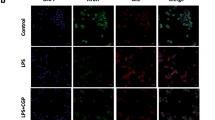
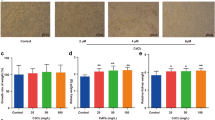
Abstract
Partial bladder outlet obstruction (pBOO) is a prevalent urological condition commonly accompanied by increased intravesical pressure, inflammation, and fibrosis. Studies have demonstrated that pBOO results in increased NLRP3 inflammasome and caspase-1 activation and that ATP is released from urothelial cells in response to elevated pressure. In the present study, we investigated the role of elevated pressure in triggering caspase-1 activation via purinergic receptors activation in urothelial cells. Rat urothelial cell line, MYP3 cells, was subjected to hydrostatic pressures of 15 cmH2O for 60 min, or 40 cmH2O for 1 min to simulate elevated storage and voiding pressure conditions, respectively. ATP concentration in the supernatant media and intracellular caspase-1 activity in cell lysates were measured. Pressure experiments were repeated in the presence of antagonists for purinergic receptors to determine the mechanism for pressure-induced caspase-1 activation. Exposure of MYP3 cells to both pressure conditions resulted in an increase in extracellular ATP levels and intracellular caspase-1 activity. Treatment with P2X7 antagonist led to a decrease in pressure-induced ATP release by MYP3 cells, while P2X4 antagonist had no effect but both antagonists inhibited pressure-induced caspase-1 activation. Moreover, when MYP3 cells were treated with extracellular ATP (500 µM), P2X4 antagonist inhibited ATP-induced caspase-1 activation, but not P2X7 antagonist. We concluded that pressure-induced extracellular ATP in urothelial cells is amplified by P2X7 receptor activation and ATP-induced-ATP release. The amplified ATP signal then activates P2X4 receptors, which mediate activation of the caspase-1 inflammatory response.






Similar content being viewed by others
References
Irwin DE, Kopp ZS, Agatep B et al (2011) Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 108:1132–1138
Metcalfe PD, Wang J, Jiao H et al (2010) Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int 106:1686–1694
Andersson K-E, Gratzke C (2007) Pharmacology of α1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol 4:368
Rossi C, Kortmann BB, Sonke GS et al (2001) alpha-Blockade improves symptoms suggestive of bladder outlet obstruction but fails to relieve it. J Urol 165:38–41
Malmgren A, Uvelius B, Andersson KE, Andersson PO (1990) On the reversibility of functional bladder changes induced by infravesical outflow obstruction in the rat. J Urol 143:1026–1031
Gabella G, Uvelius B (1994) Reversal of muscle hypertrophy in the rat urinary bladder after removal of urethral obstruction. Cell Tissue Res 277:333–339
Chai TC, Gemalmaz H, Andersson K-E et al (1999) Persistently increased voiding frequency despite relief of bladder outlet obstruction. J Urol 161:1689–1693
Jin L-H, Andersson K-E, Han J-U et al (2011) Persistent detrusor overactivity in rats after relief of partial urethral obstruction. Am J Physiol Integr Comp Physiol 301:R896–R904
Oka M, Fukui T, Ueda M et al (2009) Suppression of bladder oxidative stress and inflammation by a phytotherapeutic agent in a rat model of partial bladder outlet obstruction. J Urol 182:382–390
Hughes FM, Hill HM, Wood CM et al (2016) The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol 195:1598–1605
Shiina K, Hayashida K-I, Ishikawa K, Kawatani M (2016) ATP release from bladder urothelium and serosa in a rat model of partial bladder outlet obstruction. Biomed Res 37:299–304
Han JH, Yu HS, Lee JY et al (2015) Simple modification of the bladder outlet obstruction index for better prediction of endoscopically-proven prostatic obstruction: a preliminary study. PLoS ONE 10:e0141745
Hughes FM, Vivar NP, Kennis JG et al (2013) Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Physiol 306:F299–F308
Pandita RAJK, Fujiwara M, Alm PER, Andersson K-E (2000) Cystometric evaluation of bladder function in non-anesthetized mice with and without bladder outlet obstruction. J Urol 164:1385–1389
Beckel JM, Daugherty SL, Tyagi P et al (2015) Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593:1857–1871
Vlaskovska M, Kasakov L, Rong W et al (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–5677
Olsen SM, Stover JD, Nagatomi J (2011) Examining the role of mechanosensitive ion channels in pressure mechanotransduction in rat bladder urothelial cells. Ann Biomed Eng 39:688–697
Mochizuki T, Sokabe T, Araki I et al (2009) The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem 284:21257–21264
Birder LA, Nakamura Y, Kiss S et al (2002) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856
Riteau N, Gasse P, Fauconnier L et al (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182:774–783
Cauwels A, Rogge E, Vandendriessche B et al (2014) Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 5:e1102
Lamkanfi M, Dixit VM (2011) Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 187:597–602
Mariathasan S, Weiss DS, Newton K et al (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832
Lutolf R, Hughes FM, Purves JT (2018) NLRP3/IL-1β mediates denervation during bladder outlet obstruction in rats. Neurourol Urodyn 37:952–959
Hughes FM, Sexton SJ, Jin H et al (2017) Bladder fibrosis during outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1β. Am J Physiol Physiol 313:F603–F610
Kawamata H, Kameyama S, Nan L et al (1993) Effect of epidermal growth factor and transforming growth factor β1 on growth and invasive potentials of newly established rat bladder carcinoma cell lines. Int J Cancer 55:968–973
Cohen SM, Arnold LL, Uzvolgyi E et al (2002) Possible role of dimethylarsinous acid in dimethylarsinic acid-induced urothelial toxicity and regeneration in the rat. Chem Res Toxicol 15:1150–1157
Nascimento MG, Suzuki S, Wei M et al (2008) Cytotoxicity of combinations of arsenicals on rat urinary bladder urothelial cells in vitro. Toxicology 249:69–74
Hughes FM, Turner DP, Todd Purves J (2015) The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells). Int Urol Nephrol 47:1953–1964
Stover J, Nagatomi J (2007) Cyclic pressure stimulates DNA synthesis through the PI3K/Akt signaling pathway in rat bladder smooth muscle cells. Ann Biomed Eng 35:1585–1594
Balázs B, Dankó T, Kovács G et al (2013) Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol Biochem 32:11–24
Negoro H, Urban-Maldonado M, Liou LS et al (2014) Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling. PLoS ONE 9:e106269
Sadaharu U, Takeshi K, Tatsuo F (2018) Effects of PPADS and suramin on contractions and cytoplasmic Ca2+ changes evoked by AP4A, ATP and α,β-methylene ATP in guinea-pig urinary bladder. Br J Pharmacol 117:698–702
Wang Y, Roman R, Lidofsky SD, Fitz JG (1996) Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci 93:12020–12025
Truschel ST, Wang E, Ruiz WG et al (2002) Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13:830–846
Silverman W, Locovei S, Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Physiol 295:C761–C767
Nitti VW (2005) Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol 7:S14–S21
Adinolfi E, Raffaghello L, Giuliani AL et al (2012) Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72:2957–2969
Chen K, Zhang J, Zhang W et al (2013) ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol 45:932–943
Cook SP, Vulchanova L, Hargreaves KM et al (1997) Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 387:505–508
Nakagomi H, Yoshiyama M, Mochizuki T et al (2016) Urothelial ATP exocytosis: regulation of bladder compliance in the urine storage phase. Sci Rep 6:29761
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082
Birder LA, Ruan HZ, Chopra B et al (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol—Ren Physiol 287:F1084–F1091
McGaraughty S, Chu KL, Namovic MT et al (2007) P2X7-related modulation of pathological nociception in rats. Neuroscience 146:1817–1828
Boudreault F, Grygorczyk R (2002) Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Physiol 282:C219–C226
Boudreault F, Grygorczyk R (2004) Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561:499–513
Hazama A, Shimizu T, Ando-Akatsuka Y et al (1999) Swelling-induced, Cftr-independent Atp release from a human epithelial cell line. J Gen Physiol 114:525–533
Reigada D, Lu W, Zhang M, Mitchell CH (2008) Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience 157:396–404
Richter K, Kiefer KP, Grzesik BA et al (2013) Hydrostatic pressure activates ATP-sensitive K+ channels in lung epithelium by ATP release through pannexin and connexin hemichannels. FASEB J 28:45–55
Wang ECY, Lee J-M, Ruiz WG et al (2005) ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Investig 115:2412–2422
Sekar P, Huang D-Y, Chang S-F, Lin W-W (2018) Coordinate effects of P2X7 and extracellular acidification in microglial cells. Oncotarget 9:12718–12731
Yaron JR, Gangaraju S, Rao MY et al (2015) K+ regulates Ca2+ to drive inflammasome signaling: dynamic visualization of ion flux in live cells. Cell Death Dis 6:e1954
Draganov D, Gopalakrishna-Pillai S, Chen Y-R et al (2015) Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci Rep 5:16222
Kahlenberg JM, Dubyak GR (2004) Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Physiol 286:C1100–C1108
Mezzaroma E, Toldo S, Farkas D et al (2011) The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. PNAS 108:19725–19730
Vonend O, Turner CM, Chan CM et al (2004) Glomerular expression of the ATP-sensitive P2X7 receptor in diabetic and hypertensive rat models. Kidney Int 66:157–166
Zhao J, Wang H, Dai C et al (2013) P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum 65:3176–3185
Vilaysane A, Chun J, Seamone ME et al (2010) The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21:1732–1744
Glass R, Loesch A, Bodin P, Burnstock G (2002) P2X4 and P2X6 receptors associate with VE-cadherin in human endothelial cells. Cell Mol Life Sci C 59:870–881
LS A, AP S, XJ X et al (2011) P2X4 receptors interact with both P2X2 and P2X7 receptors in the form of homotrimers. Br J Pharmacol 163:1069–1077
Stojilkovic SS, Yan Z, Obsil T, Zemkova H (2010) Structural insights into the function of P2X4: an ATP-gated cation channel of neuroendocrine cells. Cell Mol Neurobiol 30:1251–1258
He Y, Hara H, Nunez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021
Hornung V, Latz E (2010) Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40:620–623
Inouye B, Hughes FM, Sexton S, Purves JT (2018) The emerging role of inflammasomes as central mediators in inflammatory bladder pathology. Curr Urol 11:57–72
Savage C, Lopez-Castejon G, Denes A, Brough D (2012) NLRP3-inflammasome activating DAMPs stimulate and inflammatory response in glia in the absence of priming which contributes to brain inflammation after injury. Front Immunol 3:288
Hughes FM, Hirsham N, Inouye B et al (2018) The NLRP3 inflammasome is required for the development of diabetic bladder dysfunction and causes symptom-specific changes in neuronal innervation
Franceschini A, Capece M, Chiozzi P et al (2015) The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J 29:2450–2461
Di Virgilio F (2007) Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci 28:465–472
Di Virgilio F (2013) The therapeutic potential of modifying inflammasomes and NOD-Like receptors. Pharmacol Rev 65:872–905
Grailer JJ, Canning BA, Kalbitz M et al (2014) Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 192:5974–5983
Liu H-D, Li W, Chen Z-R et al (2013) Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res 38:2072–2083
Takahashi M (2014) NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J 55:101–105
Acknowledgements
The authors would like to thank Julie Fuller and the Substrate Services Core and Research Support Services (SCRSS) in the Department of Surgery for their histological embedding and sectioning. We also thank the Light Microscopy Care Facility and Yasheng Gao for help imaging. Both core facilities are at Duke University.
Funding
Funding was provided by National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. R01DK103534), National Institutes of Health (Grant No. P20GM103444), and National Science Foundation (Grant No. 1264579).
Author information
Authors and Affiliations
Contributions
JTP, FMH, and JN conceived the project; CLD, JTP, FMH, and JN designed experiments; CLD, FMH, and HJ performed experiments; CLD analyzed data; CLD, JTP, FMH, and JN interpreted results of experiments; CLD and FMH prepared figures; CLD. drafted manuscript; CLD, JTP, FMH, and JN edited and revised manuscript. JTP, FMH, and JN approved final version of manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Dunton, C.L., Purves, J.T., Hughes, F.M. et al. Elevated hydrostatic pressure stimulates ATP release which mediates activation of the NLRP3 inflammasome via P2X4 in rat urothelial cells. Int Urol Nephrol 50, 1607–1617 (2018). https://doi.org/10.1007/s11255-018-1948-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-1948-0