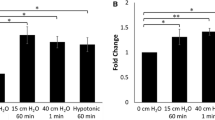
Abstract
Purpose
To determine the contributions of different durations of hypoxia to NLRP3 inflammasome activation in urothelial cells and how ischemic changes in bladder tissues is an important chemical que that leads to pathological changes seen in BOO.
Methods
A rat urothelial cell line (MYP3) was exposed to either a short duration (2 h) or long duration (6 h) of enzyme-induced hypoxia. Following exposure to a short duration of hypoxia, NO and ATP concentrations were measured from supernatant media and caspase-1 levels were measured from cell lysates. In a separate experiment, cells were fixed following hypoxia exposure and immunostained for HIF-1α stabilization.
Results
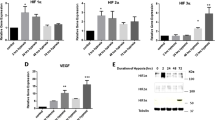
Although short exposure of low oxygen conditions resulted in a hypoxic response in MYP3 cells, as indicated by HIF-1α stabilization and increased NO activity, NLRP3 inflammasome activation was not observed as caspase-1 activity remained unchanged. However, exposure of MYP3 cells to a longer duration of hypoxia resulted in an increase in intracellular caspase-1 activity. Furthermore, treatment with antioxidant (GSH) or TXNIP inhibitor (verapamil) attenuated the hypoxia-induced increase in caspase-1 levels indicating that hypoxia primarily drives inflammation through a ROS-mediated TXNIP/NLRP3 pathway.
Conclusion
We conclude that hypoxia induced bladder damage requires a duration that is more likely related to elevated storage pressures/hypoxia, seen in later stages of BOO, as compared to shorter duration pressure elevation/hypoxia that is encountered in normal micturition cycles or early in the BOO pathology where storage pressures are still normal.







Similar content being viewed by others
Data availability
The data that support this study are available upon request from the corresponding author, JN.
References
Irwin DE, Kopp ZS, Agatep B et al (2011) Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 108:1132–1138. https://doi.org/10.1111/j.1464-410X.2010.09993.x
Nitti VW (2005) Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol 7:S14–S21
Metcalfe PD, Wang J, Jiao H et al (2010) Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int 106:1686–1694. https://doi.org/10.1111/j.1464-410X.2010.09445.x
Hughes FM, Hill HM, Wood CM et al (2016) The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol 195:1598–1605. https://doi.org/10.1016/j.juro.2015.12.068
Hughes FM, Sexton SJ, Jin H et al (2017) Bladder fibrosis during outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1β. Am J Physiol Renal Physiol 313:F603–F610. https://doi.org/10.1152/ajprenal.00128.2017
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. https://doi.org/10.3390/ijms20133328
Abais JM, Xia M, Zhang Y et al (2015) Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal 22:1111–1129. https://doi.org/10.1089/ars.2014.5994
Jo E-K, Kim JK, Shin D-M, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. https://doi.org/10.1038/cmi.2015.95
Dunton CL, Purves JT, Hughes FM et al (2018) Elevated hydrostatic pressure stimulates ATP release which mediates activation of the NLRP3 inflammasome via P2X4 in rat urothelial cells. Int Urol Nephrol 50:1607–1617. https://doi.org/10.1007/s11255-018-1948-0
Dunton CL, Purves JT, Hughes FM, Nagatomi J (2021) BOO induces fibrosis and EMT in urothelial cells which can be recapitulated in vitro through elevated storage and voiding pressure cycles. Int Urol Nephrol 53:2007–2018. https://doi.org/10.1007/s11255-021-02942-3
Koritsiadis G, Stravodimos K, Koutalellis G et al (2008) Immunohistochemical estimation of hypoxia in human obstructed bladder and correlation with clinical variables. BJU Int 102:328–332. https://doi.org/10.1111/j.1464-410X.2008.07593.x
Ghafar MA, Anastasiadis AG, Olsson LE et al (2002) Hypoxia and an angiogenic response in the partially obstructed rat bladder. Lab Invest 82:903–909. https://doi.org/10.1097/01.LAB.0000021135.87203.92
Ghafar MA, Shabsigh A, Chichester P et al (2002) Effects of chronic partial outlet obstruction on blood flow and oxygenation of the rat bladder. J Urol 167:1508–1512
Greenland JE, Hvistendahl JJ, Andersen H et al (2000) The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int 85:1109–1114. https://doi.org/10.1046/j.1464-410X.2000.00611.x
Hohlbrugger G, Frauscher F, Strasser H et al (2000) Evidence for the autoregulation of vesical circulation by intravesical potassium chloride and distension in the normal human bladder. BJU Int 85:412–415. https://doi.org/10.1046/j.1464-410x.2000.00519.x
Volikova AI, Marshall BJ, Yin JMA et al (2019) Structural, biomechanical and hemodynamic assessment of the bladder wall in healthy subjects. Res Rep Urol 11:233–245. https://doi.org/10.2147/RRU.S205383
Belenky A, Abarbanel Y, Cohen M et al (2003) Detrusor resistive index evaluated by doppler ultrasonography as a potential indicator of bladder outlet obstruction. Urology 62:647–650. https://doi.org/10.1016/S0090-4295(03)00510-7
Batista JE, Wagner JR, Azadzoi KM et al (1996) Direct measurement of blood flow in the human bladder. J Urol 155:630–633. https://doi.org/10.1016/S0022-5347(01)66471-1
Kershen RT, Azadzoi KM, Siroky MB (2002) Blood flow, pressure and compliance in the male human bladder. J Urol 168:121–125. https://doi.org/10.1016/S0022-5347(05)64843-4
Wiafe B, Adesida A, Churchill T et al (2017) Hypoxia-increased expression of genes involved in inflammation, dedifferentiation, pro-fibrosis, and extracellular matrix remodeling of human bladder smooth muscle cells. In Vitro Cell Dev Biol Anim 53:58–66. https://doi.org/10.1007/s11626-0jiji16-0085-2
Jiang Q, Geng X, Warren J et al (2020) Hypoxia inducible factor-1α (HIF-1α) mediates NLRP3 inflammasome-dependent-pyroptotic and apoptotic cell death following ischemic stroke. Neuroscience 448:126–139. https://doi.org/10.1016/j.neuroscience.2020.09.036
Minutoli L, Puzzolo D, Rinaldi M et al (2016) ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxid Med Cell Longev 2016:2183026. https://doi.org/10.1155/2016/2183026
Xin X, Yang K, Liu H, Li Y (2022) Hypobaric hypoxia triggers pyroptosis in the retina via NLRP3 inflammasome activation. Apoptosis. https://doi.org/10.1007/s10495-022-01710-7
Watanabe S, Usui-Kawanishi F, Karasawa T et al (2020) Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages. J Cell Physiol n/a: https://doi.org/10.1002/jcp.29659
Allen CB, Schneider BK, White CW (2001) Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am J Physiol-Lung Cell Mol Physiol 281:L1021–L1027. https://doi.org/10.1152/ajplung.2001.281.4.L1021
Yuan Y, Hilliard G, Ferguson T, Millhorn DE (2003) Cobalt inhibits the interaction between hypoxia-inducible factor-α and von hippel-lindau protein by direct binding to hypoxia-inducible factor-α. J Biol Chem 278:15911–15916. https://doi.org/10.1074/jbc.M300463200
Muñoz-Sánchez J, Chánez-Cárdenas ME (2019) The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol 39:556–570. https://doi.org/10.1002/jat.3749
Miyata Y, Matsuo T, Mitsunari K et al (2019) A review of oxidative stress and urinary dysfunction caused by bladder outlet obstruction and treatments using antioxidants. Antioxidants. https://doi.org/10.3390/antiox8050132
Bauer JA, Zámocká M, Majtán J, Bauerová-Hlinková V (2022) Glucose oxidase, an enzyme “ferrari”: its structure, function, production and properties in the light of various industrial and biotechnological applications. Biomolecules 12:472. https://doi.org/10.3390/biom12030472
Baumann RP, Penketh PG, Seow HA et al (2008) Generation of oxygen deficiency in cell culture using a two-enzyme system to evaluate agents targeting hypoxic tumor cells. Radiat Res 170:651–660. https://doi.org/10.1667/RR1431.1
Hudson BN, Dawes CS, Liu H-Y et al (2019) Stabilization of enzyme-immobilized hydrogels for extended hypoxic cell culture. Emergent Mater. https://doi.org/10.1007/s42247-019-00038-4
Dawes CS, Konig H, Lin C-C (2017) Enzyme-immobilized hydrogels to create hypoxia for in vitro cancer cell culture. J Biotechnol 248:25–34. https://doi.org/10.1016/j.jbiotec.2017.03.007
Kawamata H, Kameyama S, Nan L et al (1993) Effect of epidermal growth factor and transforming growth factor beta 1 on growth and invasive potentials of newly established rat bladder carcinoma cell lines. Int J Cancer 55:968–973. https://doi.org/10.1002/ijc.2910550616
Hughes FM, Turner DP, Purves JT (2015) The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells). Int Urol Nephrol 47:1953–1964. https://doi.org/10.1007/s11255-015-1126-6
Hughes FM, Vivar NP, Kennis JG et al (2014) Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol - Ren Physiol 306:F299–F308. https://doi.org/10.1152/ajprenal.00297.2013
Millonig G, Hegedüsch S, Becker L et al (2009) Hypoxia-inducible factor 1α under rapid enzymatic hypoxia: cells sense decrements of oxygen but not hypoxia per se. Free Radic Biol Med 46:182–191. https://doi.org/10.1016/j.freeradbiomed.2008.09.043
Fabian Z (2018) The signaling nature of cellular metabolism: the hypoxia signaling. Cell Signal. https://doi.org/10.5772/intechopen.79952
Packer M (2020) Mutual antagonism of hypoxia-inducible factor isoforms in cardiac, vascular, and renal disorders. JACC Basic Transl Sci 5:961–968. https://doi.org/10.1016/j.jacbts.2020.05.006
Liu Q, Palmgren VAC, Danen EH, Le Dévédec SE (2022) Acute vs. chronic vs. intermittent hypoxia in breast cancer: a review on its application in in vitro research. Mol Biol Rep 49:10961–10973. https://doi.org/10.1007/s11033-022-07802-6
Galkin A, Higgs A, Moncada S (2007) Nitric oxide and hypoxia. Essays Biochem 43:29–42. https://doi.org/10.1042/bse0430029
Hampl V, Cornfield DN, Cowan NJ, Archer SL (1995) Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur Respir J 8:515–522
Lin ATL, Ischemia J-S (2012) Hypoxia and oxidative stress in bladder outlet obstruction and bladder overdistention injury. LUTS Low Urin Tract Symptoms 4:27–31. https://doi.org/10.1111/j.1757-5672.2011.00134.x
Mao K, Chen S, Chen M et al (2013) Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res 23:201–212. https://doi.org/10.1038/cr.2013.6
Conners W, Whitebeck C, Chicester P et al (2006) L-NAME, a nitric oxide synthase inhibitor, diminishes oxidative damage in urinary bladder partial outlet obstruction. Am J Physiol Renal Physiol 290:F357-363. https://doi.org/10.1152/ajprenal.00261.2005
Azadzoi KM, Pontari M, Vlachiotis J, Siroky MB (1996) Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol 155:1459–1465. https://doi.org/10.1016/S0022-5347(01)66307-9
Chandel NS, Maltepe E, Goldwasser E et al (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95:11715–11720. https://doi.org/10.1073/pnas.95.20.11715
Guzy RD, Hoyos B, Robin E et al (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab 1:401–408. https://doi.org/10.1016/j.cmet.2005.05.001
Chen C, Ma X, Yang C et al (2018) Hypoxia potentiates LPS-induced inflammatory response and increases cell death by promoting NLRP3 inflammasome activation in pancreatic β cells. Biochem Biophys Res Commun 495:2512–2518. https://doi.org/10.1016/j.bbrc.2017.12.134
Harper SN, Leidig PD, Hughes FM et al (2019) Calcium pyrophosphate and monosodium urate activate the NLRP3 inflammasome within bladder urothelium via reactive oxygen species and TXNIP. Res Rep Urol 11:319–325. https://doi.org/10.2147/RRU.S225767
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5:a008656. https://doi.org/10.1101/cshperspect.a008656
Molla MD, Ayelign B, Dessie G et al (2020) Caspase-1 as a regulatory molecule of lipid metabolism. Lipids Health Dis 19:34. https://doi.org/10.1186/s12944-020-01220-y
Acknowledgements
The authors thank the members of the Harcum Cell-Culture Optimization Lab at Clemson University for use of their blood gas analyzer, specifically Tom Caldwell and Dr. Sarah Harcum. In addition, the authors thank the Nagatomi Mechanobiology Laboratory and lab members Cody Dunton, Julia Lunt, and Rachael Christensen. Funding was provided by NIH DK103435 and GM103444. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 2139915.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases, DK103435, J. Todd Purves, National Institute of General Medical Sciences, GM103444, Jiro Nagatomi, National Science Foundation, 2139915, Britney Hudson
Author information
Authors and Affiliations
Contributions
BNH, JTP, FMH and JN conceived the project; BNH, JTP, FMH and JN designed experiments; BNH performed experiments; BNH analyzed data; BNH, JTP, FMH and JN interpreted results of experiments; BNH prepared figures; BNH and JN drafted manuscript; BNH, JTP, FMH and JN edited and revised manuscript. BNH, JTP, FMH and JN approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2023_3900_MOESM1_ESM.docx
Supplementary file 1 Fig. 1 Glucose Levels of GOX/CAT Media. There was no difference in glucose concentrations of the 0 µg/mL GOX media and the 0.5 µg/mL GOX media. Data are the mean ± SEM analyzed using two-way ANOVA. Each group is compared to the control. N=2. Fig. 2 Nitric Oxide Levels After Treatment with L-NAME. MYP3 cells were pre-treated with 16 mM of L-NAME for 1 h before hypoxic exposure. During hypoxic exposure via GOX/CAT media, L-NAME remained in the media. Treatment with L-NAME was able to attenuate the hypoxia induced increase in NO levels at hour 1. Data are mean ± SEM and compared to the normoxic control (one-fold). Data was analyzed using two-way ANOVA. N=3. *p<0.05, **p<0.01. Fig. 3 Caspase-1 Levels After Treatment with L-NAME. MYP3 cells were pre-treated with 16 mM of L-NAME for 1 h before hypoxic exposure. L-NAME remained in the enzyme media for the duration of hypoxic exposure. Treatment with L-NAME didn’t result in any changes in intracellular caspase-1 activity. Data are mean ± SEM and compared to the normoxic control (one-fold). Data was analyzed using one-way ANOVA. N=3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hudson, B.N., Purves, J.T., Hughes, F.M. et al. Enzyme-induced hypoxia leads to inflammation in urothelial cells in vitro. Int Urol Nephrol 56, 1565–1575 (2024). https://doi.org/10.1007/s11255-023-03900-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03900-x