Abstract
The antimicrobial applications of copper (Cu) are exploited in several industries, such as agriculture and healthcare settings. While Cu is capable of efficiently killing microorganisms, sub-lethal doses can induce a viable-but-non-culturable (VBNC) state in bacteria of many distinct clades. VBNC cells cannot be detected by standard culture-based detection methods, and can become a threat to plants and animals as they often retain virulent traits upon resuscitation. Here we discuss the putative mechanisms of the Cu-induced VBNC state. Common observations in Cu-induced VBNC cells include a cellular response to reactive oxygen species, the exhaustion of energy reserves, and a reconfiguration of the proteome. While showing partial overlap with other VBNC state-inducing stressors, these changes seem to be part of an adaptive response to Cu toxicity. Furthermore, we argue that Cu resistance mechanisms such as P-type ATPases and multicopper oxidases may ward off entry into the VBNC state to some extent. The spread of these mechanisms across multi-species populations could increase population-level resistance to Cu antimicrobials. As Cu resistance mechanisms are often co-selected with antibiotic resistance mechanisms, this threat is exacerbated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The antimicrobial properties of copper (Cu) have seen renewed interest in recent decades, in part due to the widespread increase in bacterial resistance to organic antibiotics. Cu-based antimicrobials are widely applied in the agriculture industry, e.g. to combat foot-rot in cattle and sheep (Rensing et al. 2018), and downy mildew in viticulture (Komarek et al. 2010). In addition, Cu surfaces can be an integrated part of decontamination strategies in the food industry, since they effectively kill common bacterial pathogens such as Salmonella enterica and Campylobacter jejuni (Parra et al. 2018; Faúndez et al. 2004). However, the most promising applications of antimicrobial copper are found in the medical field, where antimicrobial-resistant (AMR) (opportunistic) pathogens pose severe and increasing risks to patients and personnel (Cassini et al. 2019). For instance, the prevalence of healthcare-associated infections (HAIs), often by AMR strains, has been linked to microbial contamination of high-touch surfaces (Otter et al. 2013; Boyce 2007, 2016).
Cu antimicrobials come in several distinct formulations, often tailored to the specific application. For instance, Cu salts and organic complexes are used as fungicides (Wang et al. 2018) and algicides (Shen et al. 2019), and Cu-doped zeolites are being investigated to simultaneously disinfect and remove metals from wastewater (Fanta et al. 2019). Cu salt-based formulations can be applied topically to combat infections, e.g. herpes (Clewell et al. 2012; Chen et al. 2016) and vaginitis (Abbott and Abbott 2014). In addition, several Cu salt-based therapies have been developed in recent years (Clewell et al. 2012; Styczynski et al. 2015). In parallel, a surging contribution of Cu nanoparticle (CuNP) formulations, which are intrinsically of interest due to their high surface to volume ratio, has been noted, again often in healthcare settings. For applications, CuNPs can be incorporated into polymeric matrices such as textiles, glass, and polypropylene, as reviewed by Tamayo et al. (2016) and Borkow (2014). These CuNP-infused matrices convey several key advantages, among which the controlled release of Cu ions and the increased surface area of the antimicrobial material. Such materials can be used for food packaging, water disinfection and medical applications. For example, CuNP-containing cotton and bamboo rayon clothing is toxic towards Staphylococcus aureus, which is associated with many HAIs (Anita et al. 2011; Perelshtein et al. 2009; Teli and Sheikh 2013). A final formulation of Cu antimicrobials comes in the shape of solid surfaces of Cu metal and its alloys. Noyce et al. (2006) found that a positive correlation exists between the killing rates of Cu alloy surfaces and their Cu content. The potential antimicrobial applications of Cu surfaces have been reviewed by Vincent et al. (2016), who emphasized their utility in hospital environments owing to their ability to efficiently inactivate HAI-associated strains such as methicillin-resistant S. aureus and vancomycin-resistant enterococci.
Mechanisms of copper toxicity
While highly toxic at excess amounts, Cu ions function as a micronutrient and intracellular Cu ion concentrations must consequently be carefully regulated to achieve viable homeostasis. Specifically related to bacteria, Cu ion toxicity has been shown to have multiple modes of action, and has been expertly reviewed both by Lemire et al. (2013) and Giachino and Waldron (2020). The cell envelope represents an important target since Cu can inhibit the correct enzymatic maturation of lipoproteins by binding to catalytic cysteine residues. This leads to an increased level of toxic intermediates in the inner membrane, in turn activating the envelope stress response (May et al. 2019). In a similar manner, Cu can impede the crosslinking of peptidoglycan and its binding to membrane lipoproteins, thus weakening the cell envelope (Peters et al. 2018). Cu can also oxidize thiol residues involved in the maturation of periplasmic polypeptides, again leading to the buildup of misfolded intermediates (Ito and Inaba 2008).
For CuNPs and Cu surfaces, the direct contact with bacterial cells seems to be an essential facet of toxicity, next to the release of Cu ions (Vincent et al. 2018). This phenomenon is termed ‘contact killing’, and has been elegantly demonstrated by Mathews et al. (2013), by comparing killing rates of naked Cu surfaces to Cu surfaces covered with an inert polymeric grid, inhibiting contact killing but not the release of ions into the medium. Membrane lipid peroxidation has been proposed as a key process in contact killing (Hong et al. 2012; Grass et al. 2011), but the exact mechanism of toxicity remains unclear.
Finally, another general mechanism of Cu toxicity is the generation of reactive oxygen species (ROS), such as hydroxyl and hydroperoxyl radicals, which can damage cellular components. Since it remains difficult to directly identify intracellular ROS, evidence for this toxicity mechanism has been mostly circumstantial. Consequently, the exact contribution of ROS generation to overall Cu toxicity is ambiguous. However, Macomber et al. (2007) have shown that Cu catalyzes hydroxyl radical formation in the periplasm in vivo, and Cu-catalyzed ROS generation via a Fenton-like process has been demonstrated in vitro (Valko et al. 2005; Stohs and Bagchi 1995). In addition, the high affinity of the cuprous ion for thiol groups can lead to the depletion of antioxidants like glutathione and the destruction of redox-active Fe-S clusters (Macomber and Imlay 2009; Arguello et al. 2013).
Copper toxicity induces the VBNC state
It is clear that elevated Cu concentrations are highly toxic to bacteria. In order to understand the bacterial response to this toxicity, it is relevant to study the effects of sub-lethal Cu concentrations. A curious observation in this regard is the apparent loss of culturability of Cu-exposed bacterial cells. At the same time, these cells often retain characteristics of viability such as intact cell membranes and metabolic activity. An overview of tested strains exhibiting this behavior is provided in Table 1. The latter includes human as well as plant pathogens, reflecting the medical and agricultural use of Cu antimicrobials.
The question arises whether these observations are merely a consequence of sustained cell damage, which we will denote as ‘sublethal injury’, or whether it concerns the active induction of a regulated cellular state as a response to the perceived Cu stress. This cellular state is often denoted as the ‘viable-but-non-culturable’ (VBNC) state. An excellent review on the phenotypic characteristics and the mechanisms of identification has been written by Pinto et al. (2015). VBNC cells cannot be cultured on standard laboratory media, but retain membrane integrity, and low but measurable levels of metabolism and gene expression (Davey 2011; Schottroff et al. 2018). They are formed under harsh conditions like starvation and in the presence of various chemical and physical stressors. The VBNC state displays similar traits to the so-called ‘cellular quiescence’, a noted survival strategy of Mycobacterium tuberculosis, but no in-depth comparison of these states has been performed (Rittershaus et al. 2013; Betts et al. 2002). It is often difficult to experimentally verify whether a cell population is sub-lethally injured or in the VBNC state, or both, since many measurable properties are shared by both cell states. One key distinction is the inability of sub-lethally injured cells to grow on selective media, while colony growth would be observed on non-selective media, as claimed by Bogosian and Bourneuf (2001). However, it is abundantly clear that no extant culture medium is entirely non-selective. Thus, this definition of sub-lethal injury seems untenable, especially when comparing species over multiple clades, each with their own optimal growth conditions. Consequently, many researchers do not make the above distinction and term both cell states under the VBNC denomination. Furthermore, the evolutionary conservation of entering a VBNC state in response to a range of stress conditions and the growing body of evidence elucidating its molecular mechanisms indicates that it is a cell-programmed phenomenon. Finally, there exists an ongoing discussion about the similarities between VBNC cells and persister cells. Persister cells are slow-growing, antibiotic-tolerant cells that represent a subpopulation of actively dividing cultures. This phenotypic heterogeneity allows for rapid recolonization of habitats after transient stress conditions (Fisher et al. 2017). The VBNC state shares characteristics with persister cells, such as morphology and resuscitation characteristics. Based on these similarities, Kim et al. (2018) suggested that VBNC cells and persister cells are one and the same. However, this conclusion was refuted by Ayrapetyan et al. (2018). Ultimately, an extensive comparison between the VBNC state and the persister phenotype is outside the scope of this review.
A fraction of the VBNC cell population can often be manipulated in order to make them regain culturability. This process is called resuscitation. The ability to show resuscitation is essential when arguing in favor of a programmed VBNC state, and has become a mainstay of VBNC studies (Bogosian and Bourneuf 2001; Pinto et al. 2015). Multiple resuscitation stimuli have been listed by Pinto et al. (2015), but it is difficult to distill information about putative molecular mechanisms considering the large number of experimental conditions and the phylogenetic distance of the tested strains. A note must be made that resuscitation is often difficult to distinguish from regrowth of the remaining culturable population. Protocols such as the analysis of dilution series exist to minimize the likelihood of mistaking regrowth for resuscitation, but they are not consistently used in practice (Whitesides and Oliver 1997). Consequently, this distinction must be approached with caution.
Mechanisms behind the cu‐induced VBNC state
Evidently, Cu is not the only stress capable of inducing the VBNC state. Common stressors in this regard include starvation, desiccation, low temperature, pH extremes and oxidative compounds like hypochlorous acid (Zhao et al. 2017; Pinto et al. 2015). It seems unlikely that a direct regulatory pathway exists between the sensing of each separate stressor and the induction of a general VBNC state. More plausible would be VBNC induction triggered by one or more common consequences elicited by these distinct stressors. Therefore, we may derive insights from the interpolation of these mechanisms.
Oxidative stress can induce the VBNC state in different species of bacteria (Cuny et al. 2005; Liao et al. 2020). In addition, Li et al. (2014) suggested that the oxidative stress regulator OxyR is involved in the regulation of VBNC state induction, based on work of Longkumer et al. (2014), Abe et al. (2007) and Wang et al. (2013). Oxidative stress is an important component of many conditions able to induce the VBNC state, such as desiccation (Franca et al. 2007), starvation (McDougald et al. 2002) and exposure to common disinfectants like hypochlorous acid (da Cruz Nizer et al. 2020). As mentioned before, it is also a substantial component of Cu toxicity. Kan et al. (2020) showed increased levels of superoxide dismutase and alkyl hydroperoxide reductase proteins in Cu-induced Acidovorax citrulli VBNC cells as well as during the early stages of resuscitation. In addition, an increased H2O2 content was detected in Cu-induced Ralstonia solanacearum VBNC cells (Um et al. 2013). Thus, we conclude that oxidative stress elicited by Cu is an important facet of the Cu-induced VBNC state.
The rapid response required for the detoxification of VBNC state-inducing stressors can put a strain on the cellular energy metabolism. For instance, Cu-exposed cells have a lower ATP content, and a decreased electron transport and dehydrogenase activity (Chen et al. 2019; Feng et al. 2020). In addition, this decreased ATP content has been used to define toxic CuNP levels (Reyes et al. 2016). While metabolic activity is by definition maintained in VBNC cells, it is often diminished. ATP levels are often, but not always, lower in VBNC cells (Bai et al. 2019; Kim et al. 2015; Liao et al. 2020). However, ATP content is not a straightforward indicator of general metabolic activity (Parry and Shain 2011) and ATP content in Cu-induced VBNC cells is still to be evaluated. All the while, many proteins involved in central metabolic processes were downregulated in Cu-induced VBNC Acidovorax citrulli cells (Kan et al. 2020). In addition, PHB content, which functions as a cellular energy reserve, is reduced in Cu-induced VBNC Ralstonia solanacearum cells (Um et al. 2013). Consequently, the decrease of metabolic activity due to Cu stress appears to be another vital facet of the Cu-induced VBNC state.
Degradation of the proteome can derive both directly from the interaction of proteins with Cu ions, and from the destructive action of ROS. In addition, the proteome needs some measure of reconfiguration to cope with the imposed stresses, by repairing degraded peptides and synthesizing stress defense mechanisms. Proteome adaptations are common in VBNC cells (Ramamurthy et al. 2014; Zhao et al. 2017) and in cells undergoing Cu stress (Monchy et al. 2006; Nandakumar et al. 2011). However, to date we could find only a single study investigating the proteomic composition of Cu-induced VBNC cells. In Kan et al. (2020), 3 of the 5 COG classes with the most changes in VBNC and resuscitating cells correspond to some aspect of proteome reconfiguration (posttranslational modification, protein turnover, and chaperones; amino acid and transport; translation, ribosomal structure, and biogenesis). It remains unclear to what extent these changes are due to direct changes as a result of Cu toxicity, or to the adaptive response of the cell to this toxicity. Evidence for the latter was found in the upregulation of a Cu-translocating ATPase and superoxide dismutase enzyme.
Overall, it remains clear that more research is needed to characterize Cu-induced VBNC cells. Additional whole-cell proteome, transcriptome, and metabolome studies would provide valuable insights into the complex but similar behavior exhibited by these phenotypically distinct cells. Likewise, it would be interesting to study the heterogeneity of this behavior via single-cell techniques.
Copper resistance mechanisms and their role in entry into the VBNC state
We recently showed that the activity of Cu resistance mechanisms (CRMs) affects entering the VBNC state upon exposure to sub-lethal Cu concentrations (Maertens et al. 2020). It was shown that the extent of VBNC induction was markedly decreased in Cupriavidus strains containing the pMOL30 megaplasmid, encoding many genes involved in Cu resistance. A further decrease was noted when CRM genes were pre-induced by CuSO4. In addition, higher Cu concentrations lead to a higher proportion of the initial cell population entering the VBNC state (Ordax et al. 2006; Jiang et al. 2016; del Campo et al. 2009; Grey and Steck 2001b). These results corroborate that the Cu ions are inducing the VBNC state and that CRMs play an important role in the Cu-induced VBNC state by preventing the buildup of excessive amounts of Cu in the cytoplasm. Cu detoxification occurs via efflux systems as well as chemical modification of Cu+. In Gram-negative bacteria, Cu efflux can be mediated by PI-type ATPases (e.g. CopA) and tripartite HME-RND-driven systems (Heavy Metal Efflux Resistance-Nodulation-Division) such as the CusCBA Ag/Cu efflux pump. Periplasmic multicopper oxidases such as CueO and PcoA add another layer of defense by oxidizing Cu+ to the less toxic Cu2+ (Hobman and Crossman 2015; Bondarczuk and Piotrowska-Seget 2013). These systems are commonly regulated by Cu-responsive transcriptional regulators belonging to at least nine different classes (Rademacher and Masepohl 2012). In Gram-positive bacteria, Cu efflux also seems to be largely mediated by P-type ATPases such as CopA and CopB (Solioz et al. 2010).
While it is clear that CRM activity protects the cell against Cu stress, this protection is not sufficient to maintain culturability under increased Cu concentrations. Culturability can often be regained upon chelation of excess Cu ions by EDTA, DDTC, or complex media such as LB. In the case of resuspension in growth media, we cannot rule out effects other than direct chelation of Cu ions. Conversely, spontaneous resuscitation, without the addition of chelating agents, has been observed in Cupriavidus, Ralstonia, Agrobacterium, and Rhizobium (see Table 1). This behavior occurs only upon low Cu toxicity. In Cupriavidus, spontaneous resuscitation was only observed in strains containing the pMOL30 megaplasmid, highlighting the necessity of CRM in this process. While it would be interesting to further compare the extent of VBNC state induction to the activity and complexity of CRM in the strains listed in Table 1, the multiplicity of the experimental conditions largely prohibits this analysis. Indeed, retesting these strains in more standardized conditions could provide essential results in this regard. While the role of CRM in (spontaneous) resuscitation requires further study, the risk for VBNC cells escaping common detection strategies while retaining the potential for resuscitation has been emphasized previously (Ding et al. 2017). An additional risk factor is the spread of metal resistance genes within and between bacterial populations repeatedly exposed to metal stress, which could exacerbate this effect. While the Cu-induced VBNC state has been described in (opportunistic) pathogens such as E. coli O104:H4 and P. aeruginosa PAO1, no in vivo studies have been undertaken to determine the generation of difficult-to-detect VBNC cells by existing Cu-based therapies. However, such studies could provide interesting and relevant data for the medical field.
Concluding remarks
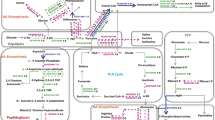
Recent years have seen the curious observation of a Cu-induced VBNC state in many phylogenetically distinct bacterial species. Here we summarized these observations and investigated the mechanisms of VBNC state induction by Cu toxicity (Fig. 1). While more research into this matter is needed, we argue that the VBNC state induced by Cu is the result of an adaptive response to this stressor. Sub-lethal Cu concentrations bring about cellular damage, rendering the cell unable to multiply. In response it will redirect its metabolism to enable reparation of sustained damage and synthesis of CRM. This response is likely under the control of alternative sigma factors. In this sense, the Cu-induced VBNC state is a programmed phenomenon. However, many different stresses can result in non-culturability, so VBNC states seem to be a common consequence of stress rather than a programmed behavior with a single and discrete set of regulators. This is evident from the observation that different stressors capable of inducing a VBNC state, such as starvation, hypochlorous acid, low temperatures and Cu, prompt the bacterial cell to activate resistance mechanisms to each of these stressors separately (Pinto et al. 2015). Thus, it seems counterintuitive to search for common regulators controlling the VBNC state induced by distinct stressors with distinct mechanisms of toxicity. Rather, we could view the VBNC state as the phenotypical result of a damaged cell opting for survival over multiplication, governed by stressor-specific regulatory mechanisms. Whatever the case, it is certain that understanding the particularities of the VBNC state is a crucial task in ensuring satisfactory biosafety and biocontrol. Several uncertainties remain at many levels, from the initial interaction of the cell with Cu ions over the type, site, and extent of damage accrued, to the molecular mechanisms governing the cellular response. Since, in most cases, only a certain fraction of the initial population can be converted to and resuscitated from the VBNC state, one research question in particular concerns the cellular heterogeneity of these processes. In this sense, single cell-oriented techniques may provide much-needed information. All in all, it is clear that these studies would benefit from a standardized multifaceted experimental approach, integrating whole-cell analyses of copper chemistry, single cell proteomics and metabolomics in order to pinpoint the metabolic status of VBNC cells and the sensory system facilitating resuscitation.
References
Abbott CL, Abbott DC (2014) Topical copper ion treatments and methods of making topical copper ion treatments for use in various anatomical areas of the body. USA Patent US 13/842(387):13–04
Abe A, Ohashi E, Ren H, Hayashi T, Endo H (2007) Isolation and characterization of a cold-induced nonculturable suppression mutant of Vibrio vulnificus. Microbiol Res 162(2):130–138. https://doi.org/10.1016/j.micres.2006.01.007
Alexander E, Pham D, Steck TR (1999) The viable-but-nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium leguminosarum. Appl Environ Microbiol 65(8):3754–3756
Anita S, Ramachandran T, Rajendran R, Koushik CV, Mahalakshmi M (2011) A study of the antimicrobial property of encapsulated copper oxide nanoparticles on cotton fabric. Text Res J 81(10):1081–1088. https://doi.org/10.1177/0040517510397577
Arguello JM, Raimunda D, Padilla-Benavides T (2013) Mechanisms of copper homeostasis in bacteria. Front Cell Infect Microbiol 3:73. https://doi.org/10.3389/fcimb.2013.00073
Aurass P, Prager R, Flieger A (2011) EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol 13(12):3139–3148. https://doi.org/10.1111/j.1462-2920.2011.02604.x
Ayrapetyan M, Williams T, Oliver JD (2018) Relationship between the viable but nonculturable state and antibiotic persister cells. J Bacteriol 200(20):e00249–18. https://doi.org/10.1128/JB.00249-18
Bai H, Zhao F, Li M, Qin L, Yu H, Lu L, Zhang T (2019) Citric acid can force Staphylococcus aureus into viable but nonculturable state and its characteristics. Int J Food Microbiol 305:108254. doi:https://doi.org/10.1016/j.ijfoodmicro.2019.108254
Bedard E, Charron D, Lalancette C, Deziel E, Prevost M (2014) Recovery of Pseudomonas aeruginosa culturability following copper- and chlorine-induced stress. FEMS Microbiol Lett 356(2):226–234. https://doi.org/10.1111/1574-6968.12494
Betts J, Lukey PT, Robb LC, McAdam RA, Duncan K (2002) Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol 43(3):717–731
Bogosian G, Bourneuf EV (2001) A matter of bacterial life and death. EMBO Rep 2(9):770–774
Bondarczuk K, Piotrowska-Seget Z (2013) Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell Biol Toxicol 29(6):397–405. https://doi.org/10.1007/s10565-013-9262-1
Borkow G (2014) Using copper to improve the well-being of the skin. Curr Chem Biol 8:89–102
Boyce JM (2007) Environmental contamination makes an important contribution to hospital infection. J Hosp Infect 65:50–54. https://doi.org/10.1016/s0195-6701(07)60015-2
Boyce JM (2016) Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control 5:10. https://doi.org/10.1186/s13756-016-0111-x
Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Strauss R, Mertens K, Struyf T, Catry B, Latour K, Ivanov IN, Dobreva EG, Tambic Andraševic A, Soprek S, Budimir A, Paphitou N, Žemlicková H, Schytte Olsen S, Wolff Sönksen U, Märtin P, Ivanova M, Lyytikäinen O, Jalava J, Coignard B, Eckmanns T, Abu Sin M, Haller S, Daikos GL, Gikas A, Tsiodras S, Kontopidou F, Tóth Á, Hajdu Á, Guólaugsson Ó, Kristinsson KG, Murchan S, Burns K, Pezzotti P, Gagliotti C, Dumpis U, Liuimiene A, Perrin M, Borg MA, de Greeff SC, Monen JCM, Koek MBG, Elstrøm P, Zabicka D, Deptula A, Hryniewicz W, Caniça M, Nogueira PJ, Fernandes PA, Manageiro V, Popescu GA, Serban RI, Schréterová E, Litvová S, Štefkovicová M, Kolman J, Klavs I, Korošec A, Aracil B, Asensio A, Pérez-Vázquez M, Billström H, Larsson S, Reilly JS, Johnson A, Hopkins S (2019) Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19(1):56–66. https://doi.org/10.1016/s1473-3099(18)30605-4
Chen MX, Alexander KS, Baki G (2016) Formulation and evaluation of antibacterial creams and gels containing metal ions for topical application. J Pharm (Cairo) 2016:5754349. https://doi.org/10.1155/2016/5754349
Chen Y, Wang L, Dai F, Tao M, Li X, Tan Z (2019) Biostimulants application for bacterial metabolic activity promotion and sodium dodecyl sulfate degradation under copper stress. Chemosphere 226:736–743. https://doi.org/10.1016/j.chemosphere.2019.03.180
Clewell A, Barnes M, Endres JR, Ahmed M, Ghambeer DKS (2012) Efficacy and tolerability assessment of a topical formulation containing copper sulfate and hypericum perforatum on patients with herpes skin lesions: a comparative, randomized controlled trial. J Drugs Dermatol 11(2):209–215
Cuny C, Dukan L, Fraysse L, Ballesteros M, Dukan S (2005) Investigation of the first events leading to loss of culturability during Escherichia coli starvation: future nonculturable bacteria form a subpopulation. J Bacteriol 187(7):2244–2248. https://doi.org/10.1128/JB.187.7.2244-2248.2005
da Cruz Nizer WS, Inkovskiy V, Overhage J (2020) Surviving reactive chlorine stress: responses of gram-negative bacteria to hypochlorous acid. Microorganisms 8(8):1220. https://doi.org/10.3390/microorganisms8081220
Davey HM (2011) Life, death, and in-between: meanings and methods in microbiology. Appl Environ Microbiol 77(16):5571–5576. https://doi.org/10.1128/AEM.00744-11
del Campo R, Russi P, Mara P, Mara H, Peyrou M, de Leon IP, Gaggero C (2009) Xanthomonas axonopodis pv. citri enters the VBNC state after copper treatment and retains its virulence. FEMS Microbiol Lett 298(2):143–148. https://doi.org/10.1111/j.1574-6968.2009.01709.x
Ding T, Suo Y, Xiang Q, Zhao X, Chen S, Ye X, Liu D (2017) Significance of viable but nonculturable Escherichia coli: induction, detection, and control. J Microbiol Biotechnol 27(3):417–428. https://doi.org/10.4014/jmb.1609.09063
Dwidjosiswojo Z, Richard J, Moritz MM, Dopp E, Flemming HC, Wingender J (2011) Influence of copper ions on the viability and cytotoxicity of Pseudomonas aeruginosa under conditions relevant to drinking water environments. Int J Hyg Environ Health 214:485–492. https://doi.org/10.1016/j.ijheh.2011.06.004
Fanta FT, Dubale AA, Bebizuh DF, Atlabachew M (2019) Copper doped zeolite composite for antimicrobial activity and heavy metal removal from waste water. BMC Chem 13(1):44. https://doi.org/10.1186/s13065-019-0563-1
Faúndez G, Troncoso M, Navarette P, Figueroa G (2004) Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol 4:19
Feng S, Hou S, Cui Y, Tong Y, Yang H (2020) Metabolic transcriptional analysis on copper tolerance in moderate thermophilic bioleaching microorganism Acidithiobacillus caldus. J Ind Microbiol Biotechnol 47(1):21–33. https://doi.org/10.1007/s10295-019-02247-6
Fisher RA, Gollan B, Helaine S (2017) Persistent bacterial infections and persister cells. Nat Rev Microbiol 15(8):453–464. https://doi.org/10.1038/nrmicro.2017.42
Franca MB, Panek AD, Eleutherio EC (2007) Oxidative stress and its effects during dehydration. Comp Biochem Physiol A Mol Integr Physiol 146(4):621–631. https://doi.org/10.1016/j.cbpa.2006.02.030
Giachino A, Waldron KJ (2020) Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol Microbiol 114(3):377-390. https://doi.org/10.1111/mmi.14522
Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77(5):1541–1547. https://doi.org/10.1128/AEM.02766-10
Grey B, Steck TR (2001) Concentrations of copper thought to be toxic to Escherichia coli can induce the viable but nonculturable condition. Appl Environ Microbiol 67(11):5325–5327. https://doi.org/10.1128/AEM.67.11.5325-5327.2001
Grey BE, Steck TR (2001) The viable but nonculturable state of Ralstonia solanacearum may be involved in long-term survival and plant infection. Appl Environ Microbiol 67(9):3866–3872. https://doi.org/10.1128/aem.67.9.3866-3872.2001
Hobman JL, Crossman LC (2015) Bacterial antimicrobial metal ion resistance. J Med Microbiol 64(Pt 5):471–497. https://doi.org/10.1099/jmm.0.023036-0
Hong R, Kang TY, Michels CA, Gadura N (2012) Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl Environ Microbiol 78(6):1776–1784. https://doi.org/10.1128/AEM.07068-11
Ito K, Inaba K (2008) The disulfide bond formation (Dsb) system. Curr Opin Struct Biol 18(4):450–458. https://doi.org/10.1016/j.sbi.2008.02.002
Jiang N, Lv QY, Xu X, Cao YS, Walcott RR, Li JQ, Luo LX (2016) Induction of the viable but nonculturable state in Clavibacter michiganensis subsp. michiganensis and in planta resuscitation of the cells on tomato seedlings. Plant Pathol 65(5):826–836. https://doi.org/10.1111/ppa.12454
Kan Y, Jiang N, Xu X, Lyu Q, Gopalakrishnan V, Walcott R, Burdman S, Li J, Luo L (2019) Induction and resuscitation of the viable but non-culturable (VBNC) state in Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbitaceous crops. Front Microbiol 10:1081. https://doi.org/10.3389/fmicb.2019.01081
Kan Y, Lyu Q, Jiang N, Han S, Li J, Burdman S, Luo L (2020) iTRAQ-based proteomic analyses of the plant-pathogenic bacterium Acidovorax citrulli during entrance into and resuscitation from the viable but nonculturable state. J Proteomics 211:103547. https://doi.org/10.1016/j.jprot.2019.103547
Kim JC, Oh E, Hwang S, Ryu S, Jeon B (2015) Non-selective regulation of peroxide and superoxide resistance genes by PerR in Campylobacter jejuni. Front Microbiol 6:126. https://doi.org/10.3389/fmicb.2015.00126
Kim JS, Chowdhury N, Yamasaki R, Wood TK (2018) Viable but non-culturable and persistence describe the same bacterial stress state. Environ Microbiol 20(6):2038–2048. https://doi.org/10.1111/1462-2920.14075
Komarek M, Cadkova E, Chrastny V, Bordas F, Bollinger JC (2010) Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environ Int 36(1):138–151. https://doi.org/10.1016/j.envint.2009.10.005
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11(6):371–384. https://doi.org/10.1038/nrmicro3028
Li L, Mendis N, Trigui H, Oliver JD, Faucher SP (2014) The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol 5:528. https://doi.org/10.3389/fmicb.2014.00258
Liao X, Liu D, Ding T (2020) Nonthermal plasma induces the viable-but-nonculturable state in Staphylococcus aureus via metabolic suppression and the oxidative stress response. Appl Environ Microbiol 86(5):e02216–e02219. https://doi.org/10.1128/AEM
Longkumer T, Parthasarathy S, Vemuri SG, Siddavattam D (2014) OxyR-dependent expression of a novel glutathione S-transferase (Abgst01) gene in Acinetobacter baumannii DS002 and its role in biotransformation of organophosphate insecticides. Microbiology 160(Pt 1):102–112. https://doi.org/10.1099/mic.0.070664-0
Macomber L, Rensing C, Imlay JA (2007) Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189(5):1616–1626. https://doi.org/10.1128/JB.01357-06
Macomber L, Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA 106(20):8344–8349
Maertens L, Coninx I, Claesen J, Leys N, Matroule J-Y, Van Houdt R (2020) Copper resistance mediates long-term survival of Cupriavidus metallidurans in wet contact with metallic copper. Front Microbiol 11:1208. https://doi.org/10.3389/fmicb.2020.01208
Mathews S, Hans M, Mucklich F, Solioz M (2013) Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl Environ Microbiol 79(8):2605–2611. https://doi.org/10.1128/AEM.03608-12
May KL, Lehman KM, Mitchell AM, Grabowicz M (2019) A stress response monitoring lipoprotein trafficking to the outer membrane. mBio 10(3):e00618-19. https://doi.org/10.1128/mBio.00618-19
McDougald D, Gong L, Srinivasan S, Hild E, Thompson L, Takayama K, Rice SA, Kjelleberg S (2002) Defences against oxidative stress during starvation in bacteria. Antonie Van Leeuwenhoek 81:3–13
Monchy S, Benotmane MA, Wattiez R, van Aelst S, Auquier V, Borremans B, Mergeay M, Taghavi S, van der Lelie D, Vallaeys T (2006) Transcriptomic and proteomic analyses of the pMOL30-encoded copper resistance in Cupriavidus metallidurans strain CH34. Microbiology 152(Pt 6):1765–1776. https://doi.org/10.1099/mic.0.28593-0
Nandakumar R, Espirito Santo C, Madayiputhiya N, Grass G (2011) Quantitative proteomic profiling of the Escherichia coli response to metallic copper surfaces. Biometals 24(3):429–444. https://doi.org/10.1007/s10534-011-9434-5
Noyce JO, Michels H, Keevil CW (2006) Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl Environ Microbiol 72(6):4239–4244. https://doi.org/10.1128/AEM.02532-05
Ordax M, Marco-Noales E, Lopez MM, Biosca EG (2006) Survival strategy of Erwinia amylovora against copper: induction of the viable-but-nonculturable state. Appl Environ Microbiol 72(5):3482–3488. https://doi.org/10.1128/AEM.72.5.3482-3488.2006
Otter JA, Yezli S, Salkeld JAG, French GL (2013) Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control 41(5):S6–S11. https://doi.org/10.1016/j.ajic.2012.12.004
Parra A, Toro M, Jacob R, Navarrete P, Troncoso M, Figueroa G, Reyes-Jara A (2018) Antimicrobial effect of copper surfaces on bacteria isolated from poultry meat. Braz J Microbiol 49(Suppl 1):113–118. https://doi.org/10.1016/j.bjm.2018.06.008
Parry BR, Shain DH (2011) Manipulations of AMP metabolic genes increase growth rate and cold tolerance in Escherichia coli: implications for psychrophilic evolution. Mol Biol Evol 28(7):2139–2145. https://doi.org/10.1093/molbev/msr038
Perelshtein I, Applerot G, Perkas N, Wehrschuetz-Sigl E, Hasmann A, Guebitz G, Gedanken A (2009) CuO-cotton nanocomposite: formation, morphology, and antibacterial activity. Surf Coat Technol 204(1–2):54–57. https://doi.org/10.1016/j.surfcoat.2009.06.028
Peters K, Pazos M, Edoo Z, Hugonnet JE, Martorana AM, Polissi A, VanNieuwenhze MS, Arthur M, Vollmer W (2018) Copper inhibits peptidoglycan LD-transpeptidases suppressing beta-lactam resistance due to bypass of penicillin-binding proteins. Proc Natl Acad Sci USA 115(42):10786–10791. https://doi.org/10.1073/pnas.1809285115
Pinto D, Santos MA, Chambel L (2015) Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit Rev Microbiol 41(1):61–76. https://doi.org/10.3109/1040841X.2013.794127
Rademacher C, Masepohl B (2012) Copper-responsive gene regulation in bacteria. Microbiology 158(Pt 10):2451–2464. https://doi.org/10.1099/mic.0.058487-0
Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S (2014) Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health 2:103. https://doi.org/10.3389/fpubh.2014.00103
Rensing C, Moodley A, Cavaco LM, McDevitt SF (2018) Resistance to metals used in agricultural production. Microbiol Spectr 6(2). https://doi.org/10.1128/microbiolspec.ARBA
Reyes VC, Spitzmiller MR, Hong-Hermesdorf A, Kropat J, Damoiseaux RD, Merchant SS, Mahendra S (2016) Copper status of exposed microorganisms influences susceptibility to metallic nanoparticles. Environ Toxicol Chem 35(5):1148–1158. https://doi.org/10.1002/etc.3254
Rittershaus ES, Baek SH, Sassetti CM (2013) The normalcy of dormancy: common themes in microbial quiescence. Cell Host Microbe 13(6):643–651. https://doi.org/10.1016/j.chom.2013.05.012
Schottroff F, Frohling A, Zunabovic-Pichler M, Krottenthaler A, Schluter O, Jager H (2018) Sublethal Injury and Viable but Non-culturable (VBNC) State in Microorganisms During Preservation of Food and Biological Materials by Non-thermal Processes. Front Microbiol 9:2773. https://doi.org/10.3389/fmicb.2018.02773
Shen X, Zhang H, He X, Shi H, Stephan C, Jiang H, Wan C, Eichholz T (2019) Evaluating the treatment effectiveness of copper-based algaecides on toxic algae Microcystis aeruginosa using single cell-inductively coupled plasma-mass spectrometry. Anal Bioanal Chem 411(21):5531–5543. https://doi.org/10.1007/s00216-019-01933-9
Solioz M, Abicht HK, Mermod M, Mancini S (2010) Response of gram-positive bacteria to copper stress. J Biol Inorg Chem 15(1):3–14. https://doi.org/10.1007/s00775-009-0588-3
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radical Bio Med 18(2):321–336
Styczynski AR, Anwar KN, Sultana H, Ghanem A, Lurain N, Chua A, Ghassemi M, Novak RM (2015) In vitro antiretroviral activity and in vivo toxicity of the potential topical microbicide copper phthalocyanine sulfate. Virol J 12:132. https://doi.org/10.1186/s12985-015-0358-5
Tamayo L, Azocar M, Kogan M, Riveros A, Paez M (2016) Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater Sci Eng C Mater Biol Appl 69:1391–1409. https://doi.org/10.1016/j.msec.2016.08.041
Teli MD, Sheikh J (2013) Modified bamboo rayon-copper nanoparticle composites as antibacterial textiles. Int J Biol Macromol 61:302–307. https://doi.org/10.1016/j.ijbiomac.2013.07.015
Um HY, Kong HG, Lee HJ, Choi HK, Park EJ, Kim ST, Murugiyan S, Chung E, Kang KY, Lee SW (2013) Altered gene expression and intracellular changes of the viable but nonculturable state in Ralstonia solanacearum by copper treatment. Plant Pathol J 29(4):374–385. https://doi.org/10.5423/PPJ.OA.07.2013.0067
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Vincent M, Hartemann P, Engels-Deutsch M (2016) Antimicrobial applications of copper. Int J Hyg Environ Health 219(7 Pt A):585–591. https://doi.org/10.1016/j.ijheh.2016.06.003
Vincent M, Duval RE, Hartemann P, Engels-Deutsch M (2018) Contact killing and antimicrobial properties of copper. J Appl Microbiol 124(5):1032–1046. https://doi.org/10.1111/jam.13681
Wang HW, Chung CH, Ma TY, Wong HC (2013) Roles of alkyl hydroperoxide reductase subunit C (AhpC) in viable but nonculturable Vibrio parahaemolyticus. Appl Environ Microbiol 79(12):3734–3743. https://doi.org/10.1128/AEM.00560-13
Wang QY, Sun JY, Xu XJ, Yu HW (2018) Integration of chemical and toxicological tools to assess the bioavailability of copper derived from different copper-based fungicides in soil. Ecotoxicol Environ Saf 161:662–668. https://doi.org/10.1016/j.ecoenv.2018.06.041
Whitesides MD, Oliver JD (1997) Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol 63(3):1002–1005
Zhao X, Zhong J, Wei C, Lin CW, Ding T (2017) Current perspectives on viable but non-culturable state in foodborne pathogens. Front Microbiol 8:580. https://doi.org/10.3389/fmicb.2017.00580
Funding
This research was supported by the European Space Agency (ESA-PRODEX) and the Belgian Science Policy (Belspo) through the BIOFILMS project (C4000129318). LM was funded by a Fonds Spécial de la Recherche of UNamur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The manuscript has been read and approved by all authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maertens, L., Matroule, JY. & Van Houdt, R. Characteristics of the copper‐induced viable‐but‐non‐culturable state in bacteria. World J Microbiol Biotechnol 37, 37 (2021). https://doi.org/10.1007/s11274-021-03006-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03006-5