Abstract
The forest canopy cover can directly and indirectly affect soil conditions and hence soil carbon emission through soil respiration. Little is known, however, on the effects of canopy cover on soil respiration under the canopy of different tree species and soil water conditions. We have examined the variation in soil respiration at different soil water conditions (dry <10 %, wet >20 %, v/v) under different tree canopy covers in comparison with the canopy interspace in a temperate coniferous (Pinus armandii Franch) and broadleaved (Quercus aliena var. acuteserrata) mixed forest in central China. The results show that soil respiration measured under tree canopy cover varied with canopy size and soil water content. Soil respiration under small-sized canopies of P. armandii (PS) was higher than that under large-sized (PL) canopies, but the difference was only significant under the dry soil condition. However, soil respiration under large-sized canopies of Q. aliena (QL) was significantly greater than that under small-sized (QS) canopies under both dry and wet soil conditions. The difference in soil respiration between differently sized canopies of Q. aliena (33.5–35.8 %) was significantly greater than that between differently sized canopies of P. armandii (2.4–8.1 %). Differences in soil respiration between inter-plant gaps and under QS canopies in both the dry and wet soil conditions were significant. Significant increases in soil respiration (9.7–32.2 %) during the transition from dry to wet conditions were found regardless of canopy size, but the increase of soil respiration was significantly lower under P. armandii canopies (9.7–17.7 %) than under Q. aliena canopies (25.9–31.5 %). Our findings that the canopy cover of different tree species influences soil respiration under different soil moisture conditions could provide useful information for parameterizing and/or calibrating carbon flux models, especially for spatially explicit carbon models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil respiration, which accounts for 30–80 % of total ecosystem respiration, is one of the major components of the terrestrial ecosystem carbon cycle (Davidson et al. 2006; Li et al. 2012). Soil respiration is the primary process by which carbon is released into the atmosphere (Lloyd and Taylor 1994; Sponseller 2007). Many researchers have shown that there are large spatio-temporal variations in soil respiration (Mäkiranta et al. 2008; Xu and Qi 2008; Ceccon et al. 2011) and that these are influenced by both biotic and abiotic factors (Luan et al. 2012). Soil temperature and moisture conditions are generally considered to be the two major environmental factors that are closely related to soil respiration (Davidson et al. 1998; Shi et al. 2011; Inoue and Koizumi 2012). However, the extent of their effects and contributions to soil respiration vary greatly depending on variations in the seasonal climate and soil conditions, especially in regions with pronounced wet–dry cycles. In a seasonally dry tropic ecosystem, respiratory activities in the soil are mostly confined to a relatively short wet season, while much lower respiration rates occur during the dry season (Holt et al. 1990). Similar results were also found in a tropical monsoon forest in northern Thailand (Hashimoto et al. 2004). In temperate forests, however, soil respiration changes seasonally with soil temperature, often decreasing with increasing soil water content in the summer (Xu et al. 2011). Therefore, there is a large variation in soil respiration due to changing environmental conditions (i.e., temperature, moisture) (Tang and Baldocchi 2005), and this leads to a poor understanding and inaccurate estimates of soil carbon fluxes in many forest ecosystems.
A number of biological factors, such as spatial distribution of individual trees (Luan et al. 2012), leaf area and litter production (Reichstein et al. 2003), and canopy cover (Concilio et al. 2005; Tedeschi et al. 2005; McCarthy and Brown 2006), can also induce temporal and spatial heterogeneity/variation in soil respiration. Variation in tree canopy cover can change soil microclimatic environment, soil physicochemical properties as well as soil carbon input and accumulation (Aponte et al. 2010; Zhang et al. 2011; Cahoon et al. 2012). For example, variations in soil temperature and moisture within the forest ecosystem are very likely caused by canopy cover due to canopy shading and rainfall interception, especially for a small precipitation event (Cable and Huxman 2004; McCarthy and Brown 2006; Potts et al. 2006b; Emmerich and Verdugo 2008). The percentage of canopy cover has also been linked to leaf litter depth, root respiration, and microbial respiration in an oak–hickory forest ecosystem (Hogberg et al. 2001; McCarthy and Brown 2006). In a Savanna ecosystem, Tang and Baldocchi (2005) found that soil respiration under tree canopies decreased with distance from its base. However, the effects of canopy cover on soil respiration and to what extent the canopy effect varies with canopy size and tree species are still unknown. Although, many studies have shown that soil temperature and water availability are key factors influencing leaf photosynthesis and soil respiration (Huxman et al. 2004; Emmerich and Verdugo 2008; Shi et al. 2011), few studies have demonstrated that canopy structure in terms of canopy cover size, leaf area and leaf litter-fall have, to some degree, have an effect on soil temperature and soil water availability, in addition to topography and soil texture. Therefore, there is a need to study the effects of canopy cover of different tree species on soil respiration and to understand to what extent these effects are the result of soil temperature and soil water content as also influenced by canopy cover.
This study was designed to explore variations in soil respiration under different canopy covers with changing soil moisture conditions in a temperate coniferous (Pinus armandii Franch) and broadleaved (Quercus aliena var. acuteserrata Max.) mixed forest ecosystem in central China. We hypothesized that soil respiration would vary with different tree canopy covers in forest ecosystems and that the effect of canopy size on soil respiration would depends on tree species and seasonal dry–wet transition. We assumed that the variation in soil respiration under different canopy covers is largely affected by soil microclimate rather than soil carbon input (litter and fine root biomass).
Materials and methods
Site description
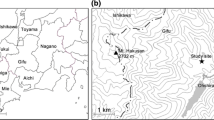
The study site is located at the Baotianman Forest Ecological Research Station in Baotianman National Nature Reserve (111°47′–112°04′E, 33°20′–33°36′N), Henan Province, PR. China. The altitude ranges from 600 to 1800 m a.s.l.. Mean annual air temperature is 15.1 °C, with monthly air temperature ranging from 1.5 °C in January to 27.8 °C in July. Mean annual precipitation is approximately 885.6 mm, of which 60 % falls in summer (from July to August). Upland soils are dominated by Haplic Luvisol (Luan et al. 2011).
The typical forest in this area is the warm-temperate forest composed mainly of temperate deciduous broadleaf trees, including some sub-tropical tree species due to its transitional location from the northern subtropical to warm-temperate climate. The dominant tree species of the warm-temperate forest is Quercus spp., accounting for approximately 70 % of the total area (Song 1994). The vertical distribution of forest communities includes Q. variabilis BI. forest at 600–1,200 m a.s.l., Q. glandulifera var. brevipetiolata (A. DC.) Nakai forest at 1,200–1,400 m a.s.l., and Q. aliena forest at >1,300 m a.s.l. In addition, several tree species are widely distributed among the interspaces of the oak forests, with the dominant species being Toxicodendron vernicifluum (Stokes) F. A. Barkley, Carpinus cordata BI, Acer spp., Platycarya strobilacea Sieb. Et. Zucc. and Pinus armandii Franch, and the secondary species being Custanea seguinii Dode, Lindera obtusiloba BI., Catalpa ovaata G. Don, and Celtis spp..
Experimental design
Experiments were carried out in a coniferous and broadleaf mixed temperate forest, about 400 m away from a weather station in the Baotianman Forest Ecological Research Station. The mixed forest covers nearly 1.5 ha (watershed area) with an average slope of 14°. All woody species within the watershed area were surveyed and recorded, including diameter at breast height (DBH), height, crown width, crown depth, and health status. The dominant species were P. armandii and Q. aliena (average DBH 23.6 ± 7.90 and 19.1 ± 8.62 cm, respectively), and these accounted for 30.4 and 34.3 % of the total canopy coverage, respectively (Table 1). The canopies were classified into two sizes: a small class and a large class. The mean canopy width of the ‘small class’ trees (DBH 15–20 cm) ranged from 1.5 to 3.0 m and that of the ‘large class’ trees (DBH 30–35 cm) ranged from 5.0 to 7.5 m. ‘Small class’ (S) trees were the most predominant in terms of density (32.6 %) and canopy coverage (35.8 %); in comparison ‘large class’ (L) trees accounted for 26.4 % of canopy coverage (Table 1). The sample trees were selected based on the following criteria: (1) healthy trees with isolated crown and exclusivity within a radius of 4 m of the sample trees; (2) similar site conditions but with a distance >15 m away from each other. The 16 sampled trees from two different canopy size classes (four replicates for each size class) for P. armandii (PS, PL) and Q. aliena (QS, QL) were randomly selected in the experiment stand (Table 1; Fig. 1). The canopy diameter of each selected sample tree was estimated from two horizontal directions (N–S, E–W) with a meter stick (Table 1; Fig. 1). The crown depth of each sample tree was calculated by the difference between the tree height and the crown base height (under the lowest whorl growing on living branches). In addition, four inter-plant gaps (IG) within the mixed forest, without canopy cover, were randomly selected for comparative measurements; these were dominated by vivacious gramineous species (Podiopogon ramosus, Carex siderosticta Hance) and wormwood (Artemisia argyi Levl. Et Vant., A. apiacea).
Soil water condition was defined as the two levels according to soil volumetric water content (v/v %) that were mainly affected by precipitation events. From May to June 2011, soil water availability was extremely low (<10 %v/v) due to no effective rainfall events (>1 mm) for nearly 40 days; this period was therefore defined as the dry condition. From June 22 to August 2, there were a total of seven rainfall events (June 20, 5.81 mm; June 24, 7.21 mm; June 27, 7.17 mm; June 30, 14.67 mm; July 10, 20.57 mm; July 19, 24.19 mm; August 2, 16.13 mm), and soil water content increased quickly following each rainfall event. This period was therefore defined as the wet condition.
Soil respiration measurement
Soil respiration (CO2 efflux) was measured using a soil respiration system (LI-8100; LI-COR, Lincoln, NB) from May to August in 2011. All measurements were made in the afternoon (13:30–17:00 pm) and executed in 2-min intervals with a 20 s deadband. Three polyvinyl chloride (PVC) collars (inside diameter 19.6 cm, height 8 cm) were inserted into the forest floor down to a depth of 5 cm around each sampled tree at an angle of 120° with 1-m distance from the bole. One PVC collar was also installed at the center of each IG. In total, 52 PVC collars were installed under different canopy cover conditions. All collars were left at the site for the whole study period; no living plants or litter were left in each collar during the whole period.
In the dry condition, soil respiration was expressed as the average value of four measurements made on May 15, May 25, June 10 and June 18, respectively. In the wet condition, soil respiration was the average of the seven measurements that were made on the day following each rainfall event. To avoid possible data variations induced by the order of making the measurements, we measured soil respiration in varying order, with sampled trees from different canopy types crossing each other one by one (PS, PL, QS, QL, and IG).
Measurements of soil temperature and moisture
Soil temperature at a soil depth of 5 cm was measured adjacent to each respiration collar with a portable temperature probe provided with the LI-8100 meter. Soil volumetric water content (VWC) at a depth of 0–5 cm was measured with a portable time domain reflectometer MPKit-B soil moisture gauge (NTZT Inc., Nantong, China) at three points close to each collar. Hourly precipitation data were collected from a nearby weather station about 400 m away from the study site.
Litter biomass and root biomass
Fresh litter biomass was collected from three leaf litter traps (50 × 50 cm) beneath each tree canopy within 1 month (November 2011). At each soil respiration collar a 20 × 20-cm square was sampled from the organic layers to a depth of 20 cm in September to estimate root biomass (Rodeghiero and Cescatti 2006). All soil samples were washed and manually sorted to separate living fine roots (<2 mm) from the dead based on morphology and color, and then measured after drying at 70 °C for 72 h.
Statistical analysis
Field data were first tested for normality and homogeneity of variance, then data from different canopy size classes were tested by one-way analysis of variance (ANOVA) with least significant difference (LSD) multiple comparison test per tree species under different soil moisture conditions. Repeated measures ANOVAs (RMANOVA) were used to test the statistical significance of soil respiration rate, soil volumetric water content and soil temperature in samples collected at a soil depth of 5 cm on different measurement dates. Regression analysis was used to determine the relationships between soil respiration and soil temperature and soil moisture. We used piecewise regression to model the relationship between soil respiration and soil moisture. Stepwise multiple regression analysis and collinearity diagnostics were then used to determine the relationships between soil respiration and each of the following variables: soil temperature, soil moisture, fine root biomass, litter biomass, and canopy sizes. The statistical analyses were performed in SPSS 16.0 for Windows (SPSS Inc., Chicago, IL).
Results
Soil microclimate under different canopy conditions
There were strong seasonal fluctuations in soil temperature and moisture during the transition from dry to wet periods (Fig. 2a, b) (P < 0.0001, RMANOVA). Regardless of the canopy cover of the different tree species, soil temperature was lower in the dry condition than in the wet condition, while soil moisture increased significantly during the dry to wet transition (Fig. 2a, b) (P < 0.0001, RMANOVA). Soil temperature in the IG was 10.4–18.6 % higher than that for the PS, PL, QS, and QL in both the dry and wet soil conditions (Fig. 3a; P < 0.05). In the dry condition, soil temperature under the PS canopy was 0.21 °C higher than that under the PL canopy (Fig. 3a; P < 0.05), while in the wet condition, soil temperature was 0.28 °C lower under the PS canopy than under the PL canopy (Fig. 3a; P < 0.05). Similar soil temperature patterns occurred under the QS and QL canopies during the whole study period in both dry and wet soil conditions (Fig. 3a; P > 0.05). Overall, regardless of canopy size and soil water condition, a significantly higher soil temperature was observed under P. armandii trees than under Q. aliena trees (Fig. 3a).
Change patterns of soil temperature (T 5 , a) and soil volumetric water content (VWC) (b) at a soil depth of 5 cm and soil respiration (Rs, c) under different canopy conditions during the investigation period. PS, PL P. armandii with small canopy size and large canopy size, respectively, QS, QL Q. aliena with small canopy size and large canopy size, respectively Q. aliena with small canopy sizes and large sizes
Mean values of soil temperature (a) and VWC (b) at a soil depth of 5 cm and soil respiration (c) for different canopy covers in the dry and wet conditions. Data are presented as the mean ± standard deviation (SD) (n = 4). Different lowercase letters indicate significant differences at P < 0.05 among different canopy covers (PS, PL, QS, QL, and IG)
Soil VWC was consistently greater (23.6–82.4 %) in the IG than that under the PS, PL, QS and QL canopies for both dry and wet soil conditions (Fig. 3b; P < 0.05). There were no statistical differences in soil VWC between canopy sizes for both Q. aliena and P. armandii in the dry soil condition (Fig. 3b; P > 0.05). In the wet soil condition, soil VWC was 11.3–20.8 % higher under the QS canopy than under the QL canopy (P < 0.05). In the dry soil condition, the mean soil VWC was 17–28 % lower under the small canopy-sized classes (PS and QS) than that under large ones (PL and QL) (Fig. 3b).
Relative changes in soil temperature were significantly lower under the PS canopy than under the PL canopy during the dry–wet transition, while no significant difference occurred between QS and QL (Table 2). Changes in soil moisture (VWC) under the small canopy-sized classes (PS, QS) were significantly greater than those under the large ones (PL, QL) (Table 2; P < 0.05).
Variations in soil respiration under different canopy sizes and soil water contents
Soil respiration under different canopy covers was higher in the wet condition than in dry condition (Fig. 2c). For P. armandii, soil respiration was 2.4–8.1 % higher under the PS canopy than under the PL canopy, and differences in soil respiration between canopy size classes were significant in the dry soil condition (Fig. 3c; P < 0.05), but not in wet condition (Fig. 3c; P > 0.05). For Q. aliena, however, soil respiration under the QS canopy was significantly lower than that under the large canopy (QL) in both the dry (33.5 %) and wet soil conditions (35.8 %) (Fig. 3c; P < 0.05). Soil respiration was significantly higher in the IG than under the QS canopy (P < 0.05) in the dry condition (Fig. 3) and was 13.4, 13.8, and 32.0 % higher in the IG than under the PS, PL, and QS canopies in the wet condition (Fig. 3c, P < 0.05), respectively.
Larger increases in soil respiration occurred under the large canopy cover (PL, QL) relative to under the small ones (PS, QS) (Table 2), and the increase in soil respiration was significantly lower under the P. armandii (9.7–17.7 %) canopy than under the Q. aliena canopy (25.9–31.5 %) regardless of canopy size (Table 2; P < 0.05).
Correlation of soil respiration with biotic and abiotic factors in relation to canopy cover
Soil respiration rate was negatively correlated with soil temperature under canopy cover regardless of tree species and canopy size (Fig. 4a, c, e), with significantly higher correlations under the large canopy sizes (Fig. 4a, c; PL P = 0.02; QL P = 0.02) than under the small ones (Fig. 4a, c; PS P = 0.04; QS P = 0.06). In contrast, a marginally significant correlation between soil respiration and soil temperature occurred in the IG (Fig. 4e; P = 0.08).
A positive correlation between soil moisture and soil respiration occurred in the dry soil condition without effective rainfall, while a negative relationship occurred when soil moisture exceeded the thresholds (17.6, 18.3, 18.2, 14.8, 31.5 % for PS, PL, QS, QL, and IG, respectively (Fig. 4b, d, f).
Based on the multiple regression analysis (Table 3), soil moisture and soil temperature accounted for 71.2 % of the variation in soil respiration under the Q. aliena canopy, while canopy sizes and litter biomass explained only 25.7 % of the variation. Among the variables, soil temperature had the closest relationship with soil respiration (52.8 % of the variance) for P. armandii, and fine root biomass explained 4.5 % of the variation. Regardless of tree species, soil temperature and soil moisture accounted for 22.7 and 36.5 % of the variation in soil respiration, respectively. For biotic factors, tree canopy size and litter biomass explained 4.5 and 15.1 %, respectively, of the variation in soil respiration (Table 3). Considering the collinearity of variables, 31 % of the variance in soil respiration was explained by canopy size, while 65 % of variance was co-shared by one or several factors of soil temperature, soil moisture, and litter biomass.
Discussion
Canopy covers affect soil respiration by regulating soil microclimate
The differences we observed in soil respiration under the canopies of different sizes and of different tree species indicate that there was a large spatial heterogeneity in soil respiration due primarily to variability of soil environmental conditions modulated by the aboveground canopy structure and plant distribution. Thus, our findings support our first hypothesis that soil respiration will vary with canopy cover and that the effects of canopy cover on soil respiration depend on tree species and the seasonal dry–wet transition. Variations in soil temperature and moisture induced by canopy cover play a greater role in affecting soil respiration than the effects of leaf litter and fine root biomass (Table 3). Q. aliena has a greater canopy width but smaller crown depth than P. armandii (Table 1), and, therefore, a lower canopy interception under the canopy of Q. aliena, leading to a relatively higher soil water availability prone to microbial metabolism (Xu et al. 2010) compared to that under the P. armandii canopy.
As described by Shi et al. (2011), the slope of linear regression between soil respiration and soil temperature can be used to interpret the sensitivity of soil respiration to temperature. We therefore assumed that soil respiration beneath the large canopies is more sensitive to temperature than that under the small ones for both P. armandii and Q. aliena (Fig. 4). Previous studies generally report a positive relationship between soil temperature and soil respiration. In our study, however, negatively linear relationships between soil respiration rate and soil temperature were found for both tree species beneath the canopies and the IGs (Fig. 4). Similar results were also reported by Xu and Wan (2008) and Shi et al. (2011). Two factors may explain our results. First, the measured soil temperature underwent only a small variation during the dry–wet transition, showing that soil moisture, relative to soil temperature, plays a dominant role in regulating soil respiration during the transition process. Secondly, high soil temperature is generally accompanied by low soil moisture, and temperature exerts a suppressive effect on soil respiration under soil water deficit conditions (Wan and Luo 2003; Inoue and Koizumi 2012).
The relationship between soil respiration and soil moisture according to piecewise regression suggests a significant effect of soil moisture on soil respiration, especially under the large canopy size classes (Fig. 4). This may partly explain why a higher soil respiration occurred beneath large canopy covers. In our study, increasing soil moisture during the transition from dry to wet soil conditions was accompanied by increasing soil respiration, which may be attributable to increased microbial activity (Ryan and Law 2005; Carbone et al. 2011). However, the opposite change occurred with a subsequent continuous increase in soil moisture in parallel with declining soil temperature (Sponseller 2007). This observation is in accordance with results from previous studies showing that soil water content is negatively correlated with soil respiration at moderate to high water content (Davidson et al. 1998; Sponseller 2007), probably due to an evolutionary adaptation of soil microorganisms to a long-time mean meteorological situation. Moreover, it has long been established that rates of aerobic processes in soils increase with water content up to the point at which microbial activity becomes limited by the diffusion of oxygen through water-filled pore spaces (Miller and Johnson 1964). Some studies have found that approximately 60 % of the water-holding capacity was the threshold at which soil CO2 production begins to decline with increased soil water content (Shi et al. 2011). Our findings support the notion that soil moisture availability is more important than soil temperature in regulating soil respiration under dry soil conditions (Thomey et al. 2011).
Canopy cover influences soil respiration by changing leaf litter and fine root biomass
Biotic factors such as fine root biomass and stand structure parameters have significant effects on the spatial variation of soil respiration in the study area (Luan et al. 2012). Litterfall is a key biological pathway for element transfer from vegetation to soils, acting as a major source of soil organic matter (SOM). The light fraction of SOM is a direct substrate of microbial respiration and has been found to accumulate under the canopy of Q. aliena (Luan et al. 2012), since the leaf litter of broadleaved species is much easier to decompose into soils than that of coniferous species (Kuiters and Sarink 1986; Lin et al. 2004; Wu et al. 2009). Deciduous trees have also been shown to have a faster decay rate of litter than pine trees (Jandl et al. 2007; Wang et al. 2010), and the rapid turnover of leaf litter could directly contribute to increased soil carbon content (Cheng et al. 2003; Lin et al. 2004; Wu et al. 2009). For the large-sized class of P. armandii, greater leaf litter biomass (Fig. 5) is likely to allow less water infiltration due to forest floor interception (Cheng et al. 2003), which may partly explain the differences in soil respiration between the PS and PL canopies. With respect to broadleaved trees of Q. aliena, however, a greater leaf litter (Fig. 5) could directly contribute to increased soil carbon content due to its rapid carbon decomposition and turnover rate (Cheng et al. 2003; Lin et al. 2004; Wu et al. 2009). Thus, higher soil respiration under the QL canopy is likely caused by greater substrate availability resulting from inputs of root production and leaf litter (Raich and Tufekciogul 2000; Tomotsune et al. 2013).
Fresh litter biomass and fine root biomass at a depth of 0–20 cm beneath the canopies of the different species and different canopy size classes. Data are presented at the mean ± SD (n = 4). Different lowercase letters indicate significant differences (P < 0.05) between different canopy covers (PS, PL, QS, and QL)
Multi-regression analysis revealed that litter biomass had larger effects on soil respiration than fine root biomass. Thus, the contribution of stimulated heterotrophic respiration to the total soil respiration was likely a reason for the higher soil respiration under the Q. aliena canopy. In addition, increased soil respiration in the wet condition may also be primarily due to stimulated microbial respiration; for example, Huxman et al. (2004) found that the response of heterotrophic respiration may be more quick and sensitive to biophysical environments during the transition from the dry to wet condition. Coniferous species with shallow roots tend to accumulate SOM in the forest floor, but less in the mineral soil, compared with deciduous trees (Jandl et al. 2007). Therefore, we assumed that the effect of fine root biomass and corresponding processes on soil respiration may be greater under the P. armandii canopy than under the Q. aliena canopy (Fig. 5).
Stimulated soil respiration during the soil dry–wet transition
Soil respiration under different soil water conditions has been widely studied in a variety of ecosystems (Holt et al. 1990; Fang et al. 1998; Dong et al. 2002; Hashimoto et al. 2004; Lee et al. 2009). Many studies have shown that soil respiration increases with increasing soil moisture (Sponseller 2007; Munson et al. 2010; Shi et al. 2011; Thomey et al. 2011). In addition, some studies have also reported a significant decline of soil respiration after rainfall (Ball et al. 1999).
In our study, we found an elevated soil respiration during the transition from dry to wet conditions; for example, higher increases in soil respirations were found under large canopy covers for both Q. aliena and P. armandii, possibly attributable to a rapid upregulation of soil microbe activity and physical displacement of CO2 from soil pores following shallow soil wetting (Potts et al. 2006a; Sponseller 2007; Thomey et al. 2011). Pore space in soil and litter can store an amount of CO2 roughly equal to 1 day’s production; therefore, emptying or filling the pore space may enhance or diminish the measured flux relative to biological production (Ryan and Law 2005). In addition, changes in soil respiration in the wet condition were greater in the IG than beneath canopy covers (Table 2), due partly to the stimulation of soil microbe activities (Chen et al. 2008) resulting from higher soil water availability in the IG (Fig. 2). However, Potts et al. (2008) recently reported that open areas in forests tended to have consistently smaller soil respiration than the sub-canopy (beneath the canopy), particularly after the monsoon period. Differences in soil types, the quality and quantity of litter, and the type of climate may (partly) explain these inconsistent results (Jandl et al. 2007).
Temporarily stimulated soil respiration during the dry–wet transition is a small relative to the annual carbon loss, but it may play an important role in affecting ecosystem carbon balance because there is a high occurrence frequency of short-term dry–wet cycles in the study region. Based on local meteorological observations from 2003 to 2010, rainfall events of <5 mm accounted for approximately 60 % of the total precipitation events, which is considered to be the lowest threshold for plant response to soil water availability (Reynolds et al. 2004). Based on this threshold, about 60 % of the precipitation events are too small to induce plant responses, but they are sufficient to elicit microbe-based CO2 losses. This means that a greater soil CO2 emission could be induced as the frequent dry–wet alternation can produce larger accumulating effects through each relatively small magnitude of increased CO2 emission (9.7–32.2 %) and, therefore, the forest ecosystem under dry–wet cycles might act as net sources of CO2 to the atmosphere (Sponseller 2007).
Conclusions
In our study canopy cover had direct and indirect effects on soil respiration under the tree canopy by changing soil biotic and abiotic conditions. Variations in soil respiration under canopy covers depended on tree species and seasonal soil water availability, and soil microclimate had larger effects on soil respiration than a number of biotic factors indirectly regulated by canopy cover. Our findings suggest that characteristics of the canopy morphology (e.g., canopy width, canopy depth, size class, leaf shape) of tree species play an important role in regulating soil respiration by directly and indirectly altering soil environmental factors. An increased number of frequent soil dry–wet transition cycles could stimulate soil respiration and greatly affect ecosystem carbon balance. Therefore, spatial variations in soil respiration induced by canopy structure and soil water condition need to be taken account in assessments of the forest carbon budget.
References
Aponte C, Marañón T, García LV (2010) Microbial C, N and P in soils of Mediterranean oak forests: influence of season, canopy cover and soil depth. Biogeochemistry 101:77–92
Ball BC, Scott A, Parker JP (1999) Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Till Res 53:29–39
Cable JM, Huxman TE (2004) Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia 141:317–324
Cahoon SM, Sullivan PF, Shaver GR, Welker JM, Post E (2012) Interactions among shrub cover and the soil microclimate may determine future Arctic carbon budgets. Ecol Lett 15:1415–1422
Carbone MS, Still CJ, Ambrose AR, Dawson TE, Williams AP, Boot CM, Schimel JP (2011) Seasonal and episodic moisture controls on plant and microbial contributions to soil respiration. Oecologia 167:265–278
Ceccon C, Panzacchi P, Scandellari F, Prandi L, Ventura M, Russo B, Millard P, Tagliavini M (2011) Spatial and temporal effects of soil temperature and moisture and the relation to fine root density on root and soil respiration in a mature apple orchard. Plant Soil 342:195–206
Chen S, Lin G, Huang J, He M (2008) Responses of soil respiration to simulated precipitation pulses in semiarid steppe under different grazing regimes. J Plant Ecol 1:237–246
Cheng JH, Zhang HJ, Shi YH, Pan L, Qi SL, Cheng Y, He F (2003) Hydrological process of litter on forest stands in the Three Gorges area. J BJ For Univ 25:8–13
Concilio A, Ma S, Li Q, LeMoine J, Chen J, North M, Moorhead D, Jensen R (2005) Soil respiration response to prescribed burning and thinning in mixed-conifer and hardwood forests. Can J For Res 35:1581–1591
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Change Biol 4:217–227
Davidson EA, Richardson AD, Savage KE, Hollinger DY (2006) A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Glob Change Biol 12:230–239
Dong Y, Scharffe D, Lobert J, Crutzen P, Sanhueza E (2002) Fluxes of CO2, CH4 and N2O from a temperate forest soil: the effects of leaves and humus layers. Tellus B 50:243–252
Emmerich WE, Verdugo CL (2008) Precipitation thresholds for CO2 uptake in grass and shrub plant communities on Walnut Gulch Experimental Watershed. Water Resour Res 44:W05S16
Fang C, Moncrieff JB, Gholz HL, Clark KL (1998) Soil CO2 efflux and its spatial variation in a Florida slash pine plantation. Plant Soil 205:135–146
Hashimoto S, Tanaka N, Suzuki M, Inoue A, Takizawa H, Kosaka I, Tanaka K, Tantasirin C, Tangtham N (2004) Soil respiration and soil CO2 concentration in a tropical forest, Thailand. J For Res 9:75–79
Hogberg P, Nordgren A, Buchmann N, Taylor AF, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Holt J, Hodgen M, Lamb D (1990) Soil respiration in the seasonally dry tropics near Townsville, North-Queensland. Soil Res 28:737–745
Huxman TE, Snyder KA, Tissue D, Leffler AJ, Ogle K, Pockman WT, Sandquist DR, Potts DL, Schwinning S (2004) Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141:254–268
Inoue T, Koizumi H (2012) Effects of environmental factors upon variation in soil respiration of a Zoysia japonica grassland, central Japan. Ecol Res 27:445–452
Jandl R, Lindner M, Vesterdal L, Bauwens B, Baritz R, Hagedorn F, Johnson DW, Minkkinen K, Byrne KA (2007) How strongly can forest management influence soil carbon sequestration? Geoderma 137:253–268
Kuiters A, Sarink H (1986) Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biol Biochem 18:475–480
Lee X, Wu HJ, Sigler J, Oishi C, Siccama T (2009) Rapid and transient response of soil respiration to rain. Glob Change Biol 10:1017–1026
Li Q, Chen J, Moorhead DL (2012) Respiratory carbon losses in a managed oak forest ecosystem. For Ecol Manage 279:1–10
Lin B, Liu Q, Wu Y, He H (2004) Advances in the studies of forest litter. Chin J Ecol 23:60–65 (in Chinese)
Lloyd J, Taylor J (1994) On the temperature dependence of soil respiration. Function Ecol 8:315–323
Luan J, Liu S, Wang J, Zhu X, Shi Z (2011) Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol Biochem 43:503–512
Luan J, Liu S, Zhu X, Wang J, Liu K (2012) Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biol Biochem 44:143–150
Mäkiranta P, Minkkinen K, Hytönen J, Laine J (2008) Factors causing temporal and spatial variation in heterotrophic and rhizospheric components of soil respiration in afforested organic soil croplands in Finland. Soil Biol Biochem 40:1592–1600
McCarthy DR, Brown KJ (2006) Soil respiration responses to topography, canopy cover, and prescribed burning in an oak-hickory forest in southeastern Ohio. For Ecol Manage 237:94–102
Miller RD, Johnson D (1964) The effect of soil moisture tension on carbon dioxide evolution, nitrification, and nitrogen mineralization. Soil Sci Soc Am Proc 24:644–647
Munson SM, Benton TJ, Lauenroth WK, Burke IC (2010) Soil carbon flux following pulse precipitation events in the shortgrass steppe. Ecol Res 25:205–211
Potts DL, Huxman TE, Cable JM, English NB, Ignace DD, Eilts JA, Mason MJ, Weltzin JF, Williams DG (2006a) Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in a semi-arid grassland. New Phytol 170:849–860
Potts DL, Huxman TE, Scott R, Williams DG, Goodrich D (2006b) The sensitivity of ecosystem carbon exchange to seasonal precipitation and woody plant encroachment. Oecologia 150:453–463
Potts DL, Scott RL, Cable JM, Huxman TE, Williams DG (2008) Sensitivity of mesquite shrubland CO2 exchange to precipitation in contrasting landscape settings. Ecology 89:2900–2910
Raich JW, Tufekciogul A (2000) Vegetation and soil respiration: correlations and controls. Biogeochemistry 48:71–90
Reichstein M, Rey A, Freibauer A, Tenhunen J, Valentini R, Banza J, Casals P, Cheng Y, Grünzweig JM, Irvine J (2003) Modeling temporal and large-scale spatial variability of soil respiration from soil water availability, temperature and vegetation productivity indices. Global Biogeochem Cycles 17:1104
Reynolds JF, Kemp PR, Ogle K, Fernández RJ (2004) Modifying the ‘pulse–reserve’ paradigm for deserts of North America: precipitation pulses, soil water, and plant responses. Oecologia 141:194–210
Rodeghiero M, Cescatti A (2006) Indirect partitioning of soil respiration in a series of evergreen forest ecosystems. Plant Soil 284:7–22
Ryan MG, Law BE (2005) Interpreting, measuring, and modeling soil respiration. Biogeochemistry 73:3–27
Shi WY, Tateno R, Zhang JG, Wang YL, Yamanaka N, Du S (2011) Response of soil respiration to precipitation during the dry season in two typical forest stands in the forest–grassland transition zone of the Loess Plateau. Agric For Meteorol 151:854–863
Song CS (1994) Scientific survey of the Baotianman Mountial national nature reserve. China Forestry publishing House, Beijing (in Chinese)
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob Change Biol 13:426–436
Tang J, Baldocchi DD (2005) Spatial–temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 73:183–207
Tedeschi V, Rey A, Manca G, Valentini R, Jarvis PG, Borghetti M (2005) Soil respiration in a Mediterranean oak forest at different developmental stages after coppicing. Glob Change Biol 12:110–121
Thomey ML, Collins SL, Vargas R, Johnson JE, Brown RF, Natvig DO, Friggens MT (2011) Effect of precipitation variability on net primary production and soil respiration in a Chihuahuan Desert grassland. Glob Change Biol 17:1505–1515
Tomotsune M, Yoshitake S, Watanabe S, Koizumi H (2013) Separation of root and heterotrophic respiration within soil respiration by trenching, root biomass regression, and root excising methods in a cool-temperate deciduous forest in Japan. Ecol Res 28:259–269
Wan S, Luo Y (2003) Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochem Cycles 17:1054
Wang H, Liu SR, Mo JM, Wang JX, Franz M, Maria W (2010) Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol Res 25:1071–1079
Wu YX, Han SJ, Lin L (2009) Litterfall decomposition in four forest types in Changbai Mountains of China. Chin J Ecol 28:400–404 (in Chinese)
Xu M, Qi Y (2008) Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob Change Biol 7:667–677
Xu W, Wan S (2008) Water-and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semiarid grassland in northern China. Soil Biol Biochem 40:679–687
Xu LH, Shi ZJ, Wang YH, Xiong P, Yu PH (2010) Canopy interception characteristics of main vegetation types in LiuPan Mountain of China. Chin J Appl Ecol 21:2487–2493 (in Chinese)
Xu J, Chen J, Brosofske K, Li Q, Weintraub M, Henderson R, Wilske B, John R, Jensen R, Li H (2011) Influence of timber harvesting alternatives on forest soil respiration and its biophysical regulatory factors over a 5-year period in the Missouri Ozarks. Ecosystems 14:1310–1327
Zhang J, Shangguan T, Meng Z (2011) Changes in soil carbon flux and carbon stock over a rotation of poplar plantations in northwest China. Ecol Res 26:153–161
Acknowledgments
We gratefully acknowledge the support from the Baotianman Forest Ecological Research Station for field monitoring and sampling. We also thank Drs. Junwei Luan, Damon Scott Hartley, and two anonymous reviewers for their valuable comments and suggestions on the manuscript. This research was jointly supported by China National Science Foundation (No. 31290223) and the Special Research Program for Public-Welfare Forestry (No. 201104006, 200804001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, Y., Liu, S., Wang, J. et al. Variation in soil respiration under the tree canopy in a temperate mixed forest, central China, under different soil water conditions . Ecol Res 29, 133–142 (2014). https://doi.org/10.1007/s11284-013-1110-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-013-1110-5