Abstract
The P2X7 plasma membrane receptor is an intriguing molecule that is endowed with the ability to kill cells, as well as to activate many responses and even stimulate proliferation. Here, the authors give an overview on the multiplicity and complexity of P2X7-mediated responses, discussing recent information on this receptor. Particular attention has been paid to early and late signs of apoptosis and necrosis linked to activation of the receptor and to the emerging field of P2X7 function in carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
P2X7: From an elusive receptor to an ATP-gated channel/pore
The P2X7 receptor (P2X7R) for extracellular ATP is expressed by a variety of cell types as different as neurons, macrophages, dendritic and microglial cells, fibroblasts, lymphocytes, and endothelial cells. At least a subpopulation of human osteoblasts also express P2X7 [1]. Expression of the receptor has been demonstrated in human fibroblasts [2, 3] and epithelia from the human bladder [4, 5], human and rat uterus [6, 7], male genital organs of the rats [8], human fetal keratinocytes [9] and mouse parotid acinar and duct cells [10].
Although interest in this molecule has increased exponentially since the late 1990s, initial observations on its peculiar biochemical properties go back to the 1970s, when Cockcroft and Gomperts reported that extracellular ATP caused degranulation and striking morphological changes in rat mast cells [11]. These authors also hypothesized that cell responses triggered by ATP, such as histamine secretion and phosphatidyl inositol formation, were due to activation of an as yet unknown receptor (the ‘ATP receptor’) activated by the fully dissociated ATP form (ATP4−) [12]. Based on its intriguing ability to cause a reversible permeabilization of the plasma membrane, this receptor was also referred to as the ‘permeabilizing ATP receptor’ later named P2Z [13]. P2Z was described not only in rat mast cells but also in mouse macrophages [14, 15]. Following these early observations, the peculiar pharmacological and biochemical properties of the P2Z receptor were recognized in many other cells such as human macrophages, human B lymphocytes, mouse microglial cells, mouse and human monocyte-derived dendritic cells.
The permeabilizing ATP receptor was cloned in 1996 from a rat brain library and named P2X7 for its homology with the other P2X receptors [16]. P2X7 is a 595 AA protein with a predicted structure comprising two transmembrane domains and a bulky extracellular cysteine rich region, with conserved lysine and glycine residues and several potential N-linked glycosylation sites, followed by a long stretch (from Phe188 to Val321) forming six putative antiparallel β sheets. The amino and carboxyl-terminal domains are both cytoplasmic. Similarities have been found between the β sheets region and the catalytic domains of class II aminoacyl-tRNA synthetases [17]. These β sheets are likely to include residues comprising the ATP-binding site, since substitution of two aminoacids comprised in this region, Lys193 and Lys311, completely abrogates ethidium and barium uptake [18].
P2X7 is an ionotropic, ligand-gated, cation channel [19, 20]. Stimulation of the receptor with low ATP doses, reversibly opens a membrane channel permeable to small cations (Na+, Ca2+, K+), while sustained stimulation with higher ATP doses or repeated stimulation with sequential ATP pulses, induces the formation of a pore permeable to large molecular weight molecules such as choline (100 Da), methylglucamine (190 Da), ethidium (314 Da), YO-PRO-1 (376 Da), propidium (414 Da), lucifer yellow (457 Da) [21–24]. ATP generates in B and T lymphocytes a pore with a smaller molecular cut-off of slightly over 300 Da (ethidium is admitted, propidium is excluded) [25–27].
Alkalinization of the medium or removal of Mg2+ and Ca2+ increases the apparent affinity of ATP and BzATP for the native and recombinant P2X7 receptor [11, 28, 29]. Metal ions such as Cu2+, Ni2+, Cd2+, Zn2+, Co2+ inhibit P2X7 evoked currents [30, 31]. The widely used calmodulin antagonist calmidazolium, inhibits BzATP-induced currents in HEK293 cells stably expressing the rat P2X7 receptor while, it has no effect on YO-PRO-1 uptake. This evidence was interpreted as proof that it is possible to dissociate the channel from pore function, and therefore these might be two separate molecular entities [24].
The carboxyl-terminal cytoplasmic domain of P2X7 (AA 352–595) is longer than in other members of the P2X subtype. This domain is crucial for P2X7 pore formation, transduction and signalling [16, 29, 32]. Allelic mutations, leading to loss of function, have been identified both in the human and mouse receptor. A mutation (P451L) in the P2X7 cytoplasmic tail occurring in some mice strains reduces the capacity of ATP to induce pore formation [33, 34]. It has been suggested that pore formation requires over 95% of the C-terminal tail of the receptor. Experiments performed with truncated receptors expressed in HEK-293 cells and Xenopus oocytes show that truncation of the protein at residue 581 allows only negligible influx of ethidium while, surprisingly, cells expressing a receptor truncated at position 582 have unchanged uptake [35]. In contrast, formation of the ionic channel occurs even in cells expressing receptors truncated at position 380, suggesting that only a limited portion of the cytosolic region is needed for channel activity [35].
The glutamic acid 496 seems to be important for the pore-forming activity of P2X7, and substitution of Glu496 with Ala (E496A), occurring in the ankyrin repeat motif of the carboxyl-terminal domain of the receptor, leads to loss of function of the receptor in homozygous individuals and around 50% reduction in heterozygous individuals [36]. The reversible ATP-induced permeabilization of human erythrocytes to Rb+, K+ and Na+ depends on P2X7 receptor activation and is impaired in cells from subjects with inherited loss of function polymorphisms at amino acid positions 307 and 496 [37]. The first polymorphism substitutes an uncharged glutamine for a highly positive charged Arg307 (R307Q) [38]. This loss of function polymorphism likely blocks the binding of ATP to the extracellular domain of the receptor. Another known loss of function polymorphism to P2X7 is due to the substitution of Ile568 with Asn (I568N) and is located in a tracking motif in the carboxyl terminus; this polymorphism blocks normal trafficking and membrane expression of the receptor [35, 39].
The Hill coefficients obtained from the ATP dose-dependency curves are consistent with multiple ATP-binding sites [28, 40]. Studies performed with P2X7 receptors fused with enhanced green fluorescent protein (EGFP), reveal that there are no large scale changes in P2X7 receptor density when pore formation occurs [41].
Li and colleagues postulated a differential assembling of the P2X7 receptor complex in diverse cell types. Experiments performed in rat parotid duct and acinar cells would indicate that while in the first cell type, P2X7 gating is fast and independent on cytoskeleton, in acinar cells, activation of the receptor is slower and requires actin polymerisation [10]. It has been suggested that the ATP-induced P2X7 pores increase or decrease by very small units [42]. It is not known why in some cell types P2X7 expression gives rise to the ATP-dependent pore, while in other cells (human lymphoblastoid cells, human and rat fibroblasts) the channel-pore transition does not occur [2, 25, 43, 44].
A subject that has interested numerous authors in the last few years is the membrane blebbing/vesiculation associated with P2X7 activation. Among other features, the vesicles have been shown to contain active IL-1β, an important proinflammatory cytokine. P2X7 activation leads to loss of plasma membrane and to phospholipid flip. Upon a brief stimulation with P2X7 agonists, phosphatidylserine exposure reverses within hours, without concomitant cell death [45]. The ROCKI kinase pathway plays a key role in the cytoskeletal rearrangement following P2X7 activation [46, 47].
Western blot analysis of native proteins shows that P2X7 forms multimeric complexes in rat bone marrow cells and peritoneal macrophages, while it is present as a monomer in brain glia or astrocyte lysates [48]. This could be due to expression of regulatory proteins modulating the channel-pore transition process. Different P2X7-interacting proteins have been identified. Mass spectrometry on immunoprecipitates showed that a complex comprising 11 proteins interacts with the receptor: laminin α3, integrin β2, β-actin, α-actinin, supervillin, matrix activated MAGuK (membrane-associated guanylate kinase P55), heat shock proteins 70 and 90 (Hsp70, Hsp90), heat shock cognate protein 71 (Hsc71), phosphatidylinositol 4-kinase (PI4K) and receptor protein tyrosine phosphatase-beta (RPTPβ). Among those RPTPβ and Hsp90 have been proposed to functionally modulate P2X7 [49, 50]. To isolate other potentially P2X7 related proteins the yeast two hybrid approach has also been used, resulting in isolation of various epithelial membrane protein family members (EMPs). The EMPs have been linked to cell blebbing and in general to receptor-mediated apoptosis [51].
Putative LPS-binding domains have been identified in the C-terminal domain. This seems to fit with the property of P2X7 to modulate secretion of different immunomodulatory molecules (IL-1β, TNF-α, NO) in LPS-stimulated macrophages. Protein-protein and protein-lipid interaction motifs within the receptor tail were also identified [52]. P2X7-derived peptides are able to bind LPS and block two LPS-modulated intracellular events, i.e. the capacity of activating extracellular signal-regulated kinases 1 and 2 (ERK1, ERK2) and stimulation of IkappaB-alpha degradation. In human leukaemic lymphocytes, P2X7 ion channel activation by ATP stimulates phospholipase D (PLD) through the influx of bivalent cations [53].
Although most of the studies on P2X7 have been performed in the immune system, recent reports showed expression and putative functions of the receptor also in other cell types such as the human neuroblastoma cell line SH-SY5Y [54], glial Müller cells from the human retina [55], mouse Schwann cells [56], and rat pituitary cells [57].
The presence of transcripts for the P2X7 subtype has been detected in preparations of medulla oblongata, spinal cord, and nodose ganglion. P2X7 protein has been found in the presynaptic terminals in the central nervous system [58]. The receptor is localized to the excitatory terminals in the hippocampus and its stimulation induces the release of glutamate and GABA [59]; primary astrocytes cultures from rat hippocampus are also immunopositive for P2X7. Contrasting observations have been recently published on involvement of P2X7 in mossy fiber-CA3 sinaptic responses. Activation of this receptor has been found to depress mossy fiber-CA3 synaptic transmission through activation of p38 MAP kinase [60], but BzATP-mediated decrease of mossy fiber-C3 potential was shown not to be mediated by P2X7 [61]. The authors hypothesize that BzATP is extracellularly catabolized to Bz-adenosine and subsequently hetero-exchanged for intracellular adenosine that would be responsible for depressing mossy fibers potential through presynaptic A1 receptors [61]. Accordingly, Sim and colleagues failed to detect P2X7 receptor in hippocampal neurons [62].
P2X7 receptor stimulation provides a new route for excitatory amino acid release (l-glutamate and d-aspartate) from murine cortical astrocytes [63]. Activation of the receptor is also coupled to the phosphoinositide 3-kinase (PI3K)/Akt pathway in rat cortical astrocytes and in P2X7-expressing 1321N1 cells [64].
Death or life?
It was soon evident that extracellular ATP was a potent permeabilizing and cytotoxic factor for macrophages. Steinberg and Silverstein demonstrated that, upon exposure of macrophages to high ATP concentrations, most of the cells died and the surviving ones were insensitive to ATP-mediated permeabilization and refractory to ATP cytotoxic effects [14]. These findings suggested that ATP-mediated responses were due to expression of cytotoxic receptor/s and not to the mere perturbation of the plasma membrane integrity by ATP [14, 21]. These observations were later confirmed by experiments performed with pharmacological inhibitors of the receptor having different properties and showing diverse species/specificity, i.e. periodate oxidized-ATP (oATP), 1-[N,O-Bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), and brilliant blue G. Oxidized ATP [65], is active not only at P2X7 but also at other P2X subtypes. KN-62 only inhibits human P2X7 [66], while brilliant blue G is active at the murine subtype [67]. A further proof of the existence of the receptor was given by the demonstration that blockade of P2X7 by a specific monoclonal antibody fully abrogated ATP-mediated cytotoxicity. Furthermore, cells lacking reactivity to anti-P2X7 antibodies were also resistant to the ATP permeabilizing and cytotoxic effects [68].
P2X7 would thus be a cytotoxic receptor, capable of killing the cell by forming membrane pores, and ATP not just an intermediate of intracellular energy transactions but a potent cytotoxic molecule. Since the cytotoxic effect occurs only at high extracellular ATP concentrations, massive release of the nucleotide due to cell damage or lysis, or decreased activity of the ATP hydrolysing enzymes (ecto-ATPases and ecto-nucleotidases) have to be postulated. For example, hematopoietic precursor cells isolated from mouse bone marrow, which express P2X7 receptors, are very sensitive to ATP and are killed by stimulation with the nucleotide. This high sensitivity has permitted the establishment of an efficient procedure to isolate highly purified marrow stromal cells, by deleting hematopoietic cell precursors [69]. Elimination of P2X7-expressing cells can also be significantly increased by incubating cells in the presence of a toxic agent such as potassium thiocyanate that enters the cell membrane upon P2X7 receptor opening [70]. This same procedure might be helpful for the treatment of tumors of hematopoietic or other origins.
ATP can induce cell death by either necrosis or apoptosis, depending upon the incubation time, ATP dose and cell type [71]. The ability of extracellular ATP to cause apoptosis of mouse cell lines was initially documented in P-815 mastocytoma and YAC lymphoid cells, and then subsequently extended to mouse thymocytes [22, 72, 73]. P2X7-expressing J774 mouse macrophages mostly die by colloido-osmotic lysis as they quickly swell, change refrangence, detach from the substrate and release cytoplasmic components. Recently, P2X7 has also been shown to cause apoptotic death of BAC1 macrophages [74]. Mouse microglial cell lines N9 and N13 also show condensation of nuclei and DNA fragmentation, which are both hallmarks of apoptosis. Furthermore, stimulation with ATP of N9 and N13 cells induces activation of different caspases. Caspase-1, 3 and 8 are activated and caspase substrates such as PARP and lamin B are processed [75, 76]. Activation of casp-3 and casp-9 has been shown in human cervical epithelial cells [77]. Triggering of P2X7 induces phosphatidylserine externalization and membrane blebbing [45]. These effects are mediated by P2X7 as they are blocked by P2X7 inhibitors and are absent in P2X7-less cells. Increased P2X7 expression has been found in the peri-infarct region in the rat brain cortex after cerebral artery occlusion; augmented P2X7 protein levels were detected in microglia, neurons and apoptotic cells [78]. Stimulation of P2X7 receptor induces release of tumor necrosis factor alpha (TNF-α) from microglia [79].
Sugiyama and colleagues reported that in pericyte-containing retinal microvessels, activation of P2Y4 receptors by UTP prevented P2X7 pores from forming. These data would point to a cross-talk between the P2X and P2Y subtypes in inducing/preventing cell death. They also showed that maximal activation of P2X7 resulted in voltage-dependent calcium channels (VDCCs) opening, exacerbating the death process [80].
The mammalian ectoenzyme ART-2 catalyzes protein ADP-ribosylation. It has been shown that exposure of T lymphocytes to NAD, the substrate of ART-2, causes P2X7 receptor ADP-ribosylation and P2X7-dependent apoptosis characterized by exposure of phosphatidylserine [81], shedding of CD62L, propidium iodide uptake and cell shrinkage [82, 83].
Human fibroblasts from diabetic patients show enhanced P2X7-mediated responses e.g. increased shape change, microvesiculation, increased IL-6 secretion and accelerated apoptosis [84]. Lymphocytes from type 1 diabetic (NOD) mice show an increased apoptosis upon stimulation of the P2X7 receptor, that also results in an augmented shedding of the lymphocyte homing receptor CD62L [85].
Purinoceptors can also be activated by spontaneous or stimulated ATP release through an autocrine or paracrine loop. A basal ATP release occurs in vitro, thus providing a chronic stimulation of P2 receptors. Murine macrophages expressing high levels of P2X7 show an unusually high rate of spontaneous cell death that can be significantly reduced by inhibiting P2X7, or by incubation of the cells in the presence of the ATP-hydrolyzing enzyme apyrase, suggesting that the P2X7 receptor is activated by constitutively released ATP [86].
Extracellular ATP induces apoptosis of human peripheral monocytes infected with Mycobacterium bovis (bacillus Calmette Guerin, BCG) as demonstrated by DNA fragmentation and nuclear condensation. Interestingly and more importantly, ATP also induces swelling of the BCG-containing vacuoles and reduces BCG viability [87]. Pore formation upon P2X7 receptor stimulation is responsible for induction of cell death of BCG-infected human macrophages. Triggering of P2X7 receptor also induces death of the intracellular bacteria, at variance with CD95 or complement activated cell death that only kills macrophages [88]. The crucial step in the killing of the intracellular parasite is fusion of the parasite-containing phagosomes with intracellular lysosomes [89].
Li and colleagues showed that heterogeneity in cell donors with respect to ATP responsiveness could depend on p2x 7 polymorphisms and suggested an association between a single-nucleotide polymorphism in the P2X7 promoter (position −762) and infection by Mycobacterium tuberculosis [90]. Another P2X7 polymorphism, this time situated in the coding region (A/C 1531), has been associated with the inability of macrophages to kill mycobacteria [91]. A putative role for P2X7 during infection has also been hypothesized since two inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) upregulate P2X7 expression [92–94].
Extracellular ATP is a powerful stimulus for IL-1β secretion [95]. Endotoxin (LPS) induced IL-1β release is in fact a very inefficient process, since it is slow and leads to secretion of a modest amount of the cytokine. Addition of extracellular ATP to endotoxin-primed macrophages or microglial cells causes a fast release of a large quantity of processed IL-1β. The process is dependent on P2X7 stimulation and ATP is an efficient stimulus for IL-1β secretion only if cells have been previously primed with LPS, pointing to a role for the nucleotide in accelerating the proteolytic maturation of the cytokine [76, 96–98]. This hypothesis was then validated by studies performed in p2x 7 −/− mice. In peritoneal macrophages obtained from these animals pro-IL-1β is not processed and externalised in response to ATP. In contrast to what is observed in cells from wild type mice. Injection of ATP in endotoxin treated p2x −/− animals also failed to increase IL-1β production [99].
p2x 7 −/− mice are also less prone to develop cartilage lesions, loss of proteoglycan content, and presence of collagen degradation products [100].
P2X7 receptor in cell growth and tumor models
In apparent contrast with the role of P2X7 receptor in necrosis and apoptosis, an increasing number of reports has also correlated this protein with increased cell proliferation and tumor transformation. The first cellular model in which a role for P2X7 in cell growth was suggested were T lymphocytes [101].
In human peripheral blood lymphocytes (PBL) and T CD4+ and CD8+ subpopulations, ATP increases mitogenic activity via the P2X7 receptor, when co-applied with anti-CD3 antibodies, phytohaemoagglutinin (PHA) or heterologous leucocytes, all stimuli that mimic TCR/MHC activation [101]. Extracellular nucleotides were known to stimulate proliferation through activation of P2Y receptors, the novelty of this study was to attribute a proliferative activity to a pro-apoptotic receptor. A further step in the analysis of the possible involvement of the receptor in promoting proliferation, was made by analysing the behaviour of human B lymphoid cells stably transfected with a P2X7 receptor cDNA [102]. In contrast to wild type cells, the P2X7 transfectants acquired the ability to survive and proliferate in the absence of serum, a hallmark of cancerous cells. Proliferation is likely supported by an autocrine/paracrine stimulation of released ATP. Indeed, P2X7-expressing cells release an amount of ATP that is four-fold larger than in control cells. Moreover incubation of the P2X7 transfectants with the ATP-hydrolyzing enzyme apyrase or pre-treatment with oxidized ATP (oATP) abrogates proliferation [101]. Recently, Budagian and coworkers dissected the signalling pathways responsible for P2X7 effects in lymphocytes, demonstrating that in Jurkat cells, a human T lymphocyte cell line, P2X7 activation results in phosphorylation and activation of p56lck [103]. p56lck is a lymphoid specific tyrosine kinase mediating the initial events of TCR/CD3 signalling leading to mitogenic activation of T lymphocytes. Active p56lck phosphorylates the TCR allowing the docking of other kinases such as Zap-70 and Syk to its complex; p56lck also stimulates MAP kinases. The P2X7 dependent activation of p56lck may offer an explanation for the proliferation-promoting effects of ATP on CD3 activated T lymphocytes [101]. Activation of P2X7 has been shown to be able to trigger the signalling pathway of mitogen-activated MAPK/ERK kinases, that has been extensively investigated in different cell types such as mouse macrophages and microglia, human astrocytoma and rat parotid ancinar salivary cells [74, 104–106]. MAP kinases are known to promote cell growth and proliferation by inducing de novo synthesis of pyrimidine nucleotides in the nucleus [107]. They also activate transcription factors, triggering expression of early response genes coding for growth-promoting proteins [108].
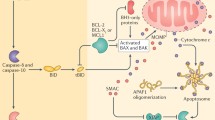
Chronic lymphocytic leukaemia B lymphocytes were one of the first cellular models in which P2X{nu7} like activity was investigated [109, 110] and are currently a hot field of investigation in view of the possible application in the prognosis and therapy of this disease [111–113]. B chronic lymphocytic leukaemia (B-CLL) is the most common leukaemia in the western world, and despite a known familial incidence and higher percentage of male individuals among patients, it has not been associated with a specific genetic ‘hallmark’ [114]. Recently it has been shown that P2X7R expression and function is higher in CLL patients with the aggressive variant of the disease, compared to those affected by the indolent form [111]. Patients affected by the aggressive form showed accordingly higher resting calcium levels and ATP evoked calcium influx as well as higher sensitivity to ATP-mediated cytotoxicity. The proposed model predicts that a tonic, low activation of P2X7 receptor will lead to an increased proliferation, while an acute stimulation with high concentrations of nucleotide causes death of tumor lymphocytes (Figure 1) [111].
Low tonic or massive stimulation with ATP of P2X7-expressing cells causes a transient or a prolonged opening of the receptor-pore, respectively; this in-turn induces a modest or high intracellular Ca2+ concentration increase. Although changes in calcium are unlikely to be solely responsible for the duality in P2X7-mediated effects, they represent physiological triggering points in diverse upstream and downstream signalling processes, activating different cell functions and responses.
More controversial at the moment is the association of a loss of function P2X7 polymorphism with B-CLL incidence and progression. This polymorphism (1513A→C) codes for glutamic acid to alanine at amino acid position 496 in the C-terminal tail of the receptor [36]. It was first identified in normal subjects in which it caused a reduced ATP-mediated barium and ethidium uptake. Subsequently, this mutation was proposed as a prognostic marker in B cells, but no consensus has been reached as yet on this matter [115–118].
Besides B chronic lymphocytic leukaemia, several other tumors show an altered expression of the P2X7 receptor. For example, non-melanoma skin cancers express this receptor and die upon massive application of ATP or BzATP [119]. In this case the receptor seems to be associated with cells undergoing apoptosis as it colocalises with TUNEL and active caspase-3 staining [119]. For other widely diffused neoplasia, such as prostate and breast cancer, the expression of P2X7R has been immunologically detected specifically in transformed cells [6, 120]. One hundred fourteen out of 116 prostate cancer biopsies stained positively for the P2X7 receptor and also cells well distinct from the tumor show expression of the receptor along with tumor progression [120]. Likewise, all cases of in situ lobular and ductal carcinoma showed intense P2X7 labelling in the nuclei and cytoplasm, while more aggressive forms tended to present the receptor at the cell surface [6]. The authors of these studies infer that the expression of the P2X7 receptor could be an attempt of cancer cells to undergo apoptosis, that fails because the receptor might be nonfunctional. However no functional studies were performed.
This increasing number of reports suggests that application of nucleotide-derived drugs, able to modulate P2X7 receptor functions, might be useful in tumour therapy [121]. To this aim, development of allosteric modulators of the P2X7 receptor that potentiate the effect of ATP might be an alternative approach to decrease ATP dose and therefore reduce the side effects of ATP break-down products or of ATP itself.
The anti-inflammatory drug tenidap synergises with extracellular ATP for activation of the P2X7 receptor, by increasing the affinity of the P2X7 for ATP [122]. The natural antibiotic polymyxin B (PMB) is a well-known agent binding and neutralizing bacterial endotoxin. It has recently been shown that PMB amplifies ATP-induced responses by acting at both recombinant or natively expressed P2X7R in several cell models [123]. Another possible therapeutical approach to neoplasias could be the use of P2X7 antagonists to counteract the proliferative advantage conferred by the receptor to the transformed cells. Several P2X7 receptors antagonist/blockers have been identified but none of them is selective [20]. Brilliant blue G [67] and KN-62 display a good potency, the latest compound is also a CaM kinase II (calcium-calmodulin dependent protein kinase type 2) inhibitor. There have been different attempts to obtain KN-62 analogues [124], more active and specific for P2X7 [125–127]. The benzophenanthridine alkaloid chelerythrine, a potent PKC inhibitor, has a noncompetitive inhibitory action on the P2X7 receptor expressed by human B lymphocytes [128]. Quite recently new adamantate amide antagonists, which show a high potency at P2X7 receptor, have been isolated by two groups at Astra Zeneca [129, 130]. Further studies on this direction could provide useful tools to better understand the physiological functions of P2X7 not only in tumour models but also in other cell types such as immune and epithelial cells.
Conclusions
In conclusion the P2X7 receptor seems to play a role in both cell death and survival. These two opposite functions are only apparently contradictory as they may depend on the specific intracellular pathway activated by P2X7 at different points or in diverse phases of the cell cycle. Low tonic stimulation by ATP is postulated to produce a low-level activation of P2X7 that supports growth. On the contrary, massive P2X7 stimulation would lead to cell death (Figure 1). In this view, it would be of great interest to measure ATP concentration at cell membrane.
Pro-apoptotic, pro-inflammatory receptors acting in some cases as growth-promoting tumorigenic proteins are not novel: TNF-a receptor (p55) for example, is known for both its cytotoxic activity and for promoting cancer cell growth [131].
References
Gartland A, Hipskind RA, Gallagher JA, Bowler WB. Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res 2001; 16: 846–56.
Solini A, Chiozzi P, Morelli A et al. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J Cell Sci 1999; 112: 297–305.
Solini A, Chiozzi P, Falzoni S et al. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia 2000; 43: 1248–56.
O’Reilly BA, Kosaka AH, Chang TK et al. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol 2001; 165: 1730–4.
O’Reilly BA, Kosaka AH, Chang TK et al. A quantitative analysis of purinoceptor expression in the bladders of patients with symptomatic outlet obstruction. BJU Int 2001; 87: 617–22.
Slater NM, Barden JA, Murphy CR. Distributional changes of purinergic receptor subtypes P2X1–7 in uterine epithelial cells during early pregnancy. Histochem J 2000; 32: 365–72.
Tassell W, Slater M, Barden JA, Murphy CR. Endometrial cell death during early pregnancy in the rat. Histochem J 2000; 32: 373–9.
Lee HY, Bardini M, Burnstock G. P2X receptor immunoreactivity in the male genital organs of the rat. Cell Tissue Res 2000; 300: 321–30.
Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation and apoptosis in human fetal epidermis. J Invest Dermatol 2003; 121: 1145–9.
Li Q, Luo X, Zeng W, Muallem S. Cell-specific behaviour of P2X7 receptors in mouse parotid acinar and duct cells. J Biol Chem 2003; 278: 47554–61.
Cockcroft S, Gomperts BD. ATP induces nucleotide permeability in rat mast cells. Nature 1979; 279: 541–2.
Cockcroft S, Gomperts BD. The ATP4‒ receptor of rat mast cells. Biochem J 1980; 188: 789–98.
Gordon JL. Extracellular ATP: Effects, sources and fate. Biochem J 1986; 233: 309–19.
Steinberg TH, Silverstein SC. Extracellular ATP4‒ promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem 1987; 262: 3118–22.
Greenberg S, Di Virgilio F, Steinberg TH, Silverstein SC. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem 1988; 263: 10337–43.
Surprenant A, Rassendren F, Kawashima E et al. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996; 272: 735–8.
Freist W, Verhey JF, Stuhmer W, Gauss DH. ATP binding site of P2X channel proteins: Structural similarities with class II aminoacyl–tRNA synthetases. FEBS Lett 1998; 434: 61–5.
Worthington RA, Smart ML, Gu BJ et al. Point mutations confer loss of ATP-induced human P2X7 receptor function. FEBS Lett 2002; 512: 43–6.
North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 2000; 40: 563–80.
North RA. The molecular physiology of P2X receptors. Physiol Rev 2002; 82: 1013–67.
Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4‒ permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem 1987; 262: 8884–8.
Di Virgilio F, Bronte V, Collavo D, Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5’-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. J Immunol 1989; 143: 1955–60.
Di Virgilio F. The P2Z purinoceptor: An intriguing role in immunity, inflammation and cell death. Immunol Today 1995; 16: 524–8.
Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 1999; 519: 335–46.
Ferrari D, Munerati M, Melchiorri L et al. Responses to extracellular ATP of lymphoblastoid cell lines from Duchenne muscular dystrophy patients. Am J Physiol 1994; 267: C886–92.
Chused TM, Apasov S, Sitkovsky M. Murine T lymphocytes modulate activity of an ATP-activated P2Z-type purinoceptor during differentiation. J Immunol 1996; 157: 1371–80.
Markwardt F, Lohn M, Bohm T, Klapperstuck M. Purinoceptor-operated cationic channels in human B lymphocytes. J Physiol 1997; 498: 143–51.
Pizzo P, Zanovello P, Bronte V, Di Virgilio F. Extracellular ATP causes lysis of mouse thymocytes and activates a plasma membrane ion channel. Biochem J 1991; 274: 139–44.
Rassendren F, Buell GN, Virginio C et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem 1997; 272: 5482–6.
Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 1997; 36: 1285–94.
Watano T, Matsuoka I, Kimura J. Inhibitory effects of metals on ATP-induced current through P2X7 receptor in NG108-15 cells. Jpn J Pharmacol 2002; 89: 296–301.
Hu Y, Fisette PL, Denlinger LC et al. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem 1998; 273: 27170–5.
Adriouch S, Dox C, Welge V et al. Cutting edge: A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 2002; 169: 4108–12.
Le Stunff H, Auger R, Kanellopoulos J, Raymond MN. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem 2004; 279: 16918–26.
Smart ML, Gu B, Panchal RG et al. P2X7 receptor cell surface expression and cytolytic pore formation are regulated by a distal C-terminal region. J Biol Chem 2003; 278: 8853–60.
Gu BJ, Zhang W, Worthington RA et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 2001; 276: 11135–42.
Sluyter R, Shemon AN, Barden JA, Wiley JS. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J Biol Chem 2004; 279: 44749–55.
Gu BJ, Sluyter R, Skarratt KK et al. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem 2004; 279: 31287–95.
Wiley JS, Dao-Ung LP, Li C et al. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem 2003; 278: 17108–13.
Klapperstuck M, Buttner C, Schmalzing G, Markwardt F. Functional evidence of distinct ATP activation sites at the human P2X7 receptor. J Physiol 2001; 534: 25–35.
Smart ML, Panchal RG, Bowser DN et al. Pore formation is not associated with macroscopic redistribution of P2X7 receptors. Am J Physiol, Cell Physiol 2002; 283: C77–84.
Tatham PE, Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol 1990; 95: 459–76.
Coutinho-Silva R, Alves LA, de Carvalho AC et al. Characterization of P2Z purinergic receptors on phagocytic cells of the thymic reticulum in culture. Biochim Biophys Acta 1996; 1280:217–22.
Kochukov MY, Ritchie AK. A P2X7 receptor stimulates plasma membrane trafficking in the FRTL rat thyrocyte cell line. Am J Physiol Cell Physiol 2004; 287: C992–1002.
MacKenzie A, Wilson HL, Kiss-Toth E et al. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 2001; 15: 825–35.
Morelli A, Chiozzi P, Chiesa A et al. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell 2003; 14: 2655–64.
Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of rho-effector kinases, caspases, and IL-1 beta release. J Immunol 2003; 170: 5728–38.
Kim M, Spelta V, Sim J et al. Differential assembly of rat purinergic P2X7 receptor in immune cells of the brain and periphery. J Biol Chem 2001; 276: 23262–7.
Kim M, Jiang LH, Wilson HL et al. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J 2001; 20: 6347–58.
Adinolfi E, Kim M, Young MT et al. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem 2003; 278: 37344–51.
Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial Membrane Proteins Induce Membrane Blebbing and Interact with the P2X7 receptor C Terminus. J Biol Chem 2002; 277: 34017–23.
Denlinger LC, Fisette PL, Sommer JA et al. Cutting edge: The nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol 2001; 167: 1871–6.
Gargett CE, Cornish EJ, Wiley JS. Phospholipase D activation by P2Z-purinoceptor agonists in human lymphocytes is dependent on bivalent cation influx. Biochem J 1996; 313: 529–35.
Per Larsson K, Jon Hansen, Dissing S. The human SH-SY5Y neuroblastoma cell-line expresses a functional P2X7 purinoceptor that modulates voltage-dependent Ca2+ channel function. J Neurochem 2002; 83: 285–98.
Pannicke T, Fischer W, Biedermann B et al. P2X7 receptors in Müller glial cells from the human retina. J Neurosci 2000; 20: 5965–72.
Colomar A, Amedee T. ATP stimulation of P2X7 receptors activates three different ionic conductances on cultured mouse Schwann cells. Eur J Neurosci 2001; 14: 927–36.
Kimm-Brinson KL, Moeller PD, Barbier M et al. Identification of a P2X7 receptor in GH(4)C(1) rat pituitary cells: A potential target for a bioactive substance produced by Pfiesteria piscicida. Environ Health Perspect 2001; 109: 457–62.
Deuchars SA, Atkinson L, Brooke RE et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 2001; 21: 7143–52.
Sperlagh B, Kofalvi A, Deuchars J et al. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem 2002; 81: 1196–211.
Armstrong JN, Brust TB, Lewis RG, MacVicar BA. Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci 2002; 22: 5938–45.
Kukley M, Stausberg P, Adelmann G et al. Ecto-nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2’3’-O-(4-benzoylbenzoyl)-ATP. J Neurosci 2004; 24: 7128–39.
Sim JA, Young MT, Sung HY et al. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 2004; 24: 6307–14.
Duan S, Anderson CM, Keung EC et al. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 2003; 23: 1308–20.
Jacques-Silva MC, Rodnight R, Lenz G et al. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 2004; 141: 1106–17.
Murgia M, Hanau S, Pizzo P et al. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem 1993; 268: 8199–203.
Humphreys BD, Virginio C, Surprenant A et al. Isoquinolines as antagonists of the P2X7 nucleotide receptor: High selectivity for the human versus rat receptor homologues. Mol Pharmacol 1998; 54: 22–32.
Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol 2000; 58: 82–8.
Buell G, Chessell IP, Michel AD et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood 1998; 92: 3521–8.
Modderman WE, Vrijheid-Lammers T, Lowik CW, Nijweide PJ. Removal of hematopoietic cells and macrophages from mouse bone marrow cultures: Isolation of fibroblast-like stromal cells. Exp Hematol 1994; 194–201.
Nijweide PJ, Modderman WE, Hagenaars CE. Extracellular adenosine triphosphate: A shock to hemopoietic cells. Clin Orthop 1995; 313: 92–102.
Di Virgilio F, Chiozzi P, Falzoni S et al. Cytolytic P2X purinoceptors. Cell Death Differ 1998; 5: 191–9.
Zanovello P, Bronte V, Rosato A et al. Responses of mouse lymphocytes to extracellular ATP. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol 1990; 145: 1545–50.
Zheng LM, Zychlinsky A, Liu C et al. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 1991; 112: 279–88.
Humphreys BD, Rice J, Kertesy SB, Dubyak GR. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem 2000; 275: 26792–8.
Ferrari D, Los M, Bauer MK et al. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett 1999; 447: 71–5.
Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol 2000; 164: 4893–8.
Wang Q, Wang L, Feng YH et al. P2X7-receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol 2004; 287: C1349–58.
Franke H, Gunther A, Grosche J et al. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol 2004; 63: 686–99.
Suzuki T, Hide I, Ido K et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci 2004; 24: 1–7.
Sugiyama T, Kawamura H, Yamanishi S et al. Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol 2004 [Epub ahead of print].
Courageot MP, Lepine S, Hours M et al. Involvement of sodium in early phosphatidylserine exposure and phospholipid scrambling induced by P2X7 purinoceptor activation in thymocytes. J Biol Chem 2004; 279: 21815–23.
Seman M, Adriouch S, Scheuplein F et al. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 2003; 19: 571–82.
Scheuplein F, Adriouch S, Glowacki G et al. Triggering of T-cell apoptosis by toxin-related ecto-ADP-ribosyltransferase ART2. Ann N Y Acad Sci 2003; 1010: 296–9.
Solini A, Chiozzi P, Morelli A et al. Enhanced P2X7 activity in human fibroblasts from diabetic patients: A possible pathogenetic mechanism for vascular damage in diabetes. Arterioscler Thromb Vasc Biol 2004; 24: 1240–5.
Elliott JI, Higgins CF. Major histocompatibility complex class I shedding and programmed cell death stimulated through the proinflammatory P2X7 receptor: A candidate susceptibility gene for NOD diabetes. Diabetes 2004; 53: 2012–7.
Chiozzi P, Sanz JM, Ferrari D et al. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol 1997; 138: 697–706.
Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette–Guerin. J Exp Med 1994; 180: 1499–509.
Lammas DA, Stober C, Harvey CJ et al. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity 1997; 7: 433–44.
Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome–lysosome fusion. J Immunol 2001; 167: 3300–7.
Li CM, Campbell SJ, Kumararatne DS et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis 2002; 186: 1458–62.
Saunders BM, Fernando SL, Sluyter R et al. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol 2003; 171: 5442–6.
Falzoni S, Munerati M, Ferrari D et al. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest 1995; 95: 1207–16.
Humphreys BD, Dubyak GR. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol 1996; 157: 5627–37.
Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 1998; 64: 265–73.
Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA 1991; 88: 8485–9.
Ferrari D, Villalba M, Chiozzi P et al. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol 1996; 156: 1531–9.
Ferrari D, Chiozzi P, Falzoni S et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 1997; 159: 1451–8.
Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol 2002; 447: 261–9.
Solle M, Labasi J, Perregaux DG et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem 2001; 276: 125–32.
Labasi JM, Petrushova N, Donovan C et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 2002; 168: 6436–45.
Baricordi OR, Ferrari D, Melchiorri L et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 1996; 87: 682–90.
Baricordi OR, Melchiorri L, Adinolfi E et al. Increased proliferation rate of lymphoid cells transfected with the P2X7 ATP receptor. J Biol Chem 1999; 274: 33206–8.
Budagian V, Bulanova E, Brovko L et al. Signaling through P2X7 receptor in human T cells involves p56 lck, MAP kinases, and transcription factors AP-1 and NF-kB. J Biol Chem 2003; 278: 1549–60.
Bradford M, Soltoff SP. P2X7 receptors activate protein kinase D and p427p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J 2002; 366: 745–55.
Gendron FP, Neary JT, Theiss PM et al. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 2003; 284: C571–81.
Parvathenani LK, Tertyshnikova S, Greco CR et al. P2X7 mediates superoxide production in primary microglia and is upregulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 2003; 278: 13309–17.
Graves LM, Guy HI, Kazlowski P et al. Regulation of carbamyl phosphate synthetase by MAP kinase. Nature 2000; 403: 328–32.
Whitmarsh AJ, Davis RJ. Trascription factor AP-1 regulation by mitogen activated protein kinase signal transduction pathways. J Mol Med 1996; 74: 589–607.
Wiley JS, Dubyak GR. Extracellular adenosine triphosphate increases cation permeability of chronic lymphocytic leukemic lymphocytes. Blood 1989; 73: 1316–23.
Wiley JS, Chen R, Jamieson GP. The ATP4‒ receptor-operated channel (P2Z class) of human lymphocytes allows Ba++ and ethidium uptake: Inhibition of fluxes by suramin. Arch Biochem Biophys 1993; 305: 54–60.
Adinolfi E, Melchiorri L, Falzoni S et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 2002; 99: 706–8.
Wiley JS, Dao-Ung LP, Gu BJ et al. A loss-of-function polymorphic mutation in the cytolytic P2X7 receptor gene and chronic lymphocytic leukaemia: A molecular study. Lancet 2002; 359: 1114–9.
Thunberg U, Tobin G, Johnson A et al. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet 2002; 360: 1935–9.
Guipaud O, Deriano L, Salin H et al. B-cell chronic lymphocytic leukemia: A polymorphic family unified by genomic features. Lancet oncol 2003; 4: 505–14.
Starczynski J, Pepper C, Pratt G et al. The P2X7 receptor gene polymorphism 1513 AC has no effect on clinical prognostic markers, in vitro sensitivity to fludarabine, Bcl-2 family protein expression or survival in B-cell chronic lymphocytic leukemia. Brit J Haematol 2003; 123: 66–71.
Zhang LY, Ibbotson RE, Orchard JA et al. P2X7 polymorphism and chronic lymphocytic leukemia: Lack of correlation with incidence, survival and abnormalities of chromosome 12. Leukemia 2003; 17: 2097–100.
Nuckel H, Frey UH, Durig J et al. 1513A/C polymorphism in the P2X7 receptor gene in chronic lymphocytic leukemia: Absence of correlation with clinical outcome. Eur J Haematol 2004; 72: 259–63.
Sellick GS, Rudd M, Eve P et al. The P2X7 receptor gene A1513C polymorphism does not contribute to risk of familial or sporadic chronic lymphocytic leukemia. Cancer Epidemiol Biomarkers Prev 2004; 13: 1065–7.
Greig AV, Linge C, Healy V et al. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. J Invest Dermatol 2003; 121: 315–27.
Slater M, Danieletto S, Gidley-Baird A et al. Early prostate cancer detected using expression of non-functional cytolytic P2X7 receptors. Histopathology. 2004; 44: 206–15.
Jacobson KA, Javris MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem 2002; 45: 4058–93.
Sanz JM, Chiozzi P, Di Virgilio F. Tenidap enhances P2Z/P2X7 receptor signalling in macrophages. Eur J Pharmacol 1998; 355: 235–44.
Ferrari D, Pizzirani C, Adinolfi E et al. The antibiotic polymyxin B modulates P2X7 receptor function. J Immunol 2004; 173: 4652–60.
Gargett CE, Wiley JS. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor of human lymphocytes. Br J Pharmacol 1997; 120: 1483–90.
Baraldi PG, Romagnoli R, Tabrizi MA et al. Synthesis of conformationally constrained analogues of KN62, a potent antagonist of the P2X7 receptor. Bioorg Med Chem Lett 2000; 10: 681–4.
Baraldi PG, Makaeva R, Pavani MG et al. Synthesis, biological activity and molecular modeling studies of 1,2,3,4-tetrahydroiso-quinoline derivatives as conformationally constrained analogues of KN62, a potent antagonist of the P2X7-receptor containing a tyrosine moiety. Arzneim-Forsch Drug Res 2002; 52: 273–85.
Baraldi PG, del Carmen Nuñez M, Morelli A et al. Synthesis and biological activity of N-Arylpiperazine-modified analogues of KN-62, a potent antagonist of the purinergic P2X7 receptor. J Med Chem 2003; 46: 1318–29.
Shemon AN, Sluyter R, Conigrave AD, Wiley JS. Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor. Br J Pharmacol 2004; 142: 1015–9.
Baxter A, Bent J, Bowers K et al. Hit-to-lead studies: The discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg Med Chem Lett 2003; 13: 4047–50.
Alcaraz L, Baxter A, Bent J et al. Novel P2X7 receptor antagonists. Bioorg Med Chem Lett 2003; 13: 4043–6.
Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: A potential target for the therapy of solid tumours. Lancet Oncol 2003; 4: 565–73.
Acknowledgement
This work was supported by grants by the Italian Ministry of Education, University and Scientific Research (MIUR), the National Research Council of Italy, the Italian Association for Cancer Research (AIRC), the Italian Space Agency (ASI), and by local funds from the University of Ferrara.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Adinolfi, E., Pizzirani, C., Idzko, M. et al. P2X7 receptor: Death or life?. Purinergic Signalling 1, 219–227 (2005). https://doi.org/10.1007/s11302-005-6322-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-005-6322-x