Abstract
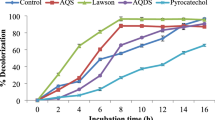
Although there have been many studies on bacterial removal of soluble azo dyes, much less information is available for biological treatment of water-insoluble azo dyes. The few bacterial species capable of removing Sudan dye generally require a long time to remove low concentrations of insoluble dye particles. The present work examined the efficient removal of Sudan I by Shewanella oneidensis MR-1 in the presence of redox mediator. It was found that the microbially reduced anthraquinone-2,6-disulfonate (AQDS) could abiotically reduce Sudan I, indicating the feasibility of microbially-mediated reduction. The addition of 100 μM AQDS and other different quinone compounds led to 4.3–54.7 % increase in removal efficiencies in 22 h. However, adding 5-hydroxy-1,4-naphthoquinone into the system inhibited Sudan I removal. The presence of 10, 50 and 100 μM AQDS stimulated the removal efficiency in 10 h from 26.4 to 42.8, 54.9 and 64.0 %, respectively. The presence of 300 μM AQDS resulted in an eightfold increase in initial removal rate from 0.19 to 1.52 mg h−1 g−1 cell biomass. A linear relationship was observed between the initial removal rates and AQDS concentrations (0–100 μM). Comparison of Michaelis–Menten kinetic constants revealed the advantage of AQDS-mediated removal over direct reduction. Different species of humic acid could also stimulate the removal of Sudan I. Scanning electronic microscopy analysis confirmed the accelerated removal performance in the presence of AQDS. These results provide a potential method for the efficient removal of insoluble Sudan dye.






Similar content being viewed by others
References
Ahlström LH, Eskilsson SC, Björklund E (2005) Determination of banned azo dyes in consumer goods. Trends Anal Chem 24:49–56
Borch T, Inskeep WP, Harwood JA, Gerlach R (2005) Impact of ferrihydrite and anthraquinone-2,6-disulfonate on the reductive transformation of 2,4,6-trinitrotoluene by a gram-positive fermenting bacterium. Environ Sci Technol 39:7126–7133
Cai P, Xiao X, He Y, Li W, Chu J, Wu C, He M, Zhang Z, Sheng G, Lam MHW, Xu F, Yu H (2012) Anaerobic biodecolorization mechanism of methyl orange by Shewanella oneidensis MR-1. Appl Microbiol Biotechnol 93:1769–1776
Cervantes FJ, Martínez CM, Gonzalez-Estrella J, Márquez A, Arriaga S (2012) Kinetics during the redox biotransformation of pollutants mediated by immobilized and soluble humic acids. Appl Microbiol Biotechnol. doi:10.1007/s00253-012-4081-5
Chailapakul O, Wonsawat W, Siangproh W, Grudpan K, Zhao Y, Zhu Z (2008) Analysis of sudan I, sudan II, sudan III, and sudan IV in food by HPLC with electrochemical detection: comparison of glassy carbon electrode with carbon nanotube-ionic liquid gel modified electrode. Food Chem 109:876–882
Chen H, Xu H, Heinze TM, Cerniglia CE (2009) Decolorization of water and oil-soluble azo dyes by Lactobacillus acidophilus and Lactobacillus fermentum. J Ind Microbiol Biotechnol 36:1459–1466
dos Santos AB, Cervantes FJ, Lier JB (2004) Azo dye reduction by thermophilic anaerobic granular sludge, and the impact of the redox mediator anthraquinone-2,6-disulfonate (AQDS) on the reductive biochemical transformation. Appl Microbiol Biotechnol 64:62–69
Dunnivant FM, Schwarzenbach RP, Macalady DL (1992) Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol 26:2133–2142
Fredrickson JK, Kostandarithes HM, Li SW, Plymale AE, Daly MJ (2000) Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl Environ Microbiol 66:2006–2011
Fries MR, Zhou J, Chee-Sanford J, Tiedje JM (1994) Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl Environ Microbiol 60:2802–2810
Gralnick JA, Newman DK (2007) Extracellular respiration. Mol Microbiol 65:1–11
He L, Su Y, Fang B, Shen X, Zeng Z, Liu Y (2007) Determination of Sudan dye residues in eggs by liquid chromatography and gas chromatography-mass spectrometry. Anal Chim Acta 594:139–146
Hong Y, Guo J, Xu Z, Xu M, Sun G (2007) Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. J Microbiol Biotechnol 17:428–437
Huang DY, Zhuang L, Cao WD, Xu W, Zhou SG, Li FB (2010) Comparison of dissolved organic matter from sewage sludge and sludge compost as electron shuttles for enhancing Fe(III) bioreduction. J Soils Sediment 10:722–729
Jeon OH, Kelly SD, Kemner KM, Barnett MO, Burgos WD, Dempsey BA, Roden EE (2004) Microbial reduction of U(VI) at the solid-water interface. Environ Sci Technol 38:5649–5655
Ji Q, Liu G, Zhou J, Wang J, Jin R, Lv H (2012) Removal of water-insoluble Sudan dyes by Shewanella oneidensis MR-1. Bioresour Technol 114:144–148
Li T, Guthrie JT (2010) Colour removal from aqueous solutions of metal-complex azo dyes using bacterial cells of Shewanella strain J18 143. Bioresour Technol 101:4291–4295
Liu G, Zhou J, Wang J, Wang X, Jin R, Lv H (2011) Decolorization of azo dyes by Shewanella oneidensis MR-1 in the presence of humic acids. Appl Microbiol Biotechnol 91:417–424
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Luan F, Burgos WD, Xie L, Zhou Q (2010) Bioreduction of nitrobenzene, natural organic matter, and hematite by Shewanella putrefaciens CN32. Envrion Sci Technol 44:184–190
O’Loughlin EJ (2008) Effects of electron transfer mediators on the bioreduction of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environ Sci Technol 42:6876–6882
Pan H, Feng J, Cerniglia CE, Chen H (2011) Effects of orange II and sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol 38:1729–1738
Pandey A, Singh P, Iyengar L (2007) Bacterial decolorization and degradation of azo dyes. Int Biodeterior Biodegrad 59:73–84
Pearce CI, Christie R, Boothman C, Canstein HV, Guthrie JT, Lloyd JR (2006) Reactive azo dye reduction by Shewanella strain J18 143. Biotechnol Bioeng 95:692–703
Pearce CI, Guthrie JT, Lloyd JR (2008) Reduction of pigment dispersions by Shewanella strain J18 143. Dyes Pigm 76:696–705
Rau J, Knackmuss HJ, Stolz A (2002) Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ Sci Technol 36:1497–1504
Royer RA, Burgos WD, Fisher AS, Jeon BH, Unz RF, Dempsey BA (2002) Enhancement of hematite bioreduction by natural organic matter. Environ Sci Technol 36:2897–2904
Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR (1998) Quinone moieties act as electron acceptors in the reduction of humic substances by humic-reducing microorganisms. Environ Sci Technol 32:2984–2989
van der Zee FP, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv 27:256–277
van der Zee FP, Bouwman RHM, Strick DPBTB, Lettinga G, Field JA (2001) Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnol Bioeng 75:691–701
van Trump JI, Sun Y, Coates JD (2006) Microbial interactions with humic substances. Adv Appl Microbiol 60:55–96
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H (2007) Anaerobic metabolism of 1-amino-2-naphthol-based azo dyes (Sudan dyes) by human intestinal microflora. Appl Environ Microbiol 73:7759–7762
Xu H, Heinze TM, Paine DD, Cerniglia CE, Chen H (2010) Sudan azo dyes and Para Red degradation by prevalent bacteria of the human gastrointestinal tract. Anaerobe 16:114–119
Yamamura S, Watanabe M, Kanzaki M, Soda S, Ike M (2008) Removal of arsenic from contaminated soils by microbial reduction of arsenate and quinone. Environ Sci Technol 42:6154–6159
Yang Y, Sun G, Guo J, Xu M (2011) Differential biofilms characteristics of Shewanella decolorationis microbial fuel cells under open and closed circuit conditions. Bioresour Technol 102:7093–7098
Acknowledgments
The work was financially supported by National Natural Science Foundation of China (No. 51008044), Fundamental Research Funds for the Central Universities, and China Postdoctoral Science Foundation (No. 201104596).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Zhou, J., Ji, Q. et al. Accelerated removal of Sudan dye by Shewanella oneidensis MR-1 in the presence of quinones and humic acids. World J Microbiol Biotechnol 29, 1723–1730 (2013). https://doi.org/10.1007/s11274-013-1336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1336-y