Abstract
Oxidative stress (OS) has been recognized as a pathophysiologic mechanism underlying the development and progression of chronic kidney disease (CKD). OS, which results from the disturbance of balance among pro-oxidants and antioxidants favoring the pro-oxidants, is present even in early CKD and increases progressively along with deterioration of kidney function to end-stage kidney disease (ESKD). In ESKD, OS is further exacerbated mainly due to dialysis procedures per se and predisposes to increased cardiovascular morbidity and mortality. Therefore, since OS plays a pivotal role in the pathogenesis and progression of atherosclerosis in uremic patients, several strategies aiming to ameliorate OS in these patients have been proposed. Among those, N-acetylcysteine (NAC), a thiol-containing antioxidant agent, has attracted special attention due to its pleiotropic functions and beneficial effect in various OS-related entities including paracetamol overdose and prevention of contrast-induced nephropathy. In this review, we present the currently available literature on the antioxidant and anti-inflammatory properties of NAC in CKD, including hemodialysis and peritoneal dialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress (OS) results from the disruption of balance between pro-oxidants (substances gaining electrons) and antioxidants (substances donating electrons) weighing in favor of the former. This balance is essential for maintaining homeostasis, and when disrupted, may lead to multiple pathological conditions, including cancer and atherosclerosis. Free radicals, including hydroxyl, superoxide anion, hydrogen peroxide, oxygen singlet, nitric oxide and peroxynitrite are independent molecular species that contain unpaired electrons in an atomic orbital. Due to their molecular structure missing electrons, free radicals are unstable and highly reactive. In an attempt to gain stability, free radicals interact and “steal” one electron from macromolecules such as nucleic acids, proteins, lipids and carbohydrates, resulting in their structural oxidative modification and dysfunction [1,2,3,4]. Antioxidants, on the other hand, are stable molecules that donate electrons and neutralize free radicals minimizing cellular damage. Naturally occurring antioxidant defense mechanisms might be either enzymatic (dismutase superoxide, catalase, and glutathione peroxidase) or non-enzymatic (uric acid, ascorbic acid, bilirubin, albumin, flavonoids, α-tocopherol, ubiquinol and carotenoids) [2, 4, 5]. Although we tend to refer to OS as a harmful condition, when maintained at low levels, free radicals are essential for human health and thus, low-level OS is crucial for maintaining homeostasis and plays a pivotal role in redox signaling, cell metabolism, immune defense, neural activity and cell reproduction.
The leading cause of mortality in chronic kidney disease (CKD) patients remains cardiovascular (CV) disease [6], which is partially attributed to OS. Compared to healthy individuals, OS along with inflammation are highly prevalent even at early stages of CKD and are gradually increased parallelly to deterioration of kidney function, as disease progresses towards end-stage renal disease (ESRD) [2, 7]. In the uremic environment, elevated OS leads to reduced bioavailability of nitric oxide (NO) resulting in decreased vascular relaxation, vascular damage, lipid peroxidation and subsequently endothelial dysfunction, the hallmark of atherosclerosis [7, 8]. The increase of OS in CKD is also attributed to the limited activity or reduced levels of antioxidants, most commonly resulting from nutritional restrictions regarding fruits and vegetables [9,10,11]. Compared to non-dialysis ESRD, those undergoing maintenance hemodialysis (HD) present significantly increased OS status. This is due to several factors. The HD procedure per se aggravates OS status; during a dialysis session, reactive oxygen species (ROS) accumulation begins immediately, peaking at 3 h to a 14-fold increase and decreases to pre-dialysis levels shortly after the end of the session [12]. The generated free radicals interact with multiple biomolecules altering their structural and functional integrity [11]. In addition, the protein-binding properties of multiple uremic toxins limit their removal via HD, promoting endothelial damage, further inflammation and OS generation [6]. Other factors promoting free radicals formation during a HD session are arteriovenous fistulae dysfunction, use of central venous catheters, contamination of the dialysate and intravenous administration of iron and heparin [13]. Anti-oxidant defense systems are also reduced in HD patients and contribute to the increased levels of OS [14].
Although peritoneal dialysis (PD) is considered a more compatible dialysis technique compared to HD, OS is still present in this dialysis modality and is associated with clinical adverse endpoints [13]. In PD, the mechanisms triggering OS differ significantly from HD [3, 15, 16] and are mainly attributed to the composition of low pH, lactate-buffered, hyper-osmolar and hyperglycemic PD solutions. The process of PD fluids’ heat sterilization leads to the formation of glucose degeneration products (GDP) which promote generation of advanced glycation end-products (AGEs) and pro-oxidants [17,18,19,20]. Chronic exposure of the peritoneal membrane to AGEs and ROS leads to progressive increase of peritoneal vascular permeability and cellular apoptosis [19]. These molecular and structural alterations eventually result to the occurrence of adverse clinical endpoints, including loss of residual renal function, inflammation, peritonitis, technique failure, endothelial dysfunction, atherosclerosis, CV disease and mortality [16, 21,22,23]. Since the main culprit for OS in PD is PD solutions, the strategies to reduce OS in these patients include the use of more biocompatible fluids with neutral pH, low glucose generation products with bicarbonate as buffer. In addition, volume management and strict glycemic control might also help using solutions with lower glucose concentrations [19, 24,25,26,27,28].
In ESRD patients undergoing either HD or PD, OS is increased and associated with adverse events, including development and progression of atherosclerosis, CV disease and mortality [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Therefore, there is a need for new strategies to ameliorate OS in these patients and possible protect them from CV disease. During the past decade, N-acetylcysteine (NAC) has emerged as a novel and quite powerful antioxidant agent [49]. Here, we aim to review the existing data regarding the possible antioxidant and anti-inflammatory properties of NAC in CKD and ESRD.
NAC: molecular structure and properties
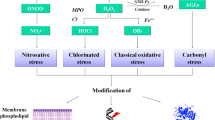
NAC was first used in the early 1960s as a mucolytic agent in patients with cystic fibrosis. The acetylation of the N-terminal of cysteine provides adequate stability to the sulfur-containing molecule of cysteine to deliver a thiol group (reduced sulfhydryl moiety) and allows it to function as a mucolytic agent by disrupting the disulfide bridges within the glycoprotein matrix of mucus without being deactivated by metabolism and rapid oxidation in the solution [50]. NAC has been also used as an effective antidote in paracetamol overdose acting as a precursor of the substrate (l-cysteine) in synthesis of hepatic glutathione (GSH) which might be depleted due to conjugation with paracetamol. GSH is the most important intracellular, endogenous antioxidant comprising of glutamic acid (E), glycine (G), and cysteine (C). The rate of GSH synthesis depends on the activity of glutamate-cysteine ligase. GSH has multiple functions including protein thiolation, drug detoxification and antioxidative protection of cellular components. The antioxidative properties of GSH derive from the free sulfhydryl group that directly interacts with free radicals as well as its role as a substrate of co-factor for various enzymes including glutathione reductase, glutaredoxin, glyoxalases 1 and 2, glutathione transferase, and membrane-associated proteins with divergent functions in Eicosanoid and Glutathione metabolism (MAPEG) [51, 52]. The anti-inflammatory and antioxidant molecular mechanisms of NAC are shown in Fig. 1.
During the past decade, research has focused on the possible beneficial antioxidant effects of NAC in multiple conditions where OS is involved [50]. NAC is believed to act as an antioxidant by several mechanisms: first, it is a direct sulfhydryl donor for the neutralization of ROS; second, it modulates extracellular glutamate and intracellular GSH levels, third, it acts as a reducing agent for protein disulfides and finally it restores thiol pools, which in turn regulate the redox state [7, 53,54,55,56]. In addition to antioxidant properties, NAC inhibits the function of pro-inflammatory transcription factors such as AP-1 (activator protein 1) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), well-known pre-cursors of OS [57, 58]. In addition, NAC is believed to exert cardioprotective properties through increasing endothelial nitric oxide synthase expression, improving nitric oxide bioavailability and suppressing angiotensin-converting enzyme activity, thus leading to vasorelaxation [7, 59,60,61,62,63,64]. Furthermore, NAC acts as a methyl donor in the conversion of homocysteine to methionine and also contributes to the displacement of homocysteine from serum albumin binding sites, a property that can be utilized during dialysis sessions to increase levels of unbound homocysteine available for plasma clearance [65,66,67,68,69,70].
NAC for the prevention of contrasted-induced nephropathy
Besides its use as a mucolytic agent, NAC has been widely used for the prevention of contrast-induced nephropathy (CIN) as various evidence suggest the involvement of OS in the pathophysiology of this condition [2, 71, 72]. The use of NAC as a preventive measure for the development of CIN relies on its antioxidant and vasorelaxant properties; NAC reduces ROS and tissue damage in the kidneys, minimizes vasoconstriction and stabilizes renal hemodynamics [71, 73]. To investigate the beneficial effect of NAC on CIN prevention, Guo et al. [74] conducted a meta-analysis including seven randomized clinical trials and 1710 ST segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention and demonstrated a 49% and 63% reduced risk of CIN and all-cause in-hospital mortality, respectively. In a subgroup analysis, the preventive effect of NAC appeared greater in patients with pre-existing impaired renal function and in those receiving higher dosages of NAC. Similarly, other meta-analyses coherently reported a 22–33% beneficial effect of NAC on preventing CIN [73, 75, 76], which was more pronounced in patients with pre-existing CKD [71]. However, the largest RCT until to date, the PRESERVE trail, failed to show any therapeutic effect of NAC regarding CIN prevention [77] and other meta-analyses providing conflicting results 12/27/2022 4:41:00 P.M. Based on the contradictory results of the existing trials and meta-analyses, current guidelines do not longer recommend NAC for CIN prevention. Since the alternatives for CIN prevention are very limited, future trials are needed examining different dosages and timing of NAC administration, combined with saline hydration in order to draw definite conclusions regarding the reno-protective effects of NAC.
NAC as an antioxidant in CKD
Accumulating preclinical data support the use of NAC in uremia and CKD. In animal models, NAC prevented GSH depletion in vascular cells exposed to uremic serum and thus diminished systemic OS that promotes CKD progression [78]. In addition, in a model of uremia-enhanced atherosclerosis, NAC reduced the progression of atheroma also by reducing OS [79]. In other in vivo studies, NAC appeared to have a protective effect on cyclosporin induced nephrotoxicity, through amelioration of local and systemic OS [80]. Experimental studies also suggested another molecular pathway through which NAC combats OS; in uremic animals, NAC administration directly attacked and neutralized AGEs that are released due to the uremic environment [81].
The clinical data regarding the effect of NAC in CKD populations are limited and have failed to show any reno-protective effect. Short-term oral NAC administration in CKD patients stage 3 showed no difference in renal function compared to placebo [82,83,84]. Similarly, NAC administration failed to show any therapeutic effect on the proteinuria levels of CKD patients with [85] and without diabetes [53]. However, in a cohort of CKD patients (stages 3–4) that received intravenous iron infusion for anemia correction, NAC resulted in a significant reduction of OS [86]. In kidney transplant recipients, the data are extremely limited; only a double-blinded, placebo-controlled randomized controlled trial (RCT) has been performed until to date [9]. This study showed a significant reno-protective effect in the NAC group, assessed by improvement in immediate graft function (28% increase over placebo) and first week eGFR (14 ml/min higher than placebo). Interestingly, this reno-protective effect of NAC was not attributed to its’ antioxidant properties, since there was no difference on malondialdehyde (MDA) levels between the groups. The authors hypothesized that other NAC properties, such as anti-inflammatory and vasodilatory might be responsible for their findings. To investigate the possible clinical benefits of NAC supplementation, Ye et al., performed a recent meta-analysis [87] including 768 CKD patients and 20 studies and found that NAC was safe without any severe adverse events. Moreover, NAC suppressed the levels of inflammatory cytokines and homocysteine, protected kidney function and was associated with reduced CV events (relative risk = 0.60, number needed to treat = 5.29). However, the authors recognized as limitations of their study the heterogeneity and low quality of the included studies and the fact that the majority of the pooled data included only few trials.
Therefore, the majority of data supporting the antioxidant effects of NAC in CKD are derived from experimental studies. The clinical studies are very scarce and have failed to show a clear-cut clinical benefit of NAC supplementation in pre-dialysis CKD.
NAC as an antioxidant in HD
Advanced oxidation protein products (AOPPs) in uremic plasma are indicators of oxidative damage to proteins and act as inflammation mediators resulting in monocyte and polymorphonuclear (PMN) activation. Release of AOPPs promotes monocyte respiratory burst and tumor necrosis factor-a (TNF-a) synthesis while PMNs produce free radicals by the molecular pathway of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and myeloperoxidase (MPO). NAC inhibits AOPP-induced oxidation of both monocytes and PMNs in a receptor-dependent way; therefore, it is suggested that in HD, NAC’s antioxidant activity might be selective and dependent on intracellular signaling rather than nonspecific oxidant scavenging [88,89,90]. A clinical trial in 24 chronic HD patients evaluated the circulating levels of MDA (a lipid peroxidation marker formed in the tissues by exposure to free radicals) pre- and post-dialysis after NAC administration (600 mg per os twice daily for 4 weeks) demonstrating that NAC significantly reduced the levels of MDA compared to placebo. Of note, HD alone was not able to diminish elevated MDA levels in chronic HD patients suggesting that glutathione repletion by NAC might be an additional mechanism contributing to the antioxidant properties of NAC [91]. Besides attacking directly and neutralizing free radicals, a double-blind, placebo-controlled RCT supported that NAC administration might reduce OS in chronic HD patients also by restoring the antioxidant defense mechanisms, assessed by total antioxidant capacity (TAC) [2].
In HD patients, intravenous iron administration is frequent and associated with increased OS. A randomized, cross-over clinical trial divided 40 HD patients in four cross-over treatment groups of 10 patients each according to iron sucrose administration dose (50 or 100 mg) and NAC supplementation (NAC or no NAC). NAC administration resulted in significant increase in TAC, whereas MDA serum levels were only reduced in the low iron dose group [92]. Swarnalatha et al. conducted a prospective, double-blinded, randomized controlled, cross-over study with 14 HD patients treated with intravenous iron receiving either NAC or placebo. NAC reduced MDA levels that were released after administration of intravenous iron therapy [5]. Likewise, another single-arm clinical trial reported decrease in MDA and asymmetric dimethylarginine (ADMA) levels post-intervention (NAC administration 600 mg per os before meals for 6 months) [93]. Since ADMA has been repeatedly associated with mortality and CV events in HD patients, another double-blind placebo-controlled clinical trial used it as a therapeutic target and showed that intravenous administration of high dose NAC (5 g) during HD resulted in significant reduction of serum ADMA levels post-dialysis compared with HD alone [94]. Since HD is a state of increased OS and inflammation, several studies aimed to investigate whether NAC supplementation might also ameliorate inflammation in these patients. A prospective, non-randomized, non-controlled clinical trial in a cohort of HD patients suggested a decrease in inflammatory and OS biomarkers after NAC administration, including high-sensitivity C-reactive protein (hs-CRP) and interleukin 6 (IL-6) [95], which have been repeatedly reported to be indicators of CV disease in CKD. IL-6 might act as a marker of atherosclerosis as well as a pro-atherogenic cytokine affecting multiple metabolic, endothelial, and coagulant pathways. In addition, CRP activates multiple inflammatory processes underlying the development of atherosclerosis [96,97,98,99]. Since CV morbidity and mortality in CKD has been associated with OS and inflammation [100], it was hypothesized that NAC might also exert cardioprotective effects in HD patients. A prospective, randomized, placebo-controlled trial in 134 maintenance HD patients, showed that daily, oral administration of NAC (600 mg/day) for a median of 14.5 months, was accompanied by a 40% reduction in the occurrence of CV events [101].
Another beneficial effect of NAC in HD patients is improvement of anemia. Red blood cell (RBC) reductase activity and TAC increased with NAC administration, while plasma levels of 8-isoprostane and oxidized low-density lipoprotein (ox-LDL) decreased, thus suggesting that positive outcomes of uremic anemia might be linked with improvement of OS status [55]. The presence of residual renal function (RRF) is an important predictor of survival in chronic dialysis patients [102,103,104]. In a non-randomized, non-blinded study, oral NAC significantly improved RRF in a small cohort of HD patients [105]. This was also confirmed in a randomized, multi-center, parallel-group, open-label study demonstrating that oral daily supplementation with NAC at a high dose of 1200 mg significantly improved RRF, urine volume and Kt/V [10].
Another novel risk factor for CV morbidity and mortality in CKD is hyperhomocysteinaemia [106]. Increased levels of homocysteine (Hcy) in plasma are indicators of increased OS, and contribute to endothelial dysfunction [8, 31]. Bostom et al., reported a non-significant reduction of homocysteine in a cohort of 11 HD patients receiving a single dose of oral NAC; however, the sample was very small and administration timing was not closely monitored to achieve optimal pharmacokinetics of NAC [107]. From this old study and there, several other investigators examined the effect of NAC on Hcy levels in ESRD patients. In a randomized, placebo-controlled cross-over study of 20 HD patients, Scholze et al., found that iv NAC administration during HD enhanced plasma Hcy clearance and ameliorated endothelial function [68]. Another study showed that addition of NAC to HD with high-flux membranes was accompanied with a significant reduction of circulating TNF-α, interleukin 10 (IL-10), hs-CRP and plasma Hcy, which was more pronounced in patients with RRF [108]. Thaha et al. performed a randomized, placebo-controlled trial and found a reduction in plasma Hcy and pulse pressure after dialysis with NAC supplementation [106]. Similarly, in a parallel, multi-center intervention study, Perna et al., showed that combined therapy of intravenous supplementation with NAC at a high dose of 5 g with 15 mg folates (5-methyltetrahydrofolate, MTHF) for 10 HD sessions, effectively reduced plasma Hcy levels in chronic HD patients [66]. The therapeutic effect of NAC in reducing plasma Hcy is reported to be about 11% higher than placebo [109].
In HD, NAC might improve OS, inflammation and anemia status; however, the existing evidence is derived from studies with various limitations, including short duration of treatment, small sample size and heterogeneity in the design. Moreover, the data regarding the clinical effect of NAC in hard endpoints, such as mortality and CV events are extremely limited, and therefore, currently, the administration of NAC in HD patients cannot be recommended. To elucidate whether NAC might be beneficial for CKD/ESKD patients and draw more definite conclusions, future, larger, well-designed RCTs are needed.
NAC as an antioxidant in PD
Since the culprit for triggering OS in PD is PD solutions, it was interesting to hypothesize that addition of antioxidants, such as NAC, to the dialysate might improve OS status. Several experimental studies suggested the clinical stability of NAC in PD solutions [52, 110]. In vitro, generation of formaldehyde (which is toxic for the peritoneal membrane) in heat-sterilized PD solutions was reduced by the administration of reduced thiol compounds [111]. Administration of NAC in the high-glucose compartment of neutral-pH-type PD solutions prevented GDP-mediated peritoneal membrane failure in PD patients [52]. In addition, NAC appeared to reduce the generation of AGEs [112] and diminished mitochondrial oxidative injury induced by conventional peritoneal solution in human peritoneal mesothelial cells by preserving the levels of reduced glutathione [113, 114]. In uremic rat models undergoing PD treatment, NAC prevented the OS-induced structural and functional alterations of the peritoneal membrane [115], decreased inflammation and vascular injury and, therefore, preserved the integrity of the peritoneal membrane [116].
After the exciting results reported in experimental studies, several researchers designed clinical trials to explore if the beneficial effect of NAC in preclinical trials could be replicated in human subjects as well. A placebo-controlled study in PD patients found that oral intake of 600 mg of NAC twice daily for 8 weeks resulted in decreased plasma levels of IL-6 compared to controls [117]. Similarly, the administration of oral NAC significantly decreased hs-CRP levels in PD patients; this anti-inflammatory effect was more pronounced in patients with increased inflammatory status at baseline (CRP levels between 5 and 15 mg/L) [118]. Another placebo-controlled trial also examined the effect of NAC on inflammation status of chronic ambulatory PD subjects demonstrating that oral NAC administration (600 mg of NAC twice daily for 8 weeks) reduced the levels of several inflammatory biomarkers; interleukin 1 (IL-1), IL-6, hs-CRP, procalcitonin, complement C3, TNF-a and soluble intercellular adhesion molecule-1 (SICAM-1) [6]. Regarding clinical endpoints, Feldman et al. found in a small cohort of PD patients, that oral NAC (1200 mg twice daily for 4 weeks) significantly improved residual RRF [119]. Table 1 shows a summary of clinical trials investigating the use of NAC in CKD, HD and PD assessing its antioxidant and anti- inflammatory properties.
The antioxidant and anti-inflammatory effects of NAC in PD are currently supported mainly by experimental studies and, therefore, no recommendations regarding NAC administration can be supported in PD patients.
Conclusions
In CKD, OS has emerged as a novel disease-related risk factor for CV disease, mortality, and progression to ESRD. In HD and PD, OS is further exacerbated and strongly associated with adverse clinical endpoints. NAC, a thiol compound, mostly known for its potential to reduce incidence of contrast-induced nephropathy has generated a lot of interest as an antioxidant agent and a potential candidate to combat OS-induced damage. Accumulated data suggest that in CKD, HD and PD patients, NAC neutralizes pro-oxidant molecules, increases antioxidant defenses, decreases Hcy, and suppresses inflammation, and thus might be beneficial for these patients. Moreover, limited data suggest that NAC might have beneficial impact on clinical hard endpoints in these populations, including protection of kidney function and prevention of endothelial dysfunction and CV disease. Of note, NAC is a safe agent without severe side effects, simple and of low cost. However, the data regarding the association of NAC with clinical hard points remain limited and derived from small studies with heterogenous populations. Well-designed RCTs with large sample size and hard endpoints are needed to draw definite conclusions regarding the beneficial effects of NAC in uremic populations.
Data availability
All data used for this article are available.
References
Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E (2019) Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne’s thread. Int J Mol Sci 20:3711. https://doi.org/10.3390/ijms20153711
Shahbazian H, Shayanpour S, Ghorbani A (2016) Evaluation of administration of oral N-acetylcysteine to reduce oxidative stress in chronic hemodialysis patients: a double-blind, randomized, controlled clinical trial. Saudi J Kidney Dis Transplant 27:88. https://doi.org/10.4103/1319-2442.174084
Roumeliotis S, Dounousi E, Salmas M, Eleftheriadis T, Liakopoulos V (2020) Unfavorable effects of peritoneal dialysis solutions on the peritoneal membrane: the role of oxidative stress. Biomolecules 10:768. https://doi.org/10.3390/biom10050768
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4:118–126. https://doi.org/10.4103/0973-7847.70902
Swarnalatha G, Ram R, Neela P, Naidu MUR, Murty KD (2010) Oxidative stress in hemodialysis patients receiving intravenous iron therapy and the role of N-acetylcysteine in preventing oxidative stress. Saudi J Kidney Dis Transplant 21:852
Purwanto B, Prasetyo DH (2012) Effect of oral N-acetylcysteine treatment on immune system in continuous ambulatory peritoneal dialysis patients. Acta Medica Indones 44:140–144
Renke M, Tylicki L, Rutkowski P, Larczynski W, Neuwelt A, Aleksandrowicz E, Łysiak-Szydłowska W, Rutkowski B (2010) The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: a placebo-controlled, randomized, cross-over study. Med Sci Monit Int Med J Exp Clin Res 16:PI13–PI18
Roumeliotis S, Mallamaci F, Zoccali C (2020) Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: a 2020 update. J Clin Med 9:2359. https://doi.org/10.3390/jcm9082359
Modarresi A, Ziaie S, Salamzadeh J, Sahraei Z, Nafar M, Panahi Y, Parvin M, Einollahi B (2017) Study of the effects of N-acetylcysteine on oxidative stress status of patients on maintenance-hemodialysis undergoing cadaveric kidney transplantation. Iran J Pharm Res IJPR 16:1631–1638
Ahmadi F, Abbaszadeh M, Razeghi E, Maziar S, Khoidaki SD, Najafi MT, Lessan-Pezeshki M (2017) Effectiveness of N-acetylcysteine for preserving residual renal function in patients undergoing maintenance hemodialysis: multicenter randomized clinical trial. Clin Exp Nephrol 21:342–349. https://doi.org/10.1007/s10157-016-1277-5
Roumeliotis S, Roumeliotis A, Gorny X, Mertens PR (2020) Could antioxidant supplementation delay progression of cardiovascular disease in end-stage renal disease patients? Curr Vasc Pharmacol 19:41–54. https://doi.org/10.2174/1570161118666200317151553
Yang C-C, Hsu S-P, Wu M-S, Hsu S-M, Chien C-T (2006) Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int 69:706–714. https://doi.org/10.1038/sj.ki.5000109
Liakopoulos V, Roumeliotis S, Gorny X, Eleftheriadis T, Mertens PR (2017) Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev 2017:1–14. https://doi.org/10.1155/2017/3494867
Liakopoulos V, Roumeliotis S, Zarogiannis S, Eleftheriadis T, Mertens PR (2019) Oxidative stress in hemodialysis: causative mechanisms, clinical implications, and possible therapeutic interventions. Semin Dial 32:58–71. https://doi.org/10.1111/sdi.12745
Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, Siamopoulos KC, Tsakiris D (2006) Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis Off J Natl Kidney Found 48:752–760. https://doi.org/10.1053/j.ajkd.2006.08.015
Roumeliotis S, Eleftheriadis T, Liakopoulos V (2019) Is oxidative stress an issue in peritoneal dialysis? Semin Dial 32:463–466. https://doi.org/10.1111/sdi.12818
Gastaldello K, Husson C, Dondeyne J-P, Vanherweghem J-L, Tielemans C (2008) Cytotoxicity of mononuclear cells as induced by peritoneal dialysis fluids: insight into mechanisms that regulate osmotic stress-related apoptosis. Perit Dial Int J Int Soc Perit Dial 28:655–666
Yamaji Y, Nakazato Y, Oshima N, Hayashi M, Saruta T (2004) Oxidative stress induced by iron released from transferrin in low pH peritoneal dialysis solution. Nephrol Dial Transplant 19:2592–2597. https://doi.org/10.1093/ndt/gfh278
Mortier S, Faict D, Lameire NH, De Vriese AS (2005) Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 67:1559–1565. https://doi.org/10.1111/j.1523-1755.2005.00237.x
Honda K, Nitta K, Horita S, Yumura W, Nihei H, Nagai R, Ikeda K, Horiuchi S (1999) Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc 14:1541–1549. https://doi.org/10.1093/ndt/14.6.1541
Choi KC, Jeong TK, Lee SC, Kim SW, Kim NH, Lee KY (1998) Nitric oxide is a marker of peritonitis in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial Conf Perit Dial 14:173–179
Furuya R, Kumagai H, Odamaki M, Takahashi M, Miyaki A, Hishida A (2009) Impact of residual renal function on plasma levels of advanced oxidation protein products and pentosidine in peritoneal dialysis patients. Nephron Clin Pract 112:c255-261. https://doi.org/10.1159/000224792
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19. https://doi.org/10.1016/j.pneurobio.2016.07.005
Plum J, Schoenicke G, Grabensee B (1997) Osmotic agents and buffers in peritoneal dialysis solution: monocyte cytokine release and in vitro cytotoxicity. Am J Kidney Dis Off J Natl Kidney Found 30:413–422. https://doi.org/10.1016/s0272-6386(97)90287-0
Park MS, Kim JK, Holmes C, Weiss MF (2000) Effects of bicarbonate/lactate solution on peritoneal advanced glycosylation end-product accumulation. Perit Dial Int J Int Soc Perit Dial 20(Suppl 5):S33-38
Htay H, Johnson DW, Wiggins KJ, Badve SV, Craig JC, Strippoli GF, Cho Y (2018) Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev 10:CD007554. https://doi.org/10.1002/14651858.CD007554.pub3
Wang HW, Hochberg AM, Pearson RK, Hauben M (2010) An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf 33:1117–1133. https://doi.org/10.2165/11584390-000000000-00000
Thomas S, Schenk U, Fischer FP, Mettang T, Passlick-Deetjen J, Kuhlmann U (1997) In vitro effects of glucose polymer-containing peritoneal dialysis fluids on phagocytic activity. Am J Kidney Dis Off J Natl Kidney Found 29:246–253. https://doi.org/10.1016/s0272-6386(97)90037-8
Feng W, Zhang K, Liu Y, Chen J, Cai Q, He W, Zhang Y, Wang M-H, Wang J, Huang H (2018) Advanced oxidation protein products aggravate cardiac remodeling via cardiomyocyte apoptosis in chronic kidney disease. Am J Physiol-Heart Circ Physiol 314:H475–H483. https://doi.org/10.1152/ajpheart.00628.2016
Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Böger R, Investigators on behalf of TC (2002) Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62:339–345. https://doi.org/10.1046/j.1523-1755.2002.00437.x
Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM (2002) The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62:1524–1538. https://doi.org/10.1046/j.1523-1755.2002.00600.x
Roumeliotis S, Veljkovic A, Georgianos PI, Lazarevic G, Perisic Z, Hadzi-Djokic J, Liakopoulos V, Kocic G (2021) Association between biomarkers of oxidative stress and inflammation with cardiac necrosis and heart failure in non-ST segment elevation myocardial infarction patients and various degrees of kidney function. Oxid Med Cell Longev 2021:3090120. https://doi.org/10.1155/2021/3090120
Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C (2005) Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol 16:2449–2455. https://doi.org/10.1681/ASN.2005010076
Zoccali C, Benedetto FA, Maas R, Mallamaci F, Tripepi G, Malatino LS, Böger R (2002) Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol 13:490–496. https://doi.org/10.1681/ASN.V132490
Clermont G, Lecour S, Lahet J-J, Siohan P, Vergely C, Chevet D, Rifle G, Rochette L (2000) Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: a possible explanation for the increased cardiovascular risk in these patients. Cardiovasc Res 47:618–623. https://doi.org/10.1016/S0008-6363(00)00117-6
Black MJ, Briscoe TA, Dunstan HJ, Bertram JF, Johnston CI (2001) Effect of angiotensin-converting enzyme inhibition on renal filtration surface area in hypertensive rats. Kidney Int 60:1837–1843. https://doi.org/10.1046/j.1523-1755.2001.00996.x
Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T (1999) Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55:1899–1911. https://doi.org/10.1046/j.1523-1755.1999.00422.x
Zoccali C, Bode-Böger SM, Mallamaci F, Benedetto FA, Tripepi G, Malatino LS, Cataliotti A, Bellanuova I, Fermo I, Frölich JC, Böger RH (2001) Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. The Lancet 358:2113–2117. https://doi.org/10.1016/S0140-6736(01)07217-8
Sakata N, Imanaga Y, Meng J, Tachikawa Y, Takebayashi S, Nagai R, Horiuchi S (1999) Increased advanced glycation end products in atherosclerotic lesions of patients with end-stage renal disease. Atherosclerosis 142:67–77. https://doi.org/10.1016/S0021-9150(98)00192-0
Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS (2000) Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. The Lancet 356:1213–1218. https://doi.org/10.1016/S0140-6736(00)02783-5
Yeun JY, Levine RA, Mantadilok V, Kaysen GA (2000) C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35:469–476. https://doi.org/10.1016/S0272-6386(00)70200-9
Mune M, Yukawa S, Kishino M, Otani H, Kimura K, Nishikawa O, Takahashi T, Kodama N, Saika Y, Yamada Y (1999) Effect of vitamin E on lipid metabolism and atherosclerosis in ESRD patients. Kidney Int 56:S126–S129. https://doi.org/10.1016/S0085-2538(15)46619-0
Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S (1999) Comparison of hemostatic factors and serum malondialdehyde as predictive factors for cardiovascular disease in hemodialysis patients. Am J Kidney Dis 34:438–444. https://doi.org/10.1016/S0272-6386(99)70070-3
Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S (1999) Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int 56:1078–1083. https://doi.org/10.1046/j.1523-1755.1999.00613.x
Shoji T, Fukumoto M, Kimoto E, Shinohara K, Emoto M, Tahara H, Koyama H, Ishimura E, Nakatani T, Miki T, Tsujimoto Y, Tabata T, Nishizawa Y (2002) Antibody to oxidized low-density lipoprotein and cardiovascular mortality in end-stage renal disease. Kidney Int 62:2230–2237. https://doi.org/10.1046/j.1523-1755.2002.00692.x
Taki K, Takayama F, Tsuruta Y, Niwa T (2006) Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int 70:218–224. https://doi.org/10.1038/sj.ki.5000330
Bayés B, Pastor MC, Bonal J, Foraster A, Romero R (2006) Oxidative stress, inflammation and cardiovascular mortality in haemodialysis—role of seniority and intravenous ferrotherapy: analysis at 4 years of follow-up. Nephrol Dial Transplant 21:984–990. https://doi.org/10.1093/ndt/gfi294
Usberti M, Gerardi GM, Gazzotti RM, Benedini S, Archetti S, Sugherini L, Valentini M, Tira P, Bufano G, Albertini A, Lorenzo DD (2002) Oxidative stress and cardiovascular disease in dialyzed patients. Nephron 91:25–33. https://doi.org/10.1159/000057601
Bashardoust B, Alaei R, Kebar SM, Hasani S, Habibzadeh A (2017) The effect of oral N-acetylcysteine on serum high sensitive CRP and plasma hemoglobin levels in end-stage renal disease patients under routine hemodialysis; a randomized placebocontrolled clinical trial. J Nephropathol 7:268–272. https://doi.org/10.15171/jnp.2018.53
Rushworth GF, Megson IL (2014) Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 141:150–159. https://doi.org/10.1016/j.pharmthera.2013.09.006
Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830:3217–3266. https://doi.org/10.1016/j.bbagen.2012.09.018
Lee E, Seo E, Kwon Y, Ha H (2011) Rapid and reliable measurement for evaluating directly the reactivity of N-acetylcysteine with glucose degradation products in peritoneal dialysis fluids. Anal Chem 83:1518–1522. https://doi.org/10.1021/ac200046y
Renke M, Tylicki L, Rutkowski P, Larczyński W, Aleksandrowicz E, Lysiak-Szydłowska W, Rutkowski B (2008) The effect of N-acetylcysteine on proteinuria and markers of tubular injury in non-diabetic patients with chronic kidney disease. A placebo-controlled, randomized, open, cross-over study. Kidney Blood Press Res 31:404–410. https://doi.org/10.1159/000185828
Delgobo M, Agnes JP, Gonçalves RM, Dos Santos VW, Parisotto EB, Zamoner A, Zanotto-Filho A (2019) N-acetylcysteine and alpha-lipoic acid improve antioxidant defenses and decrease oxidative stress, inflammation and serum lipid levels in ovariectomized rats via estrogen-independent mechanisms. J Nutr Biochem 67:190–200. https://doi.org/10.1016/j.jnutbio.2019.02.012
Hsu S-P, Chiang C-K, Yang S-Y, Chien C-T (2010) N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin Pract 116:c207–c216. https://doi.org/10.1159/000317201
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F (2018) N-acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 52:751–762. https://doi.org/10.1080/10715762.2018.1468564
Pinkus R, Weiner LM, Daniel V (1996) Role of oxidants and antioxidants in the induction of AP-1, NF-κB, and glutathione S-transferase gene expression. J Biol Chem 271:13422–13429. https://doi.org/10.1074/jbc.271.23.13422
Palacio JR, Markert UR, Martínez P (2011) Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res Off J Eur Histamine Res Soc Al 60:695–704. https://doi.org/10.1007/s00011-011-0323-8
Zicha J, Dobesová Z, Kunes J (2006) Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens Res Off J Jpn Soc Hypertens 29:1021–1027. https://doi.org/10.1291/hypres.29.1021
Rauchová H, Pechánová O, Kunes J, Vokurková M, Dobesová Z, Zicha J (2005) Chronic N-acetylcysteine administration prevents development of hypertension in N(omega)-nitro-l-arginine methyl ester-treated rats: the role of reactive oxygen species. Hypertens Res Off J Jpn Soc Hypertens 28:475–482. https://doi.org/10.1291/hypres.28.475
Cusumano G, Romagnoli J, Liuzzo G, Ciavarella LP, Severino A, Copponi G, Manchi M, Giubilato S, Zannoni GF, Stigliano E, Caristo ME, Crea F, Citterio F (2015) N-acetylcysteine and high-dose atorvastatin reduce oxidative stress in an ischemia-reperfusion model in the rat kidney. Transplant Proc 47:2757–2762. https://doi.org/10.1016/j.transproceed.2015.09.035
Wittstock A, Burkert M, Zidek W, Tepel M, Scholze A (2009) N-acetylcysteine improves arterial vascular reactivity in patients with chronic kidney disease. Nephron Clin Pract 112:c184-189. https://doi.org/10.1159/000218107
Andrews NP, Prasad A, Quyyumi AA (2001) N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol 37:117–123. https://doi.org/10.1016/s0735-1097(00)01093-7
Efrati S, Dishy V, Averbukh M, Blatt A, Krakover R, Weisgarten J, Morrow JD, Stein MC, Golik A (2003) The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int 64:2182–2187. https://doi.org/10.1046/j.1523-1755.2003.00322.x
Dodd S, Berk M (2008) The safety of medications for the treatment of bipolar disorder during pregnancy and the puerperium. Curr Drug Saf 1:25–33. https://doi.org/10.2174/157488606775252692
Perna AF, Violetti E, Lanza D, Sepe I, Bellinghieri G, Savica V, Santoro D, Satta E, Cirillo G, Lupo A, Abaterusso C, Raiola I, Raiola P, Coppola S, Di Iorio B, Tirino G, Cirillo M, Ingrosso D, De Santo NG (2012) Therapy of hyperhomocysteinemia in hemodialysis patients: effects of folates and N-acetylcysteine. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 22:507-514.e1. https://doi.org/10.1053/j.jrn.2011.10.007
Ventura P, Panini R, Pasini MC, Scarpetta G, Salvioli G (1999) N -acetyl-cysteine reduces homocysteine plasma levels after single intravenous administration by increasing thiols urinary excretion. Pharmacol Res 40:345–350. https://doi.org/10.1006/phrs.1999.0519
Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M (2004) Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation 109:369–374. https://doi.org/10.1161/01.CIR.0000109492.65802.AD
Hultberg B, Andersson A, Masson P, Larson M, Tunek A (1994) Plasma homocysteine and thiol compound fractions after oral administration of N-acetylcysteine. Scand J Clin Lab Invest 54:417–422. https://doi.org/10.3109/00365519409085464
Wiklund O, Fager G, Andersson A, Lundstam U, Masson P, Hultberg B (1996) N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 119:99–106. https://doi.org/10.1016/0021-9150(95)05635-1
Wu M-Y, Hsiang H-F, Wong C-S, Yao M-S, Li Y-W, Hsiang C-Y, Bai C-H, Hsu Y-H, Lin Y-F, Tam K-W (2013) The effectiveness of N-acetylcysteine in preventing contrast-induced nephropathy in patients undergoing contrast-enhanced computed tomography: a meta-analysis of randomized controlled trials. Int Urol Nephrol 45:1309–1318. https://doi.org/10.1007/s11255-012-0363-1
Baliga R, Ueda N, Walker PD, Shah SV (1997) Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis Off J Natl Kidney Found 29:465–477. https://doi.org/10.1016/s0272-6386(97)90212-2
Kang X, Hu D-Y, Li C-B, Ai Z-S, Peng A (2015) N-acetylcysteine for the prevention of contrast-induced nephropathy in patients with pre-existing renal insufficiency or diabetes: a systematic review and meta-analysis. Ren Fail 37:297–303. https://doi.org/10.3109/0886022X.2015.1012985
Guo Z, Liu J, Lei L, Xue Y, Liu L, Huang H, Chen S, Liu Y, Lin Y, Tao J, Xu Q, Wu K, Zhang L, Chen J-Y (2020) Effect of N-acetylcysteine on prevention of contrast-associated acute kidney injury in patients with STEMI undergoing primary percutaneous coronary intervention: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 10:e039009. https://doi.org/10.1136/bmjopen-2020-039009
Xie W, Liang X, Lin Z, Liu M, Ling Z (2021) Latest clinical evidence about effect of acetylcysteine on preventing contrast-induced nephropathy in patients undergoing angiography: a meta-analysis. Angiology 72:105–121. https://doi.org/10.1177/0003319720950162
Wang N, Qian P, Kumar S, Yan TD, Phan K (2016) The effect of N-acetylcysteine on the incidence of contrast-induced kidney injury: a systematic review and trial sequential analysis. Int J Cardiol 209:319–327. https://doi.org/10.1016/j.ijcard.2016.02.083
Weisbord SD, Gallagher M, Jneid H, Garcia S, Cass A, Thwin S-S, Conner TA, Chertow GM, Bhatt DL, Shunk K, Parikh CR, McFalls EO, Brophy M, Ferguson R, Wu H, Androsenko M, Myles J, Kaufman J, Palevsky PM, PRESERVE Trial Group (2018) Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med 378:603–614
Rodrigues SD, França KC, Dallin FT, Fujihara CK, Nascimento AJ, Pecoits-Filho R, Nakao LS (2015) N-acetylcysteine as a potential strategy to attenuate the oxidative stress induced by uremic serum in the vascular system. Life Sci 121:110–116. https://doi.org/10.1016/j.lfs.2014.11.024
Ivanovski O, Szumilak D, Nguyen-Khoa T, Ruellan N, Phan O, Lacour B, Descamps-Latscha B, Dreeke TB, Massy ZA (2005) The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int 67:2288–2294. https://doi.org/10.1111/j.1523-1755.2005.00332.x
Tariq M, Morais C, Sobki S, Al Sulaiman M, Al Khader A (1999) N-acetylcysteine attenuates cyclosporin-induced nephrotoxicity in rats. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc 14:923–929. https://doi.org/10.1093/ndt/14.4.923
Allen MR, Wallace J, McNerney E, Nyman J, Avin K, Chen N, Moe S (2020) N-acetylcysteine (NAC), an anti-oxidant, does not improve bone mechanical properties in a rat model of progressive chronic kidney disease-mineral bone disorder. PLoS One 15:e0230379. https://doi.org/10.1371/journal.pone.0230379
Moist L, Sontrop JM, Gallo K, Mainra R, Cutler M, Freeman D, House AA (2010) Effect of N-acetylcysteine on serum creatinine and kidney function: results of a randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found 56:643–650. https://doi.org/10.1053/j.ajkd.2010.03.028
Mainra R, Gallo K, Moist L (2007) Effect of N-acetylcysteine on renal function in patients with chronic kidney disease. Nephrol Carlton Vic 12:510–513. https://doi.org/10.1111/j.1440-1797.2007.00833.x
Rehman T, Fought J, Solomon R (2008) N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD patients. Clin J Am Soc Nephrol CJASN 3:1610–1614. https://doi.org/10.2215/CJN.01560408
Hashemi SR, Noshad H, Tabrizi A, Mobasseri M, Khosroshahi HT, Heydarnejad M, Khalaj MR, Aghamohammadzadeh N (2012) Angiotensin receptor blocker and N-acetyl cysteine for reduction of proteinuria in patients with type 2 diabetes Mellitus. 6:5
Agarwal R, Vasavada N, Sachs NG, Chase S (2004) Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int 65:2279–2289. https://doi.org/10.1111/j.1523-1755.2004.00648.x
Ye M, Lin W, Zheng J, Lin S (2021) N-acetylcysteine for chronic kidney disease: a systematic review and meta-analysis. Am J Transl Res 13:2472–2485
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49:1304–1313. https://doi.org/10.1038/ki.1996.186
Witko-Sarsat V, Gausson V, Nguyen A-T, Touam M, Drüeke T, Santangelo F, Descamps-Latscha B (2003) AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int 64:82–91. https://doi.org/10.1046/j.1523-1755.2003.00044.x
Amore A, Formica M, Giacchino F, Gigliola G, Bonello F, Conti G, Camilla R, Coppo R (2013) N-acetylcysteine in hemodialysis diabetic patients resets the activation of NF-kB in lymphomonocytes to normal values. J Nephrol 26:778–786. https://doi.org/10.5301/jn.5000167
Trimarchi H, Mongitore MR, Baglioni P, Forrester M, Freixas ER, Schropp M, Pereyra H, Alonso M (2003) N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients–a pilot study. Clin Nephrol 59:441–446. https://doi.org/10.5414/cnp59441
Garcia-Fernandez N, Echeverria A, Sanchez-Ibarrola A, Páramo JA, Coma-Canella I (2010) Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrol Carlton Vic 15:178–183. https://doi.org/10.1111/j.1440-1797.2009.01174.x
Giannikouris I (2015) The effect of N-acetylcysteine on oxidative serum biomarkers of hemodialysis patients. Hippokratia 19:131–135
Thaha M, Widodo null, Pranawa W, Yogiantoro M, Tomino Y (2008) Intravenous N-acetylcysteine during hemodialysis reduces asymmetric dimethylarginine level in end-stage renal disease patients. Clin Nephrol 69:24–32. https://doi.org/10.5414/cnp69024
Saddadi F, Alatab S, Pasha F, Ganji M, Soleimanian T (2014) The effect of treatment with N-acetylcysteine on the serum levels of C-reactive protein and interleukin-6 in patients on hemodialysis. Saudi J Kidney Dis Transplant 25:66. https://doi.org/10.4103/1319-2442.124489
Ridker PM (1998) Inflammation, infection, and cardiovascular risk: how good is the clinical evidence? Circulation 97:1671–1674. https://doi.org/10.1161/01.cir.97.17.1671
Elhage R, Clamens S, Besnard S, Mallat Z, Tedgui A, Arnal J, Maret A, Bayard F (2001) Involvement of interleukin-6 in atherosclerosis but not in the prevention of fatty streak formation by 17beta-estradiol in apolipoprotein E-deficient mice. Atherosclerosis 156:315–320. https://doi.org/10.1016/s0021-9150(00)00682-1
Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V (2000) Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148:209–214. https://doi.org/10.1016/s0021-9150(99)00463-3
Pigott R, Dillon LP, Hemingway IH, Gearing AJ (1992) Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 187:584–589. https://doi.org/10.1016/0006-291x(92)91234-h
Locatelli F, Canaud B, Eckardt K-U, Stenvinkel P, Wanner C, Zoccali C (2003) Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc 18:1272–1280. https://doi.org/10.1093/ndt/gfg074
Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W (2003) The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation 107:992–995. https://doi.org/10.1161/01.cir.0000050628.11305.30
Brener ZZ, Thijssen S, Kotanko P, Kuhlmann MK, Bergman M, Winchester JF, Levin NW (2011) The impact of residual renal function on hospitalization and mortality in incident hemodialysis patients. Blood Purif 31:243–251. https://doi.org/10.1159/000322252
Shemin D, Bostom AG, Laliberty P, Dworkin LD (2001) Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis Off J Natl Kidney Found 38:85–90. https://doi.org/10.1053/ajkd.2001.25198
Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT, NECOSAD Study Group (2004) Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol JASN 15:1061–1070. https://doi.org/10.1097/01.asn.0000117976.29592.93
Feldman L, Shani M, Sinuani I, Beberashvili I, Weissgarten J (2012) N-acetylcysteine may improve residual renal function in hemodialysis patients: a pilot study. Hemodial Int Int Symp Home Hemodial 16:512–516. https://doi.org/10.1111/j.1542-4758.2012.00702.x
Thaha M, Yogiantoro M, Tomino Y (2006) Intravenous N-acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end-stage renal disease. Clin Drug Investig 26:195–202. https://doi.org/10.2165/00044011-200626040-00003
Bostom AG, Shemin D, Yoburn D, Fisher DH, Nadeau MR, Selhub J (1996) Lack of effect of oral N-acetylcysteine on the acute dialysis-related lowering of total plasma homocysteine in hemodialysis patients. Atherosclerosis 120:241–244. https://doi.org/10.1016/0021-9150(95)05705-6
Tsai J-P, Yang F-L, Wang C-H, Fang T-C, Lee R-P, Hsu B-G (2010) Effect of intravenous N-acetylcysteine on plasma total homocysteine and inflammatory cytokines during high flux hemodialysis. Tzu Chi Med J 22:90–95. https://doi.org/10.1016/S1016-3190(10)60047-X
Friedman AN, Bostom AG, Laliberty P, Selhub J, Shemin D (2003) The effect of N-acetylcysteine on plasma total homocysteine levels in hemodialysis: a randomized, controlled study. Am J Kidney Dis 41:442–446. https://doi.org/10.1053/ajkd.2003.50054
Seo E-Y, Gwak H, Lee HB, Ha H (2010) Stability of N-acetylcysteine in peritoneal dialysis solution. Perit Dial Int J Int Soc Perit Dial 30:105–108. https://doi.org/10.3747/pdi.2008.00032
Legge M, Lash GE, Bird SD, Walkerl RJ (1998) Formaldehyde in heat sterilized peritoneal dialysis solutions: scavenging system for its removal. Perit Dial Int 18:228–231. https://doi.org/10.1177/089686089801800214
Nakayama M, Izumi G, Nemoto Y, Shibata K, Hasegawa T, Numata M, Wang K, Kawaguchi Y, Hosoya T (1999) Suppression of N(epsilon)-(carboxymethyl)lysine generation by the antioxidant N-acetylcysteine. Perit Dial Int J Int Soc Perit Dial 19:207–210
Kuo H-T, Lee J-J, Hsiao H-H, Chen H-W, Chen H-C (2009) N-acetylcysteine prevents mitochondria from oxidative injury induced by conventional peritoneal dialysate in human peritoneal mesothelial cells. Am J Nephrol 30:179–185. https://doi.org/10.1159/000213502
Hung K-Y, Liu S-Y, Yang T-C, Liao T-L, Kao S-H (2014) High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxid Med Cell Longev 2014:642793. https://doi.org/10.1155/2014/642793
Noh H, Kim JS, Han K-H, Lee GT, Song JS, Chung SH, Jeon JS, Ha H, Lee HB (2006) Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int 69:2022–2028. https://doi.org/10.1038/sj.ki.5001506
Bozkurt D, Hur E, Ulkuden B, Sezak M, Nar H, Purclutepe O, Sen S, Duman S (2009) Can N-acetylcysteine preserve peritoneal function and morphology in encapsulating peritoneal sclerosis? Perit Dial Int J Int Soc Perit Dial 29(Suppl 2):S202-205
Nascimento MM, Suliman ME, Silva M, Chinaglia T, Marchioro J, Hayashi SY, Riella MC, Lindholm B, Anderstam B (2010) Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int J Int Soc Perit Dial 30:336–342. https://doi.org/10.3747/pdi.2009.00073
Najafi F, Mousavi-Roknabadi RS, Pirdehghan A, Rahimian M, Nourimajalan N (2021) Effect of N-acetylcysteine on hsCRP in patients on continues ambulatory peritoneal dialysis: a quasi-experimental study. Nephro-Urol Mon. https://doi.org/10.5812/numonthly.113990
Feldman L, Shani M, Efrati S, Beberashvili I, Yakov-Hai I, Abramov E, Sinuani I, Rosenberg R, Weissgarten J (2011) N-acetylcysteine improves residual renal function in peritoneal dialysis patients: a pilot study. Perit Dial Int J Int Soc Perit Dial 31:545–550. https://doi.org/10.3747/pdi.2009.00263
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cepaityte, D., Leivaditis, K., Varouktsi, G. et al. N-Acetylcysteine: more than preventing contrast-induced nephropathy in uremic patients—focus on the antioxidant and anti-inflammatory properties. Int Urol Nephrol 55, 1481–1492 (2023). https://doi.org/10.1007/s11255-022-03455-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03455-3