191(8):1292–300
Bavoil PM (1994) Determinants of chlamydial pathogenesis in immunity. In: Miller VL, Kaper JB, Portnoy DA, Isberg RR (eds) Molecular genetics of bacterial pathogenesis. ASM Press, Washington, DC, pp 295–08
Schachter J (1999) Infection and disease epidemiology. In: Stephens RS (ed) Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, DC, pp 139–69
Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY (1995) Global data on blindness. Bull World Health Organ 73(1):115–21
Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, Cohen MS, Harris KM, Udry JR (2004) Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291(18):2229–236
Gerbase AC, Rowley JT, Mertens TE (1998) Global epidemiology of sexually transmitted diseases. Lancet 351(Suppl 3):2–
Belland R, Ojcius DM, Byrne GI (2004) Chlamydia. Nat Rev Microbiol 2(7):530–31
Campbell LA, Kuo CC (2004) Chlamydia pneumoniae an infectious risk factor for atherosclerosis? Nat Rev Microbiol 2(1):23–2
La Verda D, Byrne GI (1994) Interactions between macrophages and chlamydiae. Immunol Ser 60:381–99
Coutinho-Silva R, Stahl L, Raymond M-N, Jungas T, Verbeke P, Burnstock G, Darville T, Ojcius DM (2003) Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity 19(3):403–12
Coutinho-Silva R, Perfettini J-L, Persechini PM, Dautry-Varsat A, Ojcius DM (2001) Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol 280(1):C81–C89
Awasthi A, Mathur RK, Saha B (2004) Immune response to Leishmania infection. Indian J Med Res 119(6):238–58
Torres-Santos EC, Mantuano MB, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B (2000) Enhanced macrophage permeabilization by ATP upon leishmania infection. Drug Dev Res 50(1):61
Moore KJ, Matlashewski G (1994) Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol 152(6):2930–937
Stafford JL, Neumann NF, Belosevic M (2002) Macrophage-mediated innate host defense against protozoan parasites. Crit Rev Microbiol 28(3):187–48
Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY (1999) Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol 163(12):6403–412
da Cruz CM, Ventura AL, Schachter J, Costa-Junior HM, Silva Souza HA, Gomes FR, Coutinho-Silva R, Ojcius DM, Persechini PM (2006) Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X7-associated pore or channel formation. Br J Pharmacol 147(3):324–34
Chimelli L, Scaravilli F (1997) Trypanosomiasis. Brain Pathol 7(1):599–11
Engman DM, Leon JS (2002) Pathogenesis of Chagas heart disease: role of autoimmunity. Acta Trop 81(2):123–32
Schmunis GA (1991) Trypanosoma cruzi, the etiologic agent of Chagas–disease: status in the blood supply in endemic and nonendemic countries. Transfusion 31(6):547–57
Leite-de-Moraes MC, Hontebeyrie-Joskowicz M, Dardenne M, Savino W (1992) Modulation of thymocyte subsets during acute and chronic phases of experimental Trypanosoma cruzi infection. Immunology 77(1):95–8
Leite de Moraes MC, Hontebeyrie-Joskowicz M, Leboulenger F, Savino W, Dardenne M, Lepault F (1991) Studies on the thymus in Chagas–disease. II. Thymocyte subset fluctuations in Trypanosoma cruzi-infected mice: relationship to stress. Scand J Immunol 33(3):267–75
Mantuano MB, Henriques-Pons A, Araujo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM (2003) Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes Infect 5(15):1363–371
Mantuano MB, Henriques-Pons A, Persechini PM, Araujo-Jorge TC, Coutinho CMLM, Coutinho-Silva R (2000) P2Z/P2X7 down-regulation: would it be evasion mechanism triggered by Trypanosoma cruzi. Drug Dev Res 50(1):61
MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15(5):825–35
Denlinger LC, Fisette PL, Sommer JA, Watters JJ, Prabhu U, Dubyak GR, Proctor RA, Bertics PJ (2001) Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol 167(4):1871–876
Humphreys BD, Dubyak GR (1998) Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukocyte Biol 64(2):265–73
Eppell BA, Newell AM, Brown EJ (1989) Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J Immunol 143(12):4141–145
Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, Celada A (1999) IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol 162(6):3607–614
Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA (2002) Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168(12):6436–445
Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P,Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB,Casula MA, Yiangou Y, Birch R, Anand P, Buell GN (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114(3):386–96
Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, Gourine VN (2005) P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol 146(1):139–45
Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ (2004) Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res 317(3):289–98
Kaufmann A, Musset B, Limberg SH, Renigunta V, Sus R, Dalpke AH, Heeg KM, Robaye B, Hanley PJ (2005) ‘Host tissue damage–signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem 280(37):32459–2467
Gallucci S, Matzinger P (2001) Danger signals: SOS to the immune system. Curr Opin Immunol 13(1):114–19
Di Virgilio F (2005) Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signalling 1(3):205–09
Byrne GI, Ojcius DM (2004) Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol 2(10):802–08
Acknowledgements
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, and the University of California.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Coutinho-Silva, R., Monteiro da Cruz, C., Persechini, P.M. et al. The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signalling 3, 83–90 (2007). https://doi.org/10.1007/s11302-006-9039-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9039-6