Abstract
Shiga toxin-producing and Enteropathogenic Escherichia coli are foodborne pathogens commonly associated with diarrheal disease in humans. This study investigated the presence of STEC and EPEC in 771 dairy cattle fecal samples which were collected from 5 abattoirs and 9 dairy farms in South Africa. STEC and EPEC were detected, isolated and identified using culture and PCR. Furthermore, 339 STEC and 136 EPEC isolates were characterized by serotype and major virulence genes including stx1, stx2, eaeA and hlyA and the presence of eaeA and bfpA in EPEC. PCR screening of bacterial sweeps which were grown from fecal samples revealed that 42.2% and 23.3% were STEC and EPEC positive, respectively. PCR serotyping of 339 STEC and 136 EPEC isolates revealed 53 different STEC and 19 EPEC serotypes, respectively. The three most frequent STEC serotypes were O82:H8, OgX18:H2, and O157:H7. Only 10% of the isolates were classified as “Top 7” STEC serotypes: O26:H2, 0.3%; O26:H11, 3.2%; O103:H8, 0.6%; and O157:H7, 5.9%. The three most frequent EPEC serotypes were O10:H2, OgN9:H28, and O26:H11. The distribution of major virulence genes among the 339 STEC isolates was as follows: stx1, 72.9%; stx2, 85.7%; eaeA, 13.6% and hlyA, 69.9%. All the 136 EPEC isolates were eaeA-positive but bfpA-negative, while 46.5% carried hlyA. This study revealed that dairy cattle are a major reservoir of STEC and EPEC in South Africa. Further comparative studies of cattle and human STEC and EPEC isolates will be needed to determine the role played by dairy cattle STEC and EPEC in the occurrence of foodborne disease in humans.Please kindly check and confirm the country and city name in affiliation [6].This affiliation is correct.Please kindly check and confirm the affiliationsConfirmed. All Affiliations are accurate
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shiga toxin-producing Escherichia coli (STEC) and Enteropathogenic Escherichia coli (EPEC) are enteric pathogens commonly associated with diarrheal disease in humans. STEC disease is characterized by mild to severe diarrhea and complications such as hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS). EPEC is one of the leading causes of acute secretory watery diarrhea in children less than two years of age, worldwide, particularly in developing countries (GDB 2018).
Ruminants including cattle are the main animal reservoirs of STEC and EPEC (Habets et al. 2020; Hussein & Sakuma 2005a, b; Singh et al. 2015). Both STEC and EPEC are shed in the feces of cattle which may end up contaminating food products, water and the environment (Farrokh et al. 2013; Kintz et al. 2017). The occurrence of STEC and EPEC in cattle can be influenced by a number of factors, including the age of the animal, diet type, season and management practices (Cray et al. 1998; Gunn et al. 2007; Venegas-Vargas et al. 2016). Humans can acquire STEC and/or EPEC infections by ingesting contaminated beef or dairy products, vegetables or water (Hernandes et al. 2009; Pires et al. 2019). Furthermore, both STEC and EPEC can be transmitted to humans through contact with infected animals and a contaminated environment or from person to person (Hernandes et al. 2009; Kintz et al. 2017).
The main virulence factors of STEC are bacteriophage-encoded Shiga toxins (Stx1 and Stx2) (O'Brien et al. 1983; Strockbine et al. 1986). Another major virulence factor of STEC and EPEC is intimin (eaeA) (Jerse et al. 1990; McDaniel et al. 1995; Tzipori et al. 1995). Intimin is an adhesin responsible for attachment of STEC and EPEC to enterocytes and formation of typical attaching and effacing lesions observed in the intestinal epithelium of humans or animal models infected with eaeA-positive STEC or EPEC strains (Jerse et al. 1990; McDaniel et al. 1995; Tzipori et al. 1995). Furthermore, STEC and EPEC may carry a plasmid-encoded hemolysin (hlyA) which has been associated with enhanced virulence and severe infection. (Schmidt & Karch 1996).
While STEC and EPEC may share identical virulence factors (eaeA and hlyA), EPEC lacks bacteriophage-encoded Shiga toxins. Specifically, some EPEC strains possess a bundle-forming pilus (bfpA) which is located on the EPEC adherence factor (EAF) virulence plasmid (Girón et al. 1991, 1993). Bfp is required for the typical localized adherence (LA) phenotype commonly observed in the intestinal epithelium of animal models experimentally infected with bfp-positive EPEC strains (Cleary et al. 2004; Tobe & Sasakawa 2001). EPEC strains that possess bfpA are “typical EPEC” (tEPEC), while bfpA-negative EPEC are termed “atypical EPEC” (aEPEC) (Kaper et al. 2004; Trabulsi et al. 2002). Humans are the main source and reservoir of typical EPEC strains, while humans and healthy and/or diseased animals are considered sources and reservoirs of atypical EPEC strains (Hernandes et al. 2009; Hu & Torres 2015).
Although STEC and EPEC have been previously detected in cattle and associated with human disease, worldwide (Alfinete et al. 2022; GDB 2018; Habets et al. 2020; Karama et al. 2019a; Mainga et al. 2018; Pires et al. 2019; Smith et al. 2019), studies on the occurrence and characteristics of STEC from dairy cattle in South Africa are scarce, while similar reports on EPEC are non-existent. Therefore, the objectives of this study were (i) to determine the occurrence of STEC and EPEC in dairy cattle in South Africa and (ii) characterize recovered STEC and EPEC isolates by serotype and major virulence-associated genes.
Materials and methods
Study population and sample collection
In this study, a total of 771 fecal samples were obtained from dairy cattle and screened for STEC and EPEC. Fecal samples were collected from all adult cattle which were present at the dairy farms and abattoirs surveyed on the day of sampling. Fecal samples were collected on 9 dairy cattle farms (n = 404) and spent dairy cattle at 5 abattoirs (n = 367) in 3 provinces (Gauteng, Eastern Cape and Free State) of South Africa. The 5 abattoirs and 9 farms are represented by letters (A–E) and (F–N), respectively. Fresh fecal samples were collected and transported on ice to the laboratory, where they were stored at 4 °C and processed within one week. This study was approved by the Faculty of Veterinary Science, University of Pretoria, Research Ethics and Animal Ethics Committees-under approval numbers REC033-23 and REC 109-19, respectively.
Sample enrichment and culture
All fecal samples were enriched by placing 5 g of feces of each sample in 45 mL of EC broth (CM0990, Oxoid, Basingtoke, UK) containing 20 mg/L of novobiocin (N1628, Sigma Aldrich, St. Louis, MO, USA) and incubated at 37 °C in a shaking incubator for 18–24 h (Mainga et al. 2018). A 100 µL aliquot of the enriched culture was spread on Drigalski lactose agar (CM0531, Oxoid, Basingtoke, UK) and CHROMagar STEC base ST162(B) containing the ST162(S) supplement (CHROMagar™, Paris, France) and incubated at 37 °C for 18–24 h.
DNA extraction, STEC and EPEC screening by PCR
DNA was extracted by the boiling method (Malahlela et al. 2022) from all Drigalski lactose agar and CHROMagar petri dishes that showed growth. Briefly, a loopful of bacterial colony sweep was obtained from each Petri dish and placed in an Eppendorf tube containing 1 mL of FA buffer (223,143, BD Difco, Sparks, MD, USA). The bacterial suspension was washed twice in FA buffer by vortexing and centrifugation (10800 × g for 5 min). The supernatant was discarded after each washing. The pellet formed after the 2nd washing cycle was resuspended in 500 µL of sterile water. The suspension was homogenized and boiled at 100 °C for 25 min in a dry heating block and cooled immediately on ice for 10 min. The lysate containing the DNA was stored at −20 °C for later use. To detect STEC and EPEC, DNA was thawed and centrifuged, and multiplex PCR (mPCR) was used to screen the DNA template for stx1, stx2, eaeA and hlyA as previously described (Paton & Paton 1998). The 25 µL PCR mixture consisted of 5 μL of supernatant DNA template, 2.5 μL of 10X Thermopol reaction buffer, 1 unit of Taq DNA polymerase, 2 μL of 2.5 mM dNTPs (deoxynucleotide triphosphates), 0.6 µL for each of the forward and reverse primers (10 μM final concentration) (Inqaba Biotec, Pretoria, South Africa) and 10.5 μL of sterile water. DNA from the STEC O157:H7 strain EDL933 (ATCC®43,895™) was used as the PCR positive control for STEC (stx1, stx2, eaeA and hlyA), and sterile water was used as the negative control. PCR products were electrophoresed in a 2% agarose gel in TAE buffer (Tris–acetate-ethylenediamine tetra acetic acid), stained in a solution of ethidium bromide and visualized under ultraviolet (UV) light in a Gel Doc™ imaging system XR+ (Bio‐Rad, Hercules, USA). All Drigalski lactose agar and CHROMagar sample plates which were positive on initial PCR screening for stx1 and/or stx2 were considered STEC positive; while those which were stx-negative but positive for eaeA, were considered EPEC-positive.
Isolation and detection of STEC and EPEC isolates
Purified suspect STEC and EPEC single colonies were obtained by streaking colony sweeps from all STEC and/or EPEC PCR-positive Drigalski lactose agar and CHROMagar STEC plates onto fresh Drigalski lactose agar and CHROMagar plates. The plates were incubated at 37 °C for 18–24 h. Up to five single colonies were picked from each plate and individually propagated and multiplied on Luria Bertani (LB) agar (Becton and Dickinson & Company, Sparks, USA). Thereafter, DNA was extracted from each purified single colony by the boiling method as described above. PCR was performed as described above to confirm the STEC or EPEC status of each single colony (Paton & Paton 1998). All purified single colonies which were PCR-positive for stx1 and/or stx2 were confirmed as STEC, while those negative for stx but positive for eaeA were considered EPEC. Furthermore, an additional PCR (Gunzburg et al. 1995) was performed to classify EPEC into typical EPEC (bfpA-positive) and atypical EPEC (bfpA-negative) strains. Polymerase chain reaction was carried out to verify whether each confirmed STEC or EPEC pure colony was indeed E. coli using a PCR protocol and primers previously described by Doumith et al., (2012). Bacterial sweeps of purified single colonies that were confirmed as STEC or EPEC were collected from LB agar plates and preserved at −80 °C in cryovials containing a freezing mixture 70% Brain heart infusion broth (53,286, Sigma-Aldrich) and 30% glycerol until further processing.
Molecular serotyping of STEC and EPEC isolates
STEC and EPEC isolates were serotyped (O:H) using previously described PCR protocols (Banjo et al. 2018; Iguchi et al. 2015, 2020; Singh et al. 2015). Previously serotyped STEC isolates in our collection (Karama et al. 2019b; Mainga et al. 2018; Malahlela et al. 2022) and additional STEC which had been previously serotyped at the Laboratorio de Referencia de Escherichia coli (LREC), Facultad de Veterinaria, Universidad de Santiago de Compostela, Lugo, Spain and the National Microbiology Laboratory, Public Health Agency of Canada, Guelph, Ontario, Canada, were used as positive controls for PCR serotyping. In addition, reference STEC strains which were kindly provided by the European Union Reference Laboratory for Escherichia coli, Istituto Superiore di Sanità, Rome Italy, were used as positive controls for serotypes belonging to the following “Top 7/Big 7” STEC serogroups: STEC-C210-03 (O157), STEC-ED476 (STEC O111), STEC-C1178-04 (STEC O145), STEC-C125-06 (STEC O103) and STEC-ED745 (O26).
Statistical analysis
Data were summarized and described using proportions and percentages in Microsoft Excel spreadsheets. The Fisher’s exact test was used to determine whether there were statistical differences between the occurrence of STEC and EPEC among cattle on dairy farms and spent dairy cattle at abattoirs. A p value of 0.05 was considered statistically significant. A 2 × 2 contingency table was used to calculate the odds ratios for the occurrence of STEC and EPEC in dairy cattle on farms and at abattoirs. The convenient sampling approach was used to determine the sample size for this study. However, to adjust for the clustering effect (intra-cluster) in the cattle herds/farms surveyed, the 95% confidence interval (CI) was calculated by taking into account the cluster/farm size and assuming an intra-class correlation coefficient of 0.1 using the formulas by Dohoo et al. (2003). Statistical analysis was performed using Python Script software: https://www.python.org/about/gettingstarted/
Results
Occurrence of STEC in dairy cattle at abattoirs and farms
Overall, STEC was detected in 42.2% (325/771) (95% Confidence interval: 38.7–45.6%) of dairy cattle faeces. Furthermore, 29.9% (110/367) (95% CI: 25.3–34.7%) of spent dairy cattle faeces samples which were collected from abattoirs (A–E) and 53.2% (215/404) (95% CI: 48.4%–58.1%) from dairy cattle on farms (F–N) were STEC positive. There was a significantly lower likelihood (Odds ratio: 0.376; p < 0.05) of finding STEC in spent dairy cattle at abattoirs compared to dairy cattle on farms. The proportion of STEC positive cattle per abattoir (A–E) and farm (F–N) tested as follows: A, 37.8% (34/90); B, 31.2% (34/107); C, 47.2% (17/36); D, 18.7% (20/107); E, 18.5% (5/27) F, 64.6% (42/65); G, 87.5% (56/64); H, 31.0% (9/29); I, 32.4% (11/34); J, 47.5% (19/40); K, 81.4% (35/43); L, 14.3% (1/7); M, 73.5% (25/34); and N, 19.3% (17/88) (Table 1, Fig. 1).
Occurrence of EPEC in dairy cattle at abattoirs and farms
EPEC was detected in 23.3% (180/771) (95% CI: 20.3%–26.4%) of all dairy cattle sampled at abattoirs and farms (A–N). In addition, EPEC was found in 19.9% (73/367) (95% CI: 15.8%–24.0%) of spent dairy cattle at abattoirs (A–E) and 26.5% (107/404) (95% CI: 22.2%–30.8%) of cattle on farms (F-N). There was a significantly lower likelihood (OR: 0.689; p < 0.05) of EPEC occurrence in spent dairy cattle at abattoirs compared to dairy cattle on farms. The proportion of EPEC positive cattle per abattoir and farm was as follows: A, 24.4% (22/90); B, 15.0% (16/107); C, 36.1% (13/36); D, 14.0% (15/107); E, 26.0% (7/27); F, 24.6% (16/65); G, 14.1% (9/64); H, 62.1% (18/29); I, 11.8% (4/34); J, 0% (0/40); K, 9.3% (4/43); L, 71.4% (5/7) M, 14.7% (5/34) and N, 52.3% (46/88) (Table 2, Fig. 1). Both STEC and EPEC were concurrently detected in 5.6% (43/771) of dairy cattle.
STEC serotypes
A total of 339 STEC isolates were recovered from 32.3% (105/325) of STEC-positive dairy cattle and 95.6% (324/339) were serotypeable by PCR (Table 1). PCR revealed 53 distinct STEC serotypes including 35 O serogroups and 16 H types while 4.4% (15/339) of the STEC isolates were O-untypable (ONT) (Fig. 2a, b). The O serogroup and H type(s) combinations for STEC can be found in the Supplementary Table S1. Among the 53 STEC serotypes, 52.8% (28/53) were each represented by a single isolate, while the remaining 47.2% (25/53) were represented by more than one isolate (Supplementary Table S3). The highest number of STEC serotypes was observed in abattoir B, with a total of 14 different serotypes, followed by abattoir A, at which recovered STEC isolates belonged to 12 distinct serotypes. Overall, the five most frequent STEC serotypes were O82:H8 (28.3%, 96/339), OgX18:H2 (9.7%, 33/339), O157:H7 (5.9%, 20/339), O2/O50:H45 (5.9%, 20/339) and O153/O178:H19 (5.6%, 19/339). Furthermore, serotypes that belong to “Top 7” STEC serogroups were recovered from 10% (34/339) of all STEC isolates which were serotyped. The following “Top 7” STEC serotypes were identified: O26:H2, 0.3% (1/339); O26:H11, 3.2% (11/339); O103:H8, 0.6% (2/339); and O157:H7, 5.9% (20/339). Top 7″ STEC serotypes were recovered from 4.3% (14/325) of STEC positive animals.
EPEC serotypes
Among the 136 EPEC isolates, 92.7% (126/136) were serotypeable by PCR. EPEC serotyping revealed 19 different O:H serotypes including 16 O serogroups and 8 H-types while 5.2% (7/136) isolates were O-nontypeable (ONT) and 2.2% (3/136) were H-nontypable (HNT) (Fig. 3a, b). The 136 EPEC isolates were recovered from 28.9% (52/180) of EPEC-positive dairy cattle. The five most frequent EPEC serotypes were O10:H2 (19.9%, 27/136), ON9:H28 (18.4%, 25/136), O26:H11 (17.6%, 24/136), O10:H25 (9.6%, 13/136) and O84:H14 (4.4%, 6/136). There were 31.6% (6/19) of the EPEC serotypes which were represented by a single isolate, while 68.4% (13/19) were represented by more than one isolate (Supplementary Table S4). The O serogroup and H type (s) combinations for EPEC can be found in Supplementary Table S2.
More than one STEC serotype was isolated from 7.4% (24/325) of STEC positive animals while more than one EPEC serotype was recovered from 2.2% (4/180) of EPEC positive cattle. Furthermore, both STEC and EPEC serotypes were concurrently recovered from 0.8% (6/771) of all animals in the following serotype combinations: STEC O157:H7, STEC O136:H16 and EPEC O153/O178:HNT (1 animal); STEC O8:H21 and EPEC O26:H2 (1 animal); STEC ONT:H19 and EPEC O10:H2 (1 animal); STEC O98:H28 and EPEC O187:H28 (1 animal); STEC O2/O50:H45 and EPEC O10:H2 (1 animal); STEC O153/O178:H19 and EPEC O10:H25 (1 animal).
Distribution of major virulence genes among dairy cattle STEC and EPEC isolates
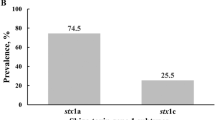
Both stx1 and stx2 were concurrently detected in 196/339 (57.8%) of the isolates, while stx1 only was detected in 15% (51/339) and stx2 only was detected in 27.1% (92/339) of isolates.
The eaeA gene was detected in 13.6% (46/339) of the STEC isolates, which corresponded to 6.2% (20/325) of STEC-positive dairy animals. Among the 46 eaeA-positive STEC isolates, 69.6% (32/46) were “Top 7” STEC serotypes: O26:H2, 2.1% (1/46); O26:H11, 23.9% (11/46); and O157:H7, 43.5% (20/46). The remaining 30.4% (14/46) of eaeA-positive STEC isolates belonged to the following serotypes: O84:H2, O98:H28, O108:H25, O136:H16, O177:H19, O182:H25, OgN3:H19 and OgX18:H8 and were recovered from 2.5% (8/325) of STEC-positive animals.
All the EPEC isolates (100%, 136/136) were eaeA positive and bfpA-negative while 45.6% (62/136) possessed hlyA (Supplementary Table S4).
Discussion
Current reports on the occurrence of STEC in dairy cattle in South Africa are non-existent. This study investigated the occurrence of STEC and EPEC in dairy cattle on farms and spent dairy animals at abattoirs. STEC was found in 42.2% of the dairy cattle population surveyed. The STEC occurrence observed in this study was higher in comparison to similar reports which reported STEC frequency ranging from 24.7 to 37.5% in different countries including Portugal, Argentina, France and Germany (Ballem et al. 2020; Fernández et al. 2010; Fremaux et al. 2006; Menrath et al. 2010). However, higher STEC occurrence ranging from 62.8 to 82% were reported in studies that investigated STEC in smaller sample sizes of dairy cattle in Brazil and Japan (Cerqueira et al. 1999; Ferreira et al. 2014; Kobayashi et al. 2001). Furthermore, wide variations in the STEC occurrence were also observed among the five abattoirs (18.5–47.2%) and nine dairy farms (14.0–87.5%) which were surveyed. Variations in STEC occurrence among abattoirs and farms may be ascribed to a number risk factors for STEC colonization in cattle including the age of animals, diet, season, health status, and various farm management and hygiene practices (e.g., housing, dry versus wet bedding, pest control, contact with wild animals, manure and slurry disposal). Additional factors which may influence variations in STEC occurrence in cattle are study designs, sampling strategies and sample handling, and laboratory methods and media which are used for enrichment, selection/isolation of STEC.
STEC occurrence was significantly lower in spent cattle at abattoirs (29.9%) in comparison to dairy cattle on farms (53%). The higher frequency of STEC in dairy cattle on farms may be attributed to the younger age of animals on farms in comparison to spent dairy cattle which are older and are sent for slaughter at the end of their productive cycle. Younger animals on farms are easily colonized by STEC because they have a less diverse intestinal microflora (Mir et al. 2016) and an immature immune system which are unable to competitively exclude STEC from the gastrointestinal tract. In contrast, spent dairy cattle are usually older, adult, slaughter age animals which have a more diverse enteric microbiota and mature immune system capable of excluding and counteracting STEC colonization competitively. In addition, previous studies have shown that the prevalence of STEC supershedding is usually greater among younger cattle, which can lead to greater STEC prevalence in cattle (Williams et al. 2015). The presence of STEC supershedders in a particular cattle operation may increase STEC contamination in the environment, which favours faster STEC transmission among animals and subsequent increase in the number of STEC-positive animals.
EPEC was detected in 23.3% of dairy cattle and there was a significantly lower likelihood of EPEC finding in spent cattle at abattoirs (19.9%) compared to dairy cattle on farms (26.5%). So far, only few studies have investigated the presence of EPEC in dairy cattle and have found variable occurrence ranging from 15 to 36% EPEC occurrence in different countries (Eldesoukey et al. 2022; Habets et al. 2020; Singh et al. 2015). While factors that influence EPEC occurrence in dairy cattle populations remain unclear, they could be similar to those that determine STEC occurrence in cattle populations.
It was possible to serotype almost all the STEC isolates (95.6%) by PCR serotyping which revealed 53 STEC serotypes (35 O serogroups and 16 H types). The number of different STEC serotypes identified in this study was higher than reported previously in similar studies on dairy cattle (Fernández et al. 2010; Irino et al. 2005; Menrath et al. 2010). This could be attributed to the use of PCR serotyping (Banjo et al. 2018; DebRoy et al. 2018; Iguchi et al. 2015, 2016, 2020; Ludwig et al. 2020; Singh et al. 2015) instead of traditional serotyping (Ørskov & Ørskov 1984). PCR serotyping has shown high sensitivity and specificity for identifying E. coli serotypes (Malahlela et al. 2022), particularly for isolates which are O nontypeable and/or H nontypeable (ONT/HNT) by traditional serotyping (Banjo et al. 2018; DebRoy et al. 2018; Iguchi et al. 2015, 2016, 2020; Ludwig et al. 2020; Singh et al. 2015). With PCR serotyping it is possible to identify E. coli strains that carry but are unable to express genes encoding somatic O and flagellar H antigens (O rough and nonmotile).
Only 10% of dairy cattle STEC isolates belonged to “Top 7” STEC serotypes, consistent with studies which have also observed low occurrence of the “Top 7” STEC, ranging from 2.4 to 13.3% in STEC from dairy cattle (Ballem et al. 2020; Bibbal et al. 2015; Cerqueira et al. 1999; Fernández et al. 2010). The following “Top 7” STEC serotypes were observed in this study: STEC O157:H7, STEC O26:H11/H2 and STEC O103:H8. While both O157:H7 and O26:H11 are considered the two most clinically relevant STEC serotypes globally including South Africa (EFSA 2013; Bettelheim and Goldwater 2019; Karama et al. 2019a; Smith et al. 2019), the clinical importance of STEC O26:H2 and STEC O103:H8 remains unclear, as both serotypes have been rarely reported in human disease (Baba et al. 2019; Bettelheim & Goldwater 2019). Furthermore, STEC O157:H7 and O26:H11 are responsible for most human STEC outbreaks and common in severe disease, and the most frequent serotypes in human STEC foodborne outbreaks linked to consumption of contaminated dairy products (Farrokh et al. 2013; Hussein & Sakuma 2005b). In addition, 46% of STEC isolates belonged to non-Top 7 serotypes which have been associated with human infections around the world including South Africa (STEC O22:H16 and O8:H19) (Karama et al. 2019a) (STEC O8:H14, O8:H21, O22:H8, O22:H11, O82:H8, O84:H2, O110:H19, O153/O178:H7, O154:H4, O171:H2 and O174:H28) (Bettelheim & Goldwater 2019; WHO 1998). The isolation of STEC serotypes which have been previously implicated in human disease supports the role of dairy cattle as a reservoir and potential source of STEC in South Africa.
Similar to STEC, almost all (92.7%) of EPEC isolates were serotypeable by PCR which revealed 19 different serotypes including EPEC O15:H2, O26:H11, O76:H7 and O177:H11 which have been previously implicated in human disease (Blanco et al. 2006). Of particular interest, was the isolation of aEPEC O26:H11 which is one of the frequent and clinically relevant EPEC serotype in infantile diarrheae, worldwide (Croxen et al. 2013; Durso et al. 2005).
Furthermore, all EPEC isolates were classified as atypical EPEC (aEPEC) bfpA-negative), in agreement with other studies which have mainly detected strains from dairy cattle (Auvray et al. 2023; Bibbal et al. 2015; Singh et al. 2015). While most human EPEC infections have been linked with typical EPEC (bfpA-positive), the association of aEPEC with infantile diarrheae remains controversial (Hernandes et al. 2009). However, some reports found various serotypes of aEPEC strains in children with acute diarrhea, in various age groups and patients with AIDS (reviewed by Hernandes et al. 2009).
Virulotyping of 339 STEC isolates revealed that stx2-positive STEC isolates were more frequent than stx1-positive among dairy STEC isolates. This finding is in agreement with previous studies that characterized STEC from dairy cattle and reported higher frequency of stx2 than stx1 (Ballem et al. 2020; Bibbal et al. 2015; Dong et al. 2017; Fernández et al. 2010; Venegas-Vargas et al. 2016). However, other studies showed that stx1 was more frequent than stx2 in dairy cattle (Cerqueira et al. 1999; Ferreira et al. 2014; Singh et al. 2015). Previous studies have shown that stx2-carrying STEC isolates are more frequent in human STEC disease, particularly severe human STEC infections characterized by bloody diarrhea, hemorrhagic colitis and the hemolytic uremic syndrome, a complication commonly associated with renal failure and dialysis, worldwide (Boerlin et al. 1999; Friedrich et al. 2002; Obrig & Karpman 2012).
While most dairy cattle STEC were eaeA-negative, eaeA was detected in only 13.6% of all STEC isolates. The eaeA gene was mainly observed among “Top 7” STEC O26:H2, O26:H11 and O157:H7 isolates and non-Top 7 including O84:H2, O98:H28, O108:H25, O136:H16, O182:H25, OgN3:H19 and OgX18:H8 (Bettelheim & Goldwater 2019; EFSA BIOHAZ Panel 2013; WHO 1998). The low occurrence of eaeA in dairy STEC isolates is in agreement with previous studies which reported low occurrence of eaeA among dairy cattle STEC (Cobbold & Desmarchelier 2001; Fernández et al. 2010; Irino et al. 2005). Considering the clinical significance and strong association of eaeA with highly virulent STEC strains (EFSA BIOHAZ Panel., 2020; Ethelberg et al. 2004), the high occurrence of eaeA among STEC isolates suggests that these eaeA-positive dairy cattle STEC isolates are of high risk to humans and will need to be closely monitored during STEC epidemiological surveillance.
Most dairy cattle STEC isolates (69.9%) and a considerable number of EPEC (46.5%) were hlyA-positive. While the significance of hlyA in STEC and EPEC virulence is not clear, some studies have suggested that hlyA is a potential EPEC and STEC virulence factor (Aldick et al. 2009; Schwidder et al. 2019). In addition, the presence of hlyA in STEC strains was associated with severe human STEC disease including hemorrhagic colitis and hemolytic uremic syndrome (Schmidt & Karch 1996). Furthermore, previous reports (Aldick et al. 2007; Bielaszewska et al. 2013) have suggested that possession and production of hlyA was associated with damage of the endothelial barrier of the vascular system through pore-formation, subsequent cell lysis and apoptosis, thereby contributing to the development of HUS.
The findings from this study demonstrated that dairy cattle in South Africa are a reservoir of STEC and EPEC. The detection of virulent STEC and EPEC serotypes which have been previously incriminated in human disease around the world, including South Africa, underscores the significance of dairy cattle as reservoir and potential source of clinically relevant STEC and EPEC. The STEC isolates recovered in this study will need to be further characterized to ascertain the full virulence potential of dairy cattle STEC and EPEC for humans. In addition, molecular characterization studies comparing cattle STEC and EPEC with human isolates will have to be carried to determine the role played by cattle in the transmission and causation of STEC and EPEC infections in humans in South Africa.
Data availability
No datasets were generated or analysed during the current study.
References
Aldick T, Bielaszewska M, Zhang W, Brockmeyer J, Schmidt H, Friedrich AW, Kim KS, Schmidt MA, Karch H (2007) Hemolysin from Shiga toxin-negative Escherichia coli O26 strains injures microvascular endothelium. Microbes Infect 9(3):282–290. https://doi.org/10.1016/j.micinf.2006.12.001
Aldick T, Bielaszewska M, Uhlin BE, Humpf HU, Wai SN, Karch H (2009) Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Mol Microbiol 71(6):1496–1508. https://doi.org/10.1111/j.1365-2958.2009.06618.x
Alfinete NW, Bolukaoto JY, Heine L, Potgieter N, Barnard TG (2022) Virulence and phylogenetic analysis of enteric pathogenic Escherichia coli isolated from children with diarrhoea in South Africa. Int J Infect Dis 114:226–232. https://doi.org/10.1016/j.ijid.2021.11.017
Auvray F, Bièche-Terrier C, Um MM, Dupouy V, Nzuzi N, David L, Allais L, Drouet M, Oswald E, Bibbal D, Brugère H (2023) Prevalence and characterization of the seven major serotypes of Shiga toxin-producing Escherichia coli (STEC) in veal calves slaughtered in France. Vet Microbiol 282:109754. https://doi.org/10.1016/j.vetmic.2023.109754
Baba H, Kanamori H, Kudo H, Kuroki Y, Higashi S, Oka K, Takahashi M, Yoshida M, Oshima K, Aoyagi T, Tokuda K, Kaku M (2019) Genomic analysis of Shiga toxin-producing Escherichia coli from patients and asymptomatic food handlers in Japan. PLoS ONE 14(11):e0225340. https://doi.org/10.1371/journal.pone.0225340
Ballem A, Gonçalves S, Garcia-Meniño I, Flament-Simon SC, Blanco JE, Fernandes C, Saavedra MJ, Pinto C, Oliveira H, Blanco J, Almeida G, Almeida C (2020) Prevalence and serotypes of Shiga toxin-producing Escherichia coli (STEC) in dairy cattle from Northern Portugal. PLoS ONE 15(12):e0244713. https://doi.org/10.1371/journal.pone.0244713
Banjo M, Iguchi A, Seto K, Kikuchi T, Harada T, Scheutz F, Iyoda S (2018) Escherichia coli H-genotyping PCR: a complete and practical platform for molecular H typing. J Clin Microbiol. https://doi.org/10.1128/jcm.00190-18
Bettelheim K, Goldwater P (2019) A compendium of Shigatoxigenic Escherichia coli: origins, bacteriological and clinical data on the serogroups, serotypes and untypeable Strains of E. coli reported between 1980 and 2017, Excluding O157:H7. Adv Microbiol 9(11):905–909. https://doi.org/10.4236/aim.2019.911056
Bibbal D, Loukiadis E, Kérourédan M, Ferré F, Dilasser F, Peytavin de Garam C, Cartier P, Oswald E, Gay E, Auvray F, Brugère H (2015) Prevalence of carriage of Shiga toxin-producing Escherichia coli serotypes O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 among slaughtered adult cattle in France. Appl Environ Microbiol 81(4):1397–1405. https://doi.org/10.1128/aem.03315-14
Bielaszewska M, Rüter C, Kunsmann L, Greune L, Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, Karch H (2013) Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog 9(12):e1003797. https://doi.org/10.1371/journal.ppat.1003797
Blanco M, Blanco JE, Dahbi G, Alonso MP, Mora A, Coira MA, Madrid C, Juárez A, Bernárdez MI, González EA, Blanco J (2006) Identification of two new intimin types in atypical enteropathogenic Escherichia coli. Int Microbiol 9(2):103–110
Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL (1999) Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol 37(3):497–503. https://doi.org/10.1128/JCM.37.3.497-503.1999
Cerqueira AM, Guth BE, Joaquim RM, Andrade JR (1999) High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State Brazil. Vet Microbiol 70(1–2):111–121. https://doi.org/10.1016/s0378-1135(99)00123-6
Cleary J, Lai L-C, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, Frankel G, Knutton S (2004) Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP) EspA Filaments and Intimin. Microbiology 150(3):527–538. https://doi.org/10.1099/mic.0.26740-0
Cobbold R, Desmarchelier P (2001) Characterisation and clonal relationships of Shiga-toxigenic Escherichia coli (STEC) isolated from Australian dairy cattle. Vet Microbiol 79(4):323–335. https://doi.org/10.1016/s0378-1135(00)00366-7
Cray WC Jr, Casey TA, Bosworth BT, Rasmussen MA (1998) Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl Environ Microbiol 64(5):1975–1979. https://doi.org/10.1128/aem.64.5.1975-1979.1998
Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB (2013) Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26(4):822–880. https://doi.org/10.1128/cmr.00022-13
DebRoy C, Fratamico PM, Roberts E (2018) Molecular serogrouping of Escherichia coli. Anim Health Res Rev 19(1):1–16. https://doi.org/10.1017/s1466252317000093
Dohoo IR, Martin W, Stryhn H (2003) Veterinary Epidemiologic Research (No. V413 DOHv). AVC Incorporated, Charlottetown, p 43
Dong H-J, Lee S, Kim W, An J-U, Kim J, Kim D, Cho S (2017) Prevalence, virulence potential, and pulsed-field gel electrophoresis profiling of Shiga toxin-producing Escherichia coli strains from cattle. Gut Pathog 9(1):22. https://doi.org/10.1186/s13099-017-0169-x
Doumith M, Day MJ, Hope R, Wain J, Woodford N (2012) Improved multiplex PCR strategy for rapid assignment of the four major Escherichia coli phylogenetic groups. J Clin Microbiol 50(9):3108–3110
Durso LM, Bono JL, Keen JE (2005) Molecular serotyping of Escherichia coli O26:H11. Appl Environ Microbiol 71(8):4941–4944. https://doi.org/10.1128/aem.71.8.4941-4944.2005
EFSA Biohaz Panel (2013) Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA J. https://doi.org/10.2903/j.efsa.2013.3138
EFSA Biohaz Panel (2020) Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. ESFA J 18(1):5967. https://doi.org/10.2903/j.efsa.2020.5967
Eldesoukey IE, Elmonir W, Alouffi A, Beleta EI, Kelany MA, Elnahriry SS, Alghonaim MI, Alzeyadi ZA, Elaadli H (2022) Multidrug-resistant enteropathogenic Escherichia coli isolated from diarrhoeic calves, milk, and workers in dairy farms: a potential public health risk. Antibiotics 11(8):999. https://doi.org/10.3390/antibiotics11080999
Ethelberg S, Olsen KE, Scheutz F, Jensen C, Schiellerup P, Enberg J, Petersen AM, Olesen B, Gerner-Smidt P, Mølbak K (2004) Virulence factors for hemolytic uremic syndrome Denmark. Emerg Infect Dis 10(5):842–847. https://doi.org/10.3201/eid1005.030576
Farrokh C, Jordan K, Auvray F, Glass K, Oppegaard H, Raynaud S, Thevenot D, Condron R, De Reu K, Govaris A, Heggum K, Heyndrickx M, Hummerjohann J, Lindsay D, Miszczycha S, Moussiegt S, Verstraete K, Cerf O (2013) Review of Shiga-toxin-producing Escherichia coli (STEC) and their significance in dairy production. Int J Food Microbiol 162(2):190–212. https://doi.org/10.1016/j.ijfoodmicro.2012.08.008
Fernández D, Irino K, Sanz ME, Padola NL, Parma AE (2010) Characterization of Shiga toxin-producing Escherichia coli isolated from dairy cows in Argentina. Lett Appl Microbiol 51(4):377–382. https://doi.org/10.1111/j.1472-765X.2010.02904.x
Ferreira MR, Freitas Filho EG, Pinto JF, Dias M, Moreira CN (2014) Isolation, prevalence, and risk factors for infection by Shiga toxin-producing Escherichia coli (STEC) in dairy cattle. Trop Anim Health Prod 46(4):635–639. https://doi.org/10.1007/s11250-014-0541-5
Fremaux B, Raynaud S, Beutin L, Rozand CV (2006) Dissemination and persistence of Shiga toxin-producing Escherichia coli (STEC) strains on French dairy farms. Vet Microbiol 117(2–4):180–191. https://doi.org/10.1016/j.vetmic.2006.04.030
Friedrich AW, Bielaszewska M, Zhang WL, Pulz M, Kuczius T, Ammon A, Karch H (2002) Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185(1):74–84. https://doi.org/10.1086/338115
GDB (2018) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18(11):1211–1228. https://doi.org/10.1016/s1473-3099(18)30362-1
Girón JA, Ho AS, Schoolnik GK (1991) An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254(5032):710–713. https://doi.org/10.1126/science.1683004
Girón JA, Ho AS, Schoolnik GK (1993) Characterization of fimbriae produced by enteropathogenic Escherichia coli. J Bacteriol 175(22):7391–7403. https://doi.org/10.1128/jb.175.22.7391-7403.1993
Gunn GJ, McKendrick IJ, Ternent HE, Thomson-Carter F, Foster G, Synge BA (2007) An investigation of factors associated with the prevalence of verocytotoxin producing Escherichia coli O157 shedding in Scottish beef cattle. Vet J 174(3):554–564. https://doi.org/10.1016/j.tvjl.2007.08.024
Gunzburg ST, Tornieporth NG, Riley LW (1995) Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J Clin Microbiol 33(5):1375–1377. https://doi.org/10.1128/jcm.33.5.1375-1377.1995
Habets A, Engelen F, Duprez JN, Devleesschauwer B, Heyndrickx M, De Zutter L, Thiry D, Cox E, Mainil J (2020) Identification of Shigatoxigenic and Enteropathogenic Escherichia coli serotypes in healthy young dairy calves in Belgium by recto-anal mucosal swabbing. Vet Sci 7(4):167. https://doi.org/10.3390/vetsci7040167
Hernandes RT, Elias WP, Vieira MA, Gomes TA (2009) An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett 297(2):137–149. https://doi.org/10.1111/j.1574-6968.2009.01664.x
Hu J, Torres AG (2015) Enteropathogenic Escherichia coli : foe or innocent bystander? Clin Microbiol Infect 21(8):729–734. https://doi.org/10.1016/j.cmi.2015.01.015
Hussein HS, Sakuma T (2005a) Shiga toxin-producing Escherichia coli : pre- and postharvest control measures to ensure safety of dairy cattle products. J Food Prot 68(1):199–207. https://doi.org/10.4315/0362-028x-68.1.199
Hussein HS, Sakuma T (2005b) Prevalence of Shiga toxin-producing Escherichia coli in dairy cattle and their products. J Dairy Sci 88(2):450–465. https://doi.org/10.3168/jds.s0022-0302(05)72706-5
Iguchi A, Iyoda S, Seto K, Morita-Ishihara T, Scheutz F, Ohnishi M (2015) Escherichia coli O-genotyping PCR: a comprehensive and practical platform for molecular O serogrouping. J Clin Microbiol 53(8):2427–2432. https://doi.org/10.1128/jcm.00321-15
Iguchi A, Iyoda S, Seto K, Nishii H, Ohnishi M, Mekata H, Ogura Y, Hayashi T (2016) Six novel O genotypes from Shiga toxin-producing Escherichia coli. Front Microbiol 7:765. https://doi.org/10.3389/fmicb.2016.00765
Iguchi A, Nishii H, Seto K, Mitobe J, Lee K, Konishi N, Obata H, Kikuchi T, Iyoda S (2020) Additional Og-typing PCR techniques targeting Escherichia coli -novel and Shigella-unique O-antigen biosynthesis gene clusters. J Clin Microbiol 58(11):e01493-e1520. https://doi.org/10.1128/jcm.01493-20
Irino K, Kato MA, Vaz TM, Ramos II, Souza MA, Cruz AS, Gomes TA, Vieira MA, Guth BE (2005) Serotypes and virulence markers of Shiga toxin-producing Escherichia coli (STEC) isolated from dairy cattle in São Paulo State Brazil. Vet Microbiol 105(1):29–36. https://doi.org/10.1016/j.vetmic.2004.08.007
Jerse AE, Yu J, Tall BD, Kaper JB (1990) A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A 87(20):7839–7843
Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2(2):123–140
Karama M, Cenci-Goga BT, Malahlela M, Smith AM, Keddy KH, El-Ashram S, Kabiru LM, Kalake A (2019a) Virulence characteristics and antimicrobial resistance profiles of Shiga toxin-producing Escherichia coli isolates from humans in South Africa: 2006–2013. Toxins 11(7):424. https://doi.org/10.3390/toxins11070424
Karama M, Mainga AO, Cenci-Goga BT, Malahlela M, El-Ashram S, Kalake A (2019b) Molecular profiling and antimicrobial resistance of Shiga toxin-producing Escherichia coli O26, O45, O103, O121, O145 and O157 isolates from cattle on cow-calf operations in South Africa. Sci Rep 9(1):11930. https://doi.org/10.1038/s41598-019-47948-1
Kintz E, Brainard J, Hooper L, Hunter P (2017) Transmission pathways for sporadic Shiga-toxin producing E. coli infections: a systematic review and meta-analysis. Int J Hyg Environ Health 220(1):57–67. https://doi.org/10.1016/j.ijheh.2016.10.011
Kobayashi H, Shimada J, Nakazawa M, Morozumi T, Pohjanvirta T, Pelkonen S, Yamamoto K (2001) Prevalence and characteristics of Shiga toxin-producing Escherichia coli from healthy cattle in Japan. Appl Environ Microbiol 67(1):484–489. https://doi.org/10.1128/aem.67.1.484-489.2001
Ludwig JB, Shi X, Shridhar PB, Roberts EL, DebRoy C, Phebus RK, Bai J, Nagaraja TG (2020) Multiplex PCR assays for the detection of one hundred and thirty seven serogroups of Shiga toxin-producing Escherichia coli associated with cattle [Methods]. Front Cell Infect Microbiol 10:378. https://doi.org/10.3389/fcimb.2020.00378
Mainga AO, Cenci-Goga BT, Malahlela MN, Tshuma T, Kalake A, Karama M (2018) Occurrence and characterization of seven major Shiga toxin-producing Escherichia coli serotypes from healthy cattle on cow-calf operations in South Africa. Zoonoses Public Health 65(7):777–789. https://doi.org/10.1111/zph.12491
Malahlela MN, Cenci-Goga BT, Marufu MC, Fonkui TY, Grispoldi L, Etter E, Kalake A, Karama M (2022) Occurrence, serotypes and virulence characteristics of Shiga-Toxin-Producing Escherichia coli isolates from goats on communal rangeland in South Africa. Toxins (Basel). https://doi.org/10.3390/toxins14050353
McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB (1995) A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 92(5):1664–1668
Menrath A, Wieler LH, Heidemanns K, Semmler T, Fruth A, Kemper N (2010) Shiga toxin producing Escherichia coli : Identification of non-O157:H7-super-shedding cows and related risk factors. Gut Pathog 2(1):7. https://doi.org/10.1186/1757-4749-2-7
Mir RA, Weppelmann TA, Elzo M, Ahn S, Driver JD, Jeong KC (2016) Colonization of beef cattle by Shiga toxin-producing Escherichia coli during the first year of life: a cohort study. PLoS ONE 11(2):e0148518. https://doi.org/10.1371/journal.pone.0148518
O’Brien AO, Lively TA, Chen ME, Rothman SW, Formal SB (1983) Escherichia coli O157:H7 strains associated with haemorrhagic colitis in the United States produce a Shigella dysenteriae 1 (SHIGA) like cytotoxin. Lancet 1(8326 Pt 1):702. https://doi.org/10.1016/s0140-6736(83)91987-6
Obrig TG, Karpman D (2012) Shiga toxin pathogenesis: kidney complications and renal failure. Curr Top Microbiol Immunol 357:105–136. https://doi.org/10.1007/82_2011_172
Ørskov F, Ørskov I (1984) Serotyping of Escherichia coli. In: Bergan T (ed) Methods in Microbiology. Academic Press, pp 43–112
Paton AW, Paton JC (1998) Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol 36(2):598–602. https://doi.org/10.1128/jcm.36.2.598-602.1998
Pires SM, Majowicz S, Gill A, Devleesschauwer B (2019) Global and regional source attribution of Shiga toxin-producing Escherichia coli infections using analysis of outbreak surveillance data. Epidemiol Infect 147:e236. https://doi.org/10.1017/s095026881900116x
Schmidt H, Karch H (1996) Enterohemolytic phenotypes and genotypes of shiga toxin-producing Escherichia coli O111 strains from patients with diarrhea and hemolytic-uremic syndrome. J Clin Microbiol 34(10):2364–2367. https://doi.org/10.1128/jcm.34.10.2364-2367.1996
Schwidder M, Heinisch L, Schmidt H (2019) Genetics, toxicity, and distribution of Enterohemorrhagic Escherichia coli hemolysin. Toxins (basel) 11(9):502. https://doi.org/10.3390/toxins11090502
Singh P, Sha Q, Lacher DW, Del Valle J, Mosci RE, Moore JA, Scribner KT, Manning SD (2015) Characterization of enteropathogenic and Shiga toxin-producing Escherichia coli in cattle and deer in a shared agroecosystem. Front Cell Infect Microbiol 5:29. https://doi.org/10.3389/fcimb.2015.00029
Smith AM, Tau NP, Kalule BJ, Nicol MP, McCulloch M, Jacobs CA, McCarthy KM, Ismail A, Allam M, Kleynhans J (2019) Shiga toxin-producing Escherichia coli O26:H11 associated with a cluster of haemolytic uraemic syndrome cases in South Africa, 2017. Access Microbiol 1(9):e000061. https://doi.org/10.1099/acmi.0.000061
Strockbine NA, Marques LR, Newland JW, Smith HW, Holmes RK, O’Brien AD (1986) Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun 53(1):135–140. https://doi.org/10.1128/iai.53.1.135-140.1986
Tobe T, Sasakawa C (2001) Role of bundle-forming pilus of enteropathogenic Escherichia coli in host cell adherence and in microcolony development. Cell Microbiol 3(9):579–585
Trabulsi LR, Keller R, Tardelli Gomes TA (2002) Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis 8(5):508–513. https://doi.org/10.3201/eid0805.010385
Tzipori S, Gunzer F, Donnenberg MS, de Montigny L, Kaper JB, Donohue-Rolfe A (1995) The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect Immun 63(9):3621–3627
Venegas-Vargas C, Henderson S, Khare A, Mosci RE, Lehnert JD, Singh P, Ouellette LM, Norby B, Funk JA, Rust S, Bartlett PC, Grooms D, Manning SD (2016) Factors associated with Shiga toxin-producing Escherichia coli shedding by dairy and beef cattle. Appl Environ Microbiol 82(16):5049–5056. https://doi.org/10.1128/aem.00829-16
WHO (1998) Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC). Report of a WHO Scientific Working Group Meeting WHO/CSR/APHI/98.8
Williams KJ, Ward MP, Dhungyel OP, Hall EJ (2015) Risk factors for Escherichia coli O157 shedding and super-shedding by dairy heifers at pasture. Epidemiol Infect 143(5):1004–1015. https://doi.org/10.1017/s0950268814001630
Acknowledgements
We would like to thank Mr. Ali Makgato for collecting a portion of the dairy cattle samples. This manuscript is part of a thesis submitted by Alaba S. Olawole in the Veterinary Public Health section, Department of Paraclinical Sciences, University of Pretoria, in partial fulfilment of the requirements for the degree of Doctor of Philosophy.
Funding
Open access funding provided by University of Pretoria. This research was funded by the National Research Foundation (NRF) of South Africa (CSRP170528234222 and SARCHI COP Grant 120317), a South African Medical Research Council Self-Initiated Research (MRC/SIR 2017–2019) grant, and the UNICEF Future Africa-UP One Health for Change Research Grants-2021.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.K. and A.S.O.; data curation, M.K and A.S.O. T.Y.F, and M.N.M.; formal analysis M.K., and M.N.M. and A.S.O.; funding acquisition, M.K., B.T.C.G., E.E., and A.S.O.; investigation, M.K., A.S.O.,T.Y.F, M.N.M., M.C.M. W.M.T.; methodology M.N.M., T.Y.F. and A.S.O.; resources, M.K., B.T.C.-G. and L.G.; Project administration, M.K.; supervision, M.K. writing original draft, M.K. and A.S.O.; Writing-review and editing M.K., A.S.O.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olawole, A.S., Malahlela, M.N., Fonkui, T.Y. et al. Occurrence, serotypes and virulence characteristics of Shiga toxin-producing and Enteropathogenic Escherichia coli isolates from dairy cattle in South Africa. World J Microbiol Biotechnol 40, 299 (2024). https://doi.org/10.1007/s11274-024-04104-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-04104-w