Abstract
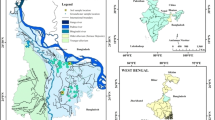
Gold mining operations in the northern Black Hills of South Dakota resulted in the discharge of arsenopyrite-bearing mine tailings into Whitewood Creek from 1876 to 1977. Those tailings were transported further downstream along the Belle Fourche River, the Cheyenne River, and the Missouri River. An estimated 110 million metric tons of tailings remain stored in alluvial deposits of the Belle Fourche and Cheyenne Rivers. Pore-water dialysis samplers were deployed in the channel and backwaters of the Belle Fourche and Cheyenne Rivers to determine temporal and seasonal changes in the geochemistry of groundwater in alluvial sediments. Alluvial sediment adjacent to the dialysis samplers were cored for geochemical analysis. In comparison to US Environmental Protection Agency drinking water standards and reference concentrations of alluvial sediment not containing mine tailings, the Belle Fourche River sites had elevated concentrations of arsenic in pore water (2570 μg/L compared to 10 μg/L) and sediment (1010 ppm compared to < 34 ppm), respectively. Pore water arsenic concentration was affected by dissolution of iron oxyhydroxides under reducing conditions. Sequential extraction of iron and arsenic from sediment cores indicates that substantial quantities of soluble metals were present. Dissolution of arsenic sorbed to alluvial sediment particles appears to be affected by changing groundwater levels that cause shifts in redox conditions. Bioreductive processes did not appear to be a substantial transport pathway but could affect speciation of arsenic, especially at the Cheyenne River sampling sites where microbial activity was determined to be greater than at Belle Fourche sampling sites.





Similar content being viewed by others
References
Bergeland, E. M., Ruth, G. R., Stack, R. L., & Emerick, R. J. (1977). Arsenic toxicosis in cattle associated with soil and water contamination from mining operations. American Association of Veterinary Laboratory Diagnosticians, 19, 311–316.
Bostick, B. C., & Fendorf, S. (2003). Arsenite sorption on troilite and pyrite. Geochimica et Cosmochimica Acta, 67(5), 909–921.
Chaerun, S., Pangesti, N., Toyota, K., & Whitman, W. (2011). Changes in microbial functional diversity and activity in paddy soils irrigated with industrial wastewaters in Bandung, West Java Province, Indonesia. Water Air Soil Pollution, 217(1), 491–502.
Cherry, J. A., Morel, F. M. M., Rouse, J. V., Schnoor, J. L., & Wolman, M. G. (1986). Hydrogeochemistry of sulfide and arsenic-rich tailings and alluvium along Whitewood Creek, South Dakota (part 1 of 3 parts). Mineral and Energy Resources, 29(4), 12.
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals:Implications for arsenic mobility. Environmental Science & Technology, 37(18), 4182–4189.
Emett, M. T., & Khoe, G. H. (2001). Photochemical oxidation of arsenic by oxygen and iron in acidic solutions. Water Research, 35(3), 649–656.
EPA. (1973). Mercury, zinc, copper, arsenic, selenium, and cyanide content of selected waters and sediment collected along whitewood creek, the Belle Fourche River, and the Cheyenne River in western South Dakota. December 1971–October 1972., U.S. Environmental Protection Agency, Denver, CO.
EPA. (2001). Method 200.7 Trace elements in water, solids, and biosolids by inductively coupled plasma-atomic emission spectrometry, U.S. Environmental Protection Agency, Washington, DC.
EPA. (2005). Ecological risk assessment for the Cheyenne River, South Dakota. Denver: U.S. Environmental Protection Agency.
EPA. (2006). Human health risk assessment for the Cheyenne River, South Dakota. Denver: U.S. Environmental Protection Agency.
EPA. (2016). Drinking water arsenic rule history. Denver: U.S. Environmental Protection Agency https://www.epa.gov/dwreginfo/drinking-water-arsenic-rule-history.
EPA. (2017). , U.S. Environmental Protection Agency, Denver.
Fendorf, S., Nico, P. S., Kocar, B. D., Masue, Y., Tufano, K. J., Balwant, S., & Markus, G. (2010). "Arsenic chemistry in soils and sediments." Developments in Soil Science (pp. 357–378). New York: Elsevier.
Fisher, J. C., Wallschlinger, D., Planer-Friedrich, B., & Hollibaugh, J. T. (2007). A new role for sulfur in arsenic cycling. Environmental Science & Technology, 42(1), 81–85.
Ford, R. G. (2002). Rates of hydrous ferric oxide crystallization and the influence on coprecipitated arsenate. Environmental Science & Technology, 36(11), 2459–2463.
Fuller, C. C., & Davis, J. A. (2003). Evaluation of the processes controlling dissolved arsenic in Whitewood Creek, South Dakota, Professional Paper 1681. Reston: U.S. Geological Survey.
Garbarino, J. R., Kanagy, L. K., & Cree, M. E. (2006). Determination of elements in natural-water, biota, sediment and soil samples using collision/reaction cell inductively coupled plasma-mass spectrometry, Book 5, Section B, Chapt 1. Washington DC: U.S. Geological Survey.
Garland, J. L., & Mills, A. L. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Applied Environmental Microbiology, 57(8), 2351–2359.
Goddard, K. E. (1989). Composition, distribution, and hydrologic effects of contaminated sediments resulting from the discharge of gold milling wastes to Whitewood Creek at Lead and Deadwood, South Dakota, Water-Resources Investigations Report 87–4051. Reston: U.S. Geological Survey.
Heiri, O., Lotter, A., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology, 25(1), 101–110.
Helsel, D.R., and Hirsch, R.M., 2002. Statistical methods in water resources: U.S. Geological Survey Techniques of Water Resources Investigations, book 4, chapter A3, 522 p., http://pubs.usgs.gov/twri/twri4a3/.
Hesslein, R. H. (1976). An in situ sampler for slose interval pore water studies. Limnology and Oceanography, 21(6), 912–914.
Hochella, M. F., Moore, J. N., Putnis, C. V., Putnis, A., Kasama, T., & Eberl, D. D. (2005). Direct observation of heavy metal-mineral association from the Clark Fork River Superfund Complex: Implications for metal transport and bioavailability. Geochimica et Cosmochimica Acta, 69(7), 1651–1663.
Horowitz, A. J., Elrick, K. A., & Callender, E. (1988). The effect of mining on the sediment - trace element geochemistry of cores from the Cheyenne River arm of Lake Oahe, South Dakota, U.S.A. Chemical Geology, 67(1–2), 17–33.
Islam, F. S., Boothman, C., Gault, A. G., Polya, D. A., & Lloyd, J. R. (2005). Potential role of the Fe(III)-reducing bacteria Geobacter and Geothrix in controlling arsenic solubility in Bengal delta sediments. Mineralogical Magazine, 69(5), 865–875.
Keon, N. E., Swartz, C. H., Brabander, D. J., Harvey, C., & Hemond, H. F. (2001). Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environmental Science & Technology, 35(13), 2778–2784.
Kirk, M. F., Holm, T. R., Park, J., Jin, Q., Sanford, R. A., Fouke, B. W., & Bethke, C. M. (2004). Bacterial sulfate reduction limits natural arsenic contamination in groundwater. Geology, 32(11), 953–956.
Kocar, B. D., Borch, T., & Fendorf, S. (2010). Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochimica et Cosmochimica Acta, 74(3), 980–994.
Kostka, J. E., Dalton, D. D., Skelton, H., Dollhopf, S., & Stucki, J. W. (2002). Growth of iron(III)-reducing bacteria on clay minerals as the sole electron acceptor and comparison of growth yields on a variety of oxidized iron forms. Applied Environmental Microbiology, 68(12), 6256–6262.
Kuwabara, J. S., Chang, C. C. Y., and Pasilis, S. P.., 2003. Section I Effects of benthic flora on arsenic transport in Whitewood Creek, South Dakota, in Kuwabara, J. S., and Fuller, C. C. (eds.), Toxic substances in surface waters and sediments—a study to assess the effects of arsenic-contaminated alluvial sediment in Whitewood Creek, South Dakota. U.S. Geological Survey Professional Paper 1681.
Larson, L. N., Kipp, G. G., Mott, H. V., & Stone, J. J. (2012). Sediment pore-water interactions associated with arsenic and uranium transport from the North Cave Hills mining region, South Dakota, USA. Applied Geochemistry, 27(4), 879–891.
Leflaive, J., Danger, M., Lacroix, G., Lyautey, E., Oumarou, C., & Ten-Hage, L. (2008). Nutrient effects on the genetic and functional diversity of aquatic bacterial communities. FEMS Microbiology Ecology, 66(2), 379–390.
Marron, D. C. (1992). Floodplain storage of mine tailings in the Belle Fourche river system: A sediment budget approach. Earth Surface Processes and Landforms, 17(7), 675–685.
Meng, X., and Wang, W. (1998). "Speciation of arsenic by disposable cartridges." Third International Conference on Arsenic Exposure and Health Effects, San Diego, CA, 1–6.
Mitchell, S. T. (2009). Nuggets to neutrinos: the Homestake story. LaVergne: Xlibris Corporation.
Morton, M. E., & Dunnette, D. A. (1994). Health effects of environmental arsenic. In J. O. Nriagu (Ed.), Arsenic in the environment part II: human health and ecosystem effects (pp. 17–34). New York: Wiley Series in Advances in Environmental Science and Technology.
Noble, J. A. (1950). Ore mineralization in the Homestake gold mine, Lead, South Dakota. Geological Society of America Bulletin, 61(3), 221–252.
O'Day, P. A., Vlassopoulos, D., Root, R., & Rivera, N. (2004). The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proceedings of the National Academy of Sciences, 101(38), 13703–13708.
Pedersen, H. D., Postma, D., & Jakobsen, R. (2006). Release of arsenic associated with the reduction and transformation of iron oxides. Geochimica et Cosmochimica Acta, 70(16), 4116–4129.
Root, R. A., Dixit, S., Campbell, K. M., Jew, A. D., Hering, J. G., & O'Day, P. A. (2007). Arsenic sequestration by sorption processes in high-iron sediments. Geochimica et Cosmochimica Acta, 71(23), 5782–5803.
Root, R. A., Vlassopoulos, D., Rivera, N. A., Rafferty, M. T., Andrews, C., & O'Day, P. A. (2009). Speciation and natural attenuation of arsenic and iron in a tidally influenced shallow aquifer. Geochimica et Cosmochimica Acta, 73(19), 5528–5553.
Salomo, S., Munch, C., & Roske, I. (2009). Evaluation of the metabolic diversity of microbial communities in four different filter layers of a constructed wetland with vertical flow by Biolog analysis. Water Research, 43(18), 4569–4578.
Saunders, J. A., Lee, M. K., Shamsudduha, M., Dhakal, P., Uddin, A., Chowdury, M. T., & Ahmed, K. M. (2008). Geochemistry and mineralogy of arsenic in (natural) anaerobic groundwaters. Applied Geochemistry, 23(11), 3205–3214.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
Stamm, J. F., and Hoogestraat, G. K. (2012). Concentrations of selected metals in Quaternary-age fluvial deposits along the lower Cheyenne and middle Belle Fourche Rivers, western South Dakota, 2009–2010, U.S. Geological Survey Data Series 695.
Stamm, J. F., Hendricks, R. R., Sawyer, J. F., Mahan, S. A., Zaprowski, B. J., Geibel, N. M., & Azzolini, D. C. (2013). Late Quaternary stream piracy and strath terrace formation along the Belle Fourche and lower Cheyenne Rivers, South Dakota and Wyoming. Geomorphology, 197(0), 10–20.
Stookey, L. L. (1970). Ferrozine-a new spectrophotometric reagent for iron. Analytical Chemistry, 42(7), 779–781.
Tourtelot, H. A. (1962). Preliminary investigation of the geologic setting and chemical composition of the Pierre Shale great plains region, Professional Paper 360. Reston: U.S. Geological Survey.
U.S. Geological Survey (2018). USGS water data for the Nation: U.S. Geological Survey National Water Information System database, accessed March 19, 2018, at https://doi.org/10.5066/F7P55KJN.
Urban, N., Dinkel, C., & Wehrli, B. (1997). Solute transfer across the sediment surface of a eutrophic lake: I. Porewater profiles from dialysis samplers. Aquatic Sciences - Research Across Boundaries, 59(1), 1–25.
Wilkin, R., Wallschlager, D., & Ford, R. (2003). Speciation of arsenic in sulfidic waters. Geochemical Transactions, 4(1), 1.
Zhou, J., Guo, W. H., Wang, R. Q., Han, X. M., & Wang, Q. (2008). Microbial community diversity in the profile of an agricultural soil in northern China. Journal of Environmental Science, 20, 981–988.
Acknowledgements
This study was conducted in cooperation with the US Geological Survey, Cheyenne River Sioux Tribe, and the US Army Corps of Engineers, and in collaboration with the South Dakota School of Mines and Technology. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Tables of data used to generate all figures, graphical site sampling locations, sequential chemical extraction results, Biolog average well color development (AWCD) trends and substrate utilization radar plots, and sieve analysis results are provided in Supporting Information.
ESM 1
(DOCX 2774 kb)
Rights and permissions
About this article
Cite this article
Pfeifle, B.D., Stamm, J.F. & Stone, J.J. Arsenic Geochemistry of Alluvial Sediments and Pore Waters Affected by Mine Tailings along the Belle Fourche and Cheyenne River Floodplains. Water Air Soil Pollut 229, 183 (2018). https://doi.org/10.1007/s11270-018-3836-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3836-8