Abstract
This study evaluates the capacity of commercial formulations of synthetic fungicides to inhibit grapevine bacterial growth when sprayed on vineyards to control diseases, such as downy mildew, powdery mildew and secondary rots. Fungicide sensitivity plate assays were carried out on bacteria isolated from vineyards that were also identified and characterized for their plant growth-promoting (PGP) traits and antifungal activity. The high taxonomic variability of bacteria screened with different chemical classes of fungicides is one new finding of this study. Seven out of 11 fungicides were able to inhibit the growth of bacteria at a concentration corresponding to the maximum dose allowed by law in spray treatments of vineyards. Bacterial sensitivity to each fungicide varied greatly. Many sensitive isolates displayed PGP traits and/or antagonistic activity. This study shows the potential impact of fungicidal treatments on grapevine bacterial microbiota. The involvement of bacteria beneficial to the growth and health of plants underlines the importance of this investigation. Our data reveal that the control of a certain disease may be possible using fungicides that have no or low impact on natural non-target microbiota. Understanding the action mechanisms of the active ingredients in these products is a priority for the development of new eco-friendly pesticides.
Highlights
Effects of synthetic fungicides on grapevine bacteria were analyzed.
Plant growth promoting (PGP) traits and antifungal activity of strains were assayed.
Great variability was observed among bacteria regarding fungicide sensitivity.
Many sensitive strains had PGP traits and antifungal activity.
Potential impact of fungicides on grapevine bacterial microbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The grapevine (Vitis vinifera) is the most widely cultivated fruit crop in the world and has enormous economic value. The susceptibility of almost every part of this crop to diseases causes substantial economic losses in the grape and wine sectors. Fungi are the most important agents of grapevine diseases, so fungicides, mostly synthetic, are frequently used to treat this crop. Grapevine synthetic fungicides are made from different chemical classes of molecules (e.g., anilinopyrimidine, dithiocarbamate, triazole, strobilurin, carboxamide and phenylamide) and new ones are being developed by companies in a bid to improve their effectiveness in controlling crop pathogens. However, the intensive application of fungicides, together with other pesticides deployed against bacteria, insects and weeds, gives rise to serious concerns about agricultural sustainability (Sergazina et al. 2021).
The accumulation of fungicides affects the biodiversity, including that of microorganisms, in the crop field and the surrounding ecosystems (Gikas et al. 2022). In fact, fungicides can alter the composition of bacterial and fungal communities due to their effects on non-target organisms (Marinho et al. 2020). Schaeffer et al. (2017) reported that fungicides reduced fungal richness and diversity in exposed flowers, but did not affect the bacterial community. Other studies confirmed the impact of fungicides on the soil microbial community, including bacteria (Sułowicz et al. 2016; Wang et al. 2020; Zhang et al. 2021). The toxicity of grapevine fungicides with regard to the cell viability and growth of various bacteria has previously been described (Marinho et al. 2020). Moreover, the effects of fungicides on wine yeasts may directly or indirectly affect the fermentation process (Oliva et al. 2020).
The impact of fungicides on non-target microorganisms is even more important when such microorganisms are pathogen antagonists and have plant growth-promoting (PGP) traits. Ahemad and Saghir Khan (2012) reported that fungicide stress can reduce the PGP activities of a Pseudomonas putida strain used as bioinoculant. The screening of pesticide-tolerant non-target PGPR bacteria for use as biofertilizers has been also recommended (Shen et al. 2019). Data on the sensitivity of bacteria to fungicides are fundamental in promoting the combined use of a bacterial inoculum for biocontrol and pesticides as sustainable disease management. Moreover, fungicides can negatively affect the composition of natural bacterial communities that otherwise could be very helpful for plant development and pathogen control. However, information about this impact on non-target microbial populations, including useful ones living on crops, is still very limited.
This study investigates the sensitivity of bacteria associated with grapevines (epiphytic, endophytic and rhizospheric strains) to some of the most common synthetic commercial fungicides used to control fungal diseases in vineyards, such as downy mildew, powdery mildew and secondary rots. Plate assays were carried out to assess the inhibitory effects of fungicides on bacterial growth at a concentration corresponding to the maximum dose allowed in spray treatments for vineyards. Several different isolates, including grapevine bacteria that were isolated and identified in this study, were analyzed and characterized for their PGP traits and antagonistic activity against fungi. To the best of author’s knowledge, this is the first investigation into the evaluation of fungicide sensitivity of high numbers of epiphytic, endophytic and rhizospheric grapevine bacteria, including those beneficial to plants. The significance of the impact of fungicides on these non-target microorganisms is discussed.
Materials and methods
Sampling and isolation of bacteria
Plants were sampled in abandoned or cultivated vineyards with organic methods where no synthetic fungicides were used, located in different areas (Verona, Vicenza and Trento) of a limited part of north Italy (the maximum distance between two areas was about 100 km as the crow flies) (Table S1). Leaves, petioles and portions of 1-, 2-, or 3-old year steams of Vitis vinifera or unidentified Vitis spp. rootstock were collected to isolate the bacteria. The isolation of endophytic bacteria, from leaves or steams was carried out using the protocol described by Andreolli et al. (2016). Briefly, plant samples were vigorously washed in distillated water for 5 min and their surface treated for 10 min with a 1% (w/v) sodium hypochlorite (NaOCl) solution. Then, after rinsed these portions three times in sterile distilled water, small pieces were collected, using a sterile blade, in sterile 2-ml Eppendorf tubes containing 0.5-1.0 mL of physiological solution (0.9% w/v NaCl). The tubes were placed for 1 h on an orbital shaker at 27 °C, then serial dilutions were plated on R2A-agar (yeast extract 0.5 g/L, peptone 0.5 g/L, casein acid hydrolysate 0.5 g/L, glucose 0.5 g/L, starch 0.5 g/L, sodium pyruvate 0.3 g/L, K2HPO4 0.3 g/L, MgSO4·7H2O 0.05 g/L, agar 15.0 g/L). All plates were incubated for 3–5 days at 27 °C, then colonies were picked up and purified by repeated streaking on the same medium. The absence of epiphytic microorganisms was verified by plating 200 µl of water derived from the third rinsing of plant portions used to isolate endophytic bacteria on the medium.
More than 100 isolates were considered and 58 out of them were selected (Table S1) according to the isolation sample, colony and cell morphology.
Identification of bacteria
Identification of isolates was carried out through the sequencing of 16 S rRNA gene. Total DNA was extracted from bacterial cultures as previously described (Andreolli et al. 2011) and 16 S rRNA gene amplification conditions were described by Andreolli et al. (2016). Amplicons were purified using a commercial kit (NucleoSpin gel and PCR Clean-up, Macherey-Nagel, Düren, Germany), then the sequencing was carried out by Eurofins Genomics (Eurofins Genomics, Edersberg, Germany) in both directions using the same primers used for amplification. Sequences were searched for similarity by relying on the EzTaxon-E (www.ezbiocloud.net/resources/16s_download). The identification was gained considering the highest score obtained in each alignment provided by EzBio-Cloud’s Identify service that searches the sequence similarity against a database of 16 S rRNA sequences quality-controlled.
Characterization of plant growth promoting traits in bacteria
The ability to solubilize phosphate by bacteria was carried out in plate assay using two media, National Botanical Research Institute’s phosphate growth medium (NBRIP) and Pikovskaya medium (PVK) (Nautiyal 1999) that were supplemented with Ca3(PO4)2 or CaHPO4. The production of siderophores by bacteria was evaluated in plate according to the protocol of Schwyn and Neilands (1987). Both assays were evaluated by measuring the clear halo size (measured along short axis) around the colony and related activity was considered as follows: 0 mm, no activity ( ̶ ); from 1 to ≤ 2 mm, weakly positive (+/̶ ), from 3 to ≤ 4 mm, positive (+), > 4 mm, strongly positive (++). Assays were performed in triplicate.
Antagonism assay against fungi
Antagonism assay was carried out to evaluate the inhibitory activity of bacteria against fungi Aspergillus uvarum An3 and Botrytis cinerea ITEM 1719 (Lorenzini and Zapparoli 2020). A suspension of 106 conidia per mL obtained from fungal culture of 7–10 days in MEA at 25 °C, were spread in Nutrient agar (Condalab, Madrid, Spain) containing 1% (w/v) malt extract and 1% (w/v) glucose. Plates were incubated at 25 °C for 24 h, then a 20 µL spot of bacterial culture grown 48–72 h in Nutrient, containing about 108 cells/mL, was poured on the medium surface. Bacterial culture spots were leave to dry for 20 min in a laminar flow hood, then plates were incubated at 25 °C for 3–4 days, and examined daily. The formation of clear zone around the bacterial colony due to the lack of mycelial growth indicated antagonistic activity of bacteria against the fungus. This activity was positive (+) or strongly positive (++) when distance between margin of bacterial colony and mycelium was at least 2 and 4 mm. respectively. Assays were performed in triplicate.
Fungicide sensitivity assay
This assay was carried out on a total of 100 bacteria, 58 isolated and identified in this study as above described and 42 isolated in previous studies (Andreolli et al. 2016; Lorenzini and Zapparoli 2020). A total of 11 commercial formulations of synthetic fungicides on sale in Italy was used (Table S2). An amount of each product (powder or concentrated emulsion), taken from the package, was diluted in water in stock solutions to use for the plate assay at desired concentration. Each fungicide was used at a concentration corresponding to the maximum dosage allowed in vineyards according to information reported in the product. Nutrient agar was used as assay medium. Final concentrations per mL of medium were: Dedalus® 2.3 µL, Lidal® 3.75 µL, Topas® 0,3 µL, Ridomil® Gold SL 0.23 µL, Cantus ® 1.20 mg, Switch® 0.8 mg, Prolectus® 50WG 1.0 mg, Tucana® 0.4 µL, Flint ® 0.25 mg, Carson® 1.35 mg, Folpan® 2.0 mg. An aliquot of diluted fungicide was poured in tube containing this medium maintained at 40 °C, then vigorously mixed and immediately poured into a sterile plate. After the solidification, plates were inoculated with 20 µL spot of bacterial culture grown 48–72 h in Nutrient, then leave to dry for 20 min in a laminar flow hood. Plates were incubated at 25 °C for 4 days, and the colony growth was examined daily. Fungicide was inhibitor when the colony diameter was shorter than 2 mm after 4 days. A plate of the same medium without fungicide was used as control. Assays were performed in triplicate.
Results
Identification of bacteria
A total of 14 genera was recognized by analyzing 16 S rRNA gene sequence of 58 bacteria isolated in this study, 6 from the rhizosphere and 52 from the phyllosphere (7 endophytic and 45 epiphytic) of grapevines, according to the comparative sequence analysis on web-accessible databases (Table 1). Bacillus and Pseudomonas were the most frequent genera (14 isolates each), while Acinetobacter, Frigoribacterium, Kasakoina, Microbacterium, Micrococcus and Staphylococcus were represented only by one isolate.
Analysis of plant growth-promoting (PGP) traits and antagonistic activity
A total of 51 out of 58 (88%) isolates exhibited PGP traits and/or antagonistic activity towards fungi. Specifically, 43 (74.1%) displayed ability to solubilize phosphate and/or produce siderophores, while 14 (24.1%) showed antifungal activity against A. uvarum An3 and B. cinerea ITEM 1719 (Table 2). Six isolates (Bacillus sp. V3Be, V5B and V82, Priestia sp. FM6 and Pseudomonas sp. PT1e) had at least one PGP trait and antagonistic activity. Twenty-six isolates were able to solubilize the phosphate in both forms, Ca3(PO4)2 and CaHPO4, while 9 isolates only in one form in NBRIP and/or PVK medium. Pantoea sp. PT2D had pronounced ability to solubilize the phosphate in both forms in the two media. Pronounced ability in either form, but only in one medium, was observed in 8 isolates (Curtobacterium sp. PT2A, Priestia sp. V13M, Priestia sp. FM1 and Pseudomonas sp. ITAVF in NBRIP medium, Erwinia sp. V13F, Pantoea sp. PT14, Priestia sp. FM6 and Pseudomonas sp. LG4M in PVK medium). Other isolates (e.g., Bacillus sp. V5B, Microbacterium sp. PT13, Paenibacillus sp. VT3) produced halos only on one specific combination of medium and phosphate source. The production of siderophores was exhibited by 20 isolates (51.7%) and 13 of them were also able to solubilize the phosphate. In particular, Pseudomonas sp. ITAVE and Pseudomonas sp. LG4T were positive in all PGP trait assays.
Most Bacillus sp. isolates displayed antagonistic activity against A. uvarum An3 and B. cinerea ITEM 1719 (Table 2). Apart from Bacillus sp. V12e, which strongly inhibited only on B. cinerea ITEM 1719, all isolates exhibited antagonism against both fungal strains. Among isolates with antagonistic activity, Pseudomonas sp. PT1e exhibited phosphate solubilization ability and siderophores production, Bacillus sp. V5B and Bacillus sp. V82, Priestia sp. FM6 and Pseudomonas sp. ITAVF displayed only phosphate solubilization, while Bacillus sp. V3Be only siderophores production.
Fungicide sensitivity assay
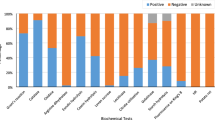
A total of 100 isolates, 58 isolated in this study and 42 previously investigated (Andreolli et al. 2016; Lorenzini and Zapparoli 2020), were tested for their sensitivity to 11 fungicides (Table 3). Four fungicides (Ridomil Gold®, Cantus®, Prolectus® and Flint®) did not inhibit the growth of any isolates, while the other 7 (Dedalus®, Lidal®, Topas®, Switch®, Tucana®, Carson® and Folpan®) were able to inhibit bacterial growth. A total of 16 isolates tolerated all these inhibitor fungicides, while the remaining 84 isolates were sensitive at least to one of them (Fig. 1). Twenty-six out of 28 genera/OUT had sensitive isolates. Nine isolates of Bacillus, Brevibacillus, Frigoribacterium, Lysinbacillus, Paenibacillus and Priestia were sensitive to all seven fungicides. The 16 isolates tolerant to all fungicides belonged to Kasakonia, Stenotrophomonas, Curtobacterium, Erwina, Massilia, Pantoea and Pseudomonas. A total of 41 isolates were sensitive to 5 or 6 fungicides. Bacillus, the most frequent genus in this study with 25 isolates out of 100 assayed for fungicide sensitivity, had 24 isolates sensitive at least to 4 fungicides. Pseudomonas, the second most represented genus with 15 isolates, had 5 tolerant and 10 sensitive isolates. Five Pseudomonas isolates were sensitive to only one fungicide.
Analyzing each inhibitory fungicide, Carson® affected the highest number of genera/OTU (24 out of 28) inhibiting 59 isolates, while Folpan® inhibited the highest number of isolates (64 out of 84) belonging to 22 genera/OTU (Fig. 2). Tucana® was the least inhibitory fungicide in terms of number of isolates and genera/OTU (18 and 10, respectively).
Based on the results of fungicide sensitivity of each isolate, 15 fungicide sensitive-isolate groups (S1A-S7) were recognized (Fig. S1). The most numerous group was S5A made up of by 13 isolates, all belonging to Bacillus, while seven groups, that is, S1B, S2B, S2C, S3, S5B, S5D and S6C, were represented only by one isolate.
Interestingly, fungicides exerted different effects on isolates with PGP traits and/or antagonistic activity, as described above. A total of 39 of 51 isolates (76%) with PGP traits and/or antagonistic activity were sensitive to fungicides. Pseudomonas sp. ITAVE, which had all PGP traits, was tolerant of all fungicides. Among isolates that were positive in all the phosphate splubilizing assays, Pseudomonas sp. VT1 and Massilia sp. LG6 were tolerant to 7 fungicides, while Pantoea sp. VO21 and Pantoea sp. PT2D were sensitive to 1 and 6 fungicides, respectively. It is worth mentioning that Pseudomonas PT1e, which is able to solubilize the phosphate in CaHPO4 form, produces siderophores and exerts a strong antagonistic activity, was also sensitive to 5 fungicides, Bacillus sp. V5B and Bacillus sp. V82, which displayed phosphate solubilization and antagonistic activity, were sensitive to 6 fungicides. The other Bacillus isolates with strong antagonistic activity (e.g., VAe, V3Be, V13C, FM5 and G2) were sensitive at least to 4 fungicides.
Most isolates (91%) with previously characterized PGP and/or antagonistic activity (Andreolli et al. 2016; Lorenzini and Zapparoli 2020) were sensitive to fungicides.
Discussion
The isolation and identification of several bacteria associated with grapevines conducted in this study, in addition to previously analyzed isolates (Andreolli et al. 2016; Lorenzini and Zapparoli 2020), enabled these to be investigated in a representative bacterial community of this agroecosystem. Since foliar fungicides were assayed, most isolates were from the phyllosphere and only a few from the rhizosphere, although non-target impacts of active ingredients in soil microbiota have also been documented (Sułowicz et al. 2016; Roman et al. 2021).
On analyzing 58 isolates, such as Bacillus, Pseudomonas, Pantoea and Curtobacterium, most of the identified genera were frequently detected in the grapevine phyllosphere, with both endophytes and epiphytes retrieved from leaves, petioles and canes, in varying degrees of abundance (Martins et al. 2012; Andreolli et al. 2017). On the other hand, to the best of author’s knowledge, no recovery has ever been documented of Kosakonia from the grapevine, a genus frequently identified in other crops like rice (Walitang et al. 2017).
The high number of bacterial isolates (88%) that displayed PGP traits and/or antagonistic activity among those analyzed in the study highlights the positive potential of the microbiota for the growth and health of plants. These beneficial features of the bacteria in the phyllosphere and rhizosphere of plants, including those of the grapevine, are well documented (Etminani and Harighi 2018; Pacifico et al. 2019). Our investigation reveals great variability among bacteria with regard to phosphate solubilization and siderophore production. This diversity was particularly noticeable within isolates belonging to the same genus, such as Pseudomonas and Pantoea, represented by a high number of isolates. In addition to confirming the results of previous investigations into the phosphate solubilization ability of these bacteria (Aarab et al. 2015; Li et al. 2019), our assays, carried out in different media and forms of phosphate, suggest the involvement of different metabolisms for mineral solubilization in isolates (Alori et al. 2017). The predominance of Bacillus isolates among those with antagonistic activity corroborates the importance of these Firmicutes in controlling fungal pathogens (Bruisson et al. 2019). The occurrence of Bacillus in the grapevine phyllosphere and rhizosphere appears even more relevant considering that only another two Pseudomonas isolates and one Priestia isolate displayed such antagonistic activity. Strains with antifungal activity among these latter genera and others found in agroecosystems have been reported (Niem et al. 2020; Shahid et al. 2022). Their low incidence seen here is therefore interesting and needs to be confirmed by further investigation.
In vitro fungicide assays displayed different bacterial behaviour, including among those with PGP traits and antagonistic activity. The high number of bacterial genera/OTU screened for sensitivity to fungicides of different chemical classes is a new finding of this study. Bacteria, mainly Firmicutes such as Bacillus, were particularly sensitive to Folpan®, the fungicide that affected the highest number of isolates. Its active principle (folpet) is a multi-site inhibitor acting on several target sites in fungi simultaneously. Folpet and its degradation product (thiophosgene) can interact with thiols such as glutathione, an essential molecule for keeping the redox homeostasis and iron metabolism (Toledano et al. 2007; Canal-Raffin et al. 2008). Anjum et al. 2011) reported sensitivity in 35 non-identified bacteria to folpet, where the MIC was up to 1600 µg/mL. This concentration, which corresponds to the maximum dose allowed per spray application in vineyards, was used in our study to identify the sensitivity of bacteria to folpen.
Carson® (cymoxanil), used primarily to control downy mildew, affected the growth of more than 50% of assayed isolates. Cymoxanil was discovered in 1972 but its primary mechanism of action on fungi is still unknown (Hillebrand et al. 2019). In this study, the assessment of the inhibitory effects of this molecule on bacterial growth is a significant advance, given that very little information is available in the literature. Previously, Marinho et al. (2020) did not find any sensitivity of Escherichia coli, Pseudomonas putida and Arthrobacter sp. to 46 mg/L cymoxanil, a concentration 13 times lower than the maximum dose allowed in vineyards.
Dedalus® (tebuconazole), Lidal® (tetraconazole) and Topas® (penconazole), used to control powdery mildew in vineyards, are 1,2,4-triazole fungicides, a class of heterocyclic rings introduced in the 1970s. They act against the biosynthesis of ergosterol, an essential compound of the fungal membrane, as a mechanism of action against the target fungus (Deising et al. 2008). However, the effects of these molecules on soil bacteria has previously been documented (Zhang et al. 2014; Baćmaga et al. 2015; Sułowicz et al. 2016). Observations reported by Zhang et al. (2014), on the decrease of the ratio of gram-negative to gram-positive bacteria in soil treated with tetraconazole, are not in accordance with our study. In fact, Lidal® and the other two triazole fungicides were particularly active against gram-positive bacteria (Firmicutes were strongly inhibited, as were more than half the Actinobacteria), while they had limited effects on gram-negative bacteria (Proteobacteria). Baćmaga et al. (2015) observed changes in the relative abundance of Proteobacteria, Firmicutes, Actinobacteria and other phyla, depending on the dose of tebuconazole in the soil, and also reported the prevalence of Bacillus, Brevibacillus and Pseudomonas in soil treated with 10 mg/kg of this molecule. Obviously, inconsistencies between the data obtained by analyzing soil microbiota and our results are not surprising since different experimental approaches were used to evaluate the fungicide impact on bacteria. In this study, over 90% of the isolates that were sensitive to one of three molecules were also sensitive to the other two. This was expected, due to the similarity of the molecular structure of tebuconazole, tetraconazole and penconazole. Although the mechanism of their action against bacteria remains unclear, investigations into 1,2,4-triazole derivates suggest that they may have inhibitory potential against enzymatic proteins that are essential for bacteria (e.g., ATPase, DNA gyrase, glucosamie-9-phosphate synthase). These derivates bear fragments, such as quinazoline and quinazolinone, that have been demonstrated to be effective against some phytopathogen gram-negative bacteria, such as Xanthomonas oryzae and Ralstonia solanacearum (Angajala et al. 2016; Yang and Bao 2017; Shi et al. 2020). As these fungicides can induce resistance in fungal pathogenic populations in agroecosystems or other environments (Bowyer and Denning 2014), it cannot be ruled out that similar effects could occur in bacteria, an important area that requires further investigation.
The two active ingredients of Switch®, cyprodinil and fludioxonil, used to control secondary rots, target the high osmolarity glycerol pathway of fungi. In this study, this fungicide was shown to inhibit several bacteria, especially Firmicutes and Actinobacteria. According to the literature, both molecules are potentially effective against bacteria and could be effective alone or in combination. Ejim et al. (2004) observed that cyprodinil inhibited Escherichia coli and Staphylococcus aureus at concentrations more than four and two times lower than allowed in vineyards (300 mg/L), respectively. Keum et al. (2010) reported that the fludioxonil molecule acts on the pyrrolnitrin biosynthesis of bacteria and, consequently, serves as a moderate inhibitor of growth. Of course, further experiments using these two active ingredients separately must be carried out to evaluate the effects of each one on bacteria.
Tucana® (pyraclostrobin) is a strobilurin fungicide that acts on the mitochondrial respiratory chain and is mainly used to control powdery mildew in vineyards. Its effects on bacteria have previously been investigated (Skandalis et al. 2016; Lu et al. 2019). However, only Lu et al. (2019) described the in vitro inhibitory activity of this molecule in a cyanobacterium culture of Microcystis aeruginosa. Although Tucana® proved to be less inhibitory in terms of the number of isolates, its effects have been deployed against the genera most frequently found in agroecosystems, such as Pseudomonas, Bacillus, Pantoea, Paenibacillus and Curtobacterium. According to Baćmaga et al. (2015), who analyzed the effects of azoxystrobin, a strobilurin fungicide similar to pyraclostrobin, these molecules could affect the activities of enzymes like dehydrogenase, phosphatase, catalase and urease that are fundamental for bacterial growth. Our study found that Flint®, a strobilurin fungicide containing trifloxystrobin used to control powdery mildew, was ineffective in inhibiting bacteria at concentrations of 250 mg/L, which suggests that strobilurins may have different specificities on target proteins or other molecules. Further investigation to individuate their possible molecular targets should consider the high structural variability of molecules of this chemical class.
Other fungicides that did not affect the growth of bacteria at the concentration corresponding to maximum dose allowed for spraying in vineyards included Cantus® (boscalid) and Prolectus® (fenpyrazamine), mainly used to control powdery mildew and/or secondary rots. The former is a carboxamide fungicide inhibitor of succinate dehydrogenase, the latter an amino-pyrazolone that inhibits ergosterol biosynthesis. Ridomil Gold ® is used mainly against downy mildews in vineyards and contains metalaxyl-M, which acts on the polymerase complex of rRNA synthesis of fungi. To the best of author’s knowledge, this is the first study on the effects of these three fungicides on bacteria. Previous investigations into the impact of boscalid and metalaxyl-M on bacteria concerned experiments with treated soil (Ahmed El-Imam and Machido 2012; Wang et al. 2020).
This work clearly demonstrates the effects of fungicides on grapevine bacteria, through in vitro experimentation. The effective impact of these molecules on bacterial microbiota in the field has yet to be evaluated. This result could be obtained with field trials by analyzing culturable and unculturable microbial communities of the grapevine.
Conclusion
This study demonstrates that fungicides designed for protecting grapevines from the most important fungal pathogens have different effects on non-target bacteria. Inter- and intra-species sensitivity of bacteria, including those with PGP traits and antifungal activity, observed by screening many genera/OTU with several chemical classes of fungicides, is a new finding. The potential negative impact of fungicidal treatments on natural bacterial populations of grapevines has been clearly highlighted by the observation that most of the isolates involved are beneficial to plant growth. The evidence that fungicides that target the same fungal pathogen or disease have different inhibitory effects on these isolates paves the way for a discussion about the use of molecules that have less impact on the bacterial microbiota. For example, carboxamide or strobilurin fungicides, in particular trifloxystrobin, may be preferable to triazolic fungicides in controlling powdery mildew. Similarly, spray applications of boscalid or fenpyrazamine against secondary rots may be advisable as well as metalaxyl-M for controlling downy mildews. This practice may increase the positive effect of either autochthonous PGP bacteria or exogenous biocontrol and biofertilizer agents. The use of selected exogenous PGP bacteria, as alternative strategies to counter phytopathogenic fungi and improve plant nutrient assimilation, is becoming increasingly widespread. In particular, appropriate experiments should be carried out in the field to assess the antagonistic activity of these bacteria against obligate phytopathogens, such as powdery and downy mildew, against which fungicide treatments are necessary. In this context, investigations into the compatibility of a certain fungicide with selected bacterial biocontrol agents must be encouraged. Since the impact of fungicides on natural bacterial populations could favour the selection of resistant strains, it appears clear that any loss of microbial biodiversity due to fungicidal treatments should be avoided. Finally, the understanding of the mechanisms of action of these molecules on non-target microorganisms is a priority in order to design new eco-friendly pesticides.
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Aarab S, Ferdaouss H-A, Laglaoui A, Bakkali M, Arakrak A (2015) Solubilization of inorganic phosphate by Pseudomonas strains isolated from rice rhizosphere. In J Biosci 6:116–124
Ahemad M, Saghir Khan M (2012) Effect of fungicides on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica campestris) rhizosphere. Chemosphere 86:945–950. https://doi.org/10.1016/j.chemosphere.2011
Ahmed El-Imam AM, Machido DA (2012) Growth of ammonium oxidizing bacteria in soil treated with some fungicides. Global J Environ Sci 11:1–7
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Andreolli M, Lampis S, Vallini G (2017) Diversity, distribution and functional role of bacterial endophytes in Vitis vinifera. In: Maheshwari DK (ed) Endophytes: Biology and Biotechnology. Springer, Cham, pp 233–266
Andreolli M, Lampis S, Zapparoli G, Angelini E, Vallini G (2016) Diversity of bacterial endophytes in 3 and 15 year-old grapevines of Vitis vinifera cv. Corvina and their potential for plant growth promotion and phytopathogen control. Microbiol Res 183:42–52
Andreolli M, Lampis S, Zenaro E, Salkinoja-Salonen M, Vallini G (2011) Burkholderia fungorum DBT1: a promising bacterial strain for bioremediation of PAHs-contaminated soils. FEMS Microbiol Lett 319:11–18
Angajala KK, Vianala S, Macha R, Raghavender M, Thupurani MK, Path PJ (2016) Synthesis, anti-inflammatory, bactericidal activities and docking studies of novel 1,2,3-triazoles derived from ibuprofen using click chemistry. Springerplus 5:423
Anjum R, Grohmann E, Malik A (2011) Molecular characterization of conjugative plasmids in pesticide tolerant and multi-resistant bacterial isolates from contaminated alluvial soil. Chemosphere 84:175–181
Baćmaga M, Kucharski J, Wyszkowska J (2015) Microbial and enzymatic activity of soil contaminated with azoxystrobin. Environ Monit Asses 187:615–629
Bowyer P, Denning DW (2014) Environmental fungicides and triazole resistance in aspergillus. Pest Manag Sci 70:173–178
Bruisson S, Zufferey M, L’Haridon F, Trutmann E, Anand A, Dutartre A, De Vrieze M, Weisskopf L (2019) Endophytes and epiphytes from the grapevine leaf microbiome as potential biocontrol agents against phytopathogens. Front Microbio 10:2726
Canal-Raffin M, l’Azou B, Jorly J, Hurtier A, Cambar J, Brochard P (2008) Cytotoxicity of folpet fungicide on human bronchial epithelial cells. Toxicology 249:160–116
Deising HB, Reimann S, Pascholati SF (2008) Mechanisms and significance of fungicide resistance. Braz J Microbiol 39:286–295
Ejim LJ, D’Costa VM, Elowe NH, Loredo-Osti JC, Malo D, Wright GD (2004) Cystathionine β-lyase is important for virulence of Salmonella enterica serovar Typhimurium. Infect Immun 72:3310–3314
Etminani F, Harighi B (2018) Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol 34:208
Gikas GD, Parlakidis P, Mavropoulos T, Vryzas Z (2022) Particularities of fungicides and factors affecting their fate and removal efficacy: a review. Sustainability 14:4056. https://doi.org/10.3390/su14074056
Hillebrand S, Tietjen K, Zundel JL (2019) Fungicides with unknown mode of action. Mod Crop Prot Compd 2:911–932
Keum YS, Lee HR, Kim J-H (2010) Effects of pesticides on the bacterial production of pyrrolnitrin. J Agric Food Chem 58:5531–5537
Li Y, Zhang J, Zhang J, Xu W, Mou Z (2019) Characteristics of inorganic phosphate-solubilizing bacteria from the sediments of a eutrophic lake. Int J Environ Res Public Health 16:2141
Lorenzini M, Zapparoli G (2020) Epiphytic bacteria from withered grapes and their antagonistic effects on grape-rotting fungi. Int J Food Microbiol 16:319. https://doi.org/10.1016/j.ijfoodmicro.2019.108505
Lu T, Zhou Z, Zhang Q, Zhang Z, Qian H (2019) Ecotoxicological effects of fungicides azoxystrobin and pyraclostrobin on freshwater aquatic bacterial communities. Bull Environ Contamin Toxicol 103:683–688
Marinho MDC, Diogo BS, Lage OM, Antunes SC (2020) Ecotoxicological evaluation of fungicides used in viticulture in non-target organisms. Environ Sci Pollut Res 27:43958–43969
Martins G, Miot-Sertier C, Lauga B, Claisse O, Lonvaud-Funel A, Soulas G, Masneuf-Pomarède I (2012) Grape berry bacterial microbiota: impact of the ripening process and the farming system. Int J Food Microbiol 158:93–100
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Niem JM, Billones-Baaijens R, Stodart B, Savocchia S (2020) Diversity profiling of grapevine microbial endosphere and antagonistic potential of endophytic Pseudomonas against grapevine trunk diseases. Front Microbiol 11:47
Oliva J, Girón F, Cayuela JM, Mulero J, Zafrilla P, Cámara M (2020) Effect of fungicides on the yeast population during spontaneous fermentation in the vinification of Monastrell grapes. LWT 131:109816
Pacifico D, Squartini A, Crucitti D, Barizza E, Lo Schiavo F, Muresu R, Carimi F, Zottini M (2019) The role of the endophytic microbiome in the grapevine response to environmental triggers. Front Plant Sci 10:1256
Roman DL, Voiculescu I, Filip M, Ostafe V, Isvoran A (2021) Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: a review. Agriculture 11:893. https://doi.org/10.3390/agriculture11090893
Schaeffer RS, Vannette RL, Brittain C, Williams NM, Fukami T (2017) Non-target effects of fungicides on nectar-inhabiting fungi of almond flowers. Environ Microbiol Rep 9:79–84. https://doi.org/10.1111/1758-2229.12501
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sergazina M, Vazquez L, Llompart M, Dagnac T (2021) Occurrence of fungicides in vineyard and the surrounding environment. Molecules 26:6152
Shahid M, Zeyad MT, Syed A, Singh UB, Mohamed A, Bahkali AH, Elgorban AM (2022) Stress-tolerant endophytic isolate Priestia aryabhattai BPR-9 modulates physio-biochemical mechanisms in wheat (Triticum aestivum L.) for enhanced salt tolerance. Int J Environ Res Public Health 19:10883. https://doi.org/10.3390/ijerph191710883
Shen F-T, Yen J-H, Liao C-S, Chen W-C, Chao Y-T (2019) Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability 11:1133. https://doi.org/10.3390/su11041133
Shi J, Ding M, Luo N, Wan S, Li P, Li J, Bao X (2020) Design, synthesis, crystal structure, and antimicrobial evaluation of 6-fluoroquinazolinylpiperidinyl-containing 1, 2, 4-triazole Mannich base derivatives against phytopathogenic bacteria and fungi. J Agric Food Chem 68:9613–9623
Skandalis N, Dimopoulou A, Beri D, Tzima A, Malandraki I, Theologidis I, Bitivanos S, Varveri C, Klitsinaris T, Vassilakos N (2016) Effect of pyraclostrobin application on viral and bacterial diseases of tomato. Plant Dis 100:1321–1330
Sułowicz S, Cycon M, Piotrowska-Seget Z (2016) Non-target impact of fungicide tetraconazole on microbial communities in soils with different agricultural management. Ecotoxicology 25:1047–1060
Toledano MB, Kumar C, Le Moan N, Spector D, Tacnet F (2007) The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett 581:3598–3607
Walitang DI, Kim K, Madhaiyan M, Kim YK, Kang Y, Sa T (2017) Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice. BMC Microbiol 17:1–13
Wang CN, Wu RL, Li YY, Qin YF, Li YL, Meng FQ, Wang L-G, Xu FL (2020) Effects of pesticide residues on bacterial community diversity and structure in typical greenhouse soils with increasing cultivation years in Northern China. Sci Total Environ 710:136321
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl) acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC advances 7:34005–34011
Zhang H, Song J, Zhang Z, Zhang Q, Chen S, Mei J, Yu Y, Fang H (2021) Exposure to fungicide difenoconazole reduces the soil bacterial community diversity and the co-occurrence network complexity. J Hazard Mat 405:124208
Zhang W, Xu J, Dong F, Liu X, Zhang Y, Wu X, Zheng Y (2014) Effect of tetraconazole application on the soil microbial community. Environ Sci Pollut Res Int 21:8323–8332. https://doi.org/10.1007/s11356-014-2844-5
Acknowledgements
Not applicable.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
“MA: data curation, methodology, writing-original draft. SL: validation, writing-review and editing. LT: methodology, resources. VM: data curation, formal analysis. GZ: conceptualization, data curation, methodology, writing-original draft, supervision.“
Corresponding author
Ethics declarations
Competing interests
The author declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andreolli, M., Lampis, S., Tosi, L. et al. Fungicide sensitivity of grapevine bacteria with plant growth-promoting traits and antagonistic activity as non-target microorganisms. World J Microbiol Biotechnol 39, 121 (2023). https://doi.org/10.1007/s11274-023-03569-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03569-5