Abstract
Gallibacterium anatis (G. anatis), a member of the Pasteurellaceae family, normally inhabits the upper respiratory and lower genital tracts of poultry. However, under certain circumstances of immunosuppression, co-infection (especially with Escherichia coli or Mycoplasma), or various stressors, G. anatis caused respiratory, reproductive, and systemic diseases. Infection with G. anatis has emerged in different countries worldwide. The bacterium affects mainly chickens; however, other species of domestic and wild birds may get infected. Horizontal, vertical, and venereal routes of G. anatis infection have been reported. The pathogenicity of G. anatis is principally related to the presence of some essential virulence factors such as Gallibacterium toxin A, fimbriae, haemagglutinin, outer membrane vesicles, capsule, biofilms, and protease. The clinical picture of G. anatis infection is mainly represented as tracheitis, oophoritis, salpingitis, and peritonitis, while other lesions may be noted in cases of concomitant infection. Control of such infection depends mainly on applying biosecurity measures and vaccination. The antimicrobial sensitivity test is necessary for the correct treatment of G. anatis. However, the development of multiple drug resistance is common. This review article sheds light on G. anatis regarding history, susceptibility, dissemination, virulence factors, pathogenesis, clinical picture, diagnosis, and control measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Certain infections of poultry have a tremendous direct adverse impact on egg production or an indirect effect on the health status of poultry. One of these bacterial infections is Gallibacterium anatis (G. anatis) infection. G. anatis is a member of the family Pasteurellaceae (Christensen et al. 2003; Bisgaard et al. 2009). In 1981, the separate taxonomy of the family Pasteurellaceae, which mainly consisted of isolated Gram-negative coccobacilli from animals, was established. To date, this family includes 15 genera: Pasteurella, Avibacterium, Actinobacillus, Gallibacterium, Haemophilus, Mannheimia, Aggregatibacter, Bibersteinia, Lonepinella, Phocoenobacter, Histophilus, Nicoletella, Volucribacter, Chelonobacter, and Basfia (Janda 2011).
According to several studies, Gallibacterium caused deaths in domestic birds and occasionally people, suggesting it was more dangerous than an opportunistic infection (Driessche et al. 2020). The importance of G. anatis was underestimated for a long time due to an incomplete understanding of the pathogenesis, virulence, and growth kinetics (Kristensen et al. 2011). However, the overall rate of G. anatis disease increased in layer, breeder, and boiler chicken flocks in previous years (Elbestawy et al. 2018; Krishnegowda et al. 2020; Algammal et al. 2022a). These days, this newly developing illness poses a grave threat to the world’s chicken meat and egg industry (Antenucci et al. 2020; Algammal et al. 2021a; Elewa, 2021; Sorescu et al. 2021). The disease is widely distributed in American, European, Australian, African, and Asian countries. Although G. anatis may be regarded as a normal component of the microbiota of the lower genital, terminal digestive, and upper respiratory tracts under certain environmental and stress circumstances, it initiates reproductive and systemic disorders (Paudel et al. 2015; Ataei et al. 2017). Domestic and non-domestic species of birds and mammals, including humans, are susceptible to G. anatis infection (Aubin et al. 2013; Krishnegowda et al. 2020).
This bacterium has several virulence factors causing reproductive and respiratory tissue damage, particularly in the presence of co-infections and other stressors related to environmental conditions (Paudel et al. 2017a,b). G. anatis infection causes either local or systemic affection and is typically accompanied by a notable decline in laying performance, 5–10% egg drop, adverse changes in the eggshell quality, increased mortality, respiratory manifestations, and diarrhea (Paudel et al. 2014a). Oophoritis, salpingitis, epididymitis, peritonitis, septicemia, respiratory tract lesions, enteritis, hepatic necrosis, and pericarditis are the most common lesions of G. anatis infections (Driessche et al. 2020; Krishnegowda et al. 2020).
Recent and advanced diagnostic and identification techniques were adopted alongside routine cultural or biochemical phenotypic assays and molecular characterization for rapidly detecting G. anatis infection (Christensen et al. 2003; Huangfu et al. 2012).
Despite the sensitivity of G. anatis to many antimicrobials, some cases are non-responsive, and disease recurrences were reported. The development of multi-drug resistance (MDR), notable antigenic variation, and ineffective elimination of G. anatis by the host are the key limitations for disease control (El-Adawy et al. 2018; Hess et al. 2019). The widespread antibiotic resistance leads to ineffective treatment (Johnson et al. 2013). The antibiotic sensitivity of G. anatis strains continuously varies; thus, frequent in vitro assessment of strains is essential (Elbestawy et al. 2018). Few vaccines were exploited against G. anatis and are being estimated. To better understand G. anatis infection in poultry, this review discusses G. anatis history, susceptibility, dissemination, virulence factors, pathogenesis, clinical picture, diagnosis, and control and preventive measures.
History
In 1950, G. anatis was found as a component of normal microbiota in the cloacae of apparently healthy chickens and designated as a “hemolytic cloaca bacterium” (Kjos-Hansen 1950). Later on, DNA hybridization demonstrated that avian Pasteurella hemolytica (P. hemolytica), Actinobacillus salpingitidis (A. salpingitidis), and P. anatis belong to different genera inside the family Pasteurellaceae (Bisgaard 1977), while Christensen et al. (2003) classified Gallibacterium into a separate and independent genus. Taxon 1, the 3rd group of strains labeled P. anatis, was closely related to A. salpingitidis and avian P. haemolytica (El-Adawy et al. 2018). P. hemolytica was then re-classified within the family Pasteurellaceae into G. anatis biovar hemolytica based on 16S rRNA gene sequences (Christensen et al. 2003; Bojesen et al. 2008; Bisgaard et al. 2009). Now, the genus consists of 4 known species [G. anatis (biovar haemolytica and biovar anatis), G. salpingitidis, G. melopsittaci, and G. trehalosi fermentans], 3 genomospecies (1, 2, and 3), and unnamed group V (Christensen et al. 2003; Bisgaard et al. 2009; Janda 2011). G. anatis is a Gram-negative pleomorphic, capsulated, non-motile, non-spore former, and facultative anaerobic bacterium [requires micro-aerophilic conditions for growth on blood agar medium supplemented with 5–10% carbon dioxide (CO2)] (Christensen et al. 2003). According to Bisgaard (1982), phenotypically, G. anatis is divided into the “hemolytica” biovar, which produces β-hemolysis, and the “anatis” biovar, which is not a hemolytic variation.
The disease emerged in several continents, such as Europe, the Americas, Australia, Africa, and Asia, indicating these bacteria’s importance and worldwide distribution. For instance, G. anatis infections have been reported in Europe, including Germany (Matthes et al. 1969; Matthes and Loliger 1976; Mraz et al. 1976; Bisgaard 1977), Denmark (Bisgaard 1977; Bojesen 2003), Austria (Mirle et al. 1991; Neubauer et al. 2007), England, Norway, Sweden, and Czech Republic (Jordan et al. 2005; Galaz-Luna et al. 2009), and Romania (Sorescu et al. 2021). Moreover, in American countries, i.e., the USA (Shaw et al. 1990; Zellner et al. 2004; Jones et al. 2013), Canada (Shapiro et al. 2013), Mexico (Vazquez et al. 2003; Bojesen et al. 2007a, 2008; Chavez et al. 2017b), and Peru (Mendoza et al. 2014), cases of G. anatis were recorded. In addition, Gallibacterium have been recorded in Australia (Gilchrist, 1963), some African countries, such as Nigeria (Addo and Mohan, 1985; Lawal et al. 2017), Egypt (Elbestawy, 2014; Sorour et al. 2015; Abd El-Hamid et al. 2016, 2018; Mataried, 2016; Elbestawy et al. 2018; Elewa, 2021; Algammal et al. 2022a), and Morocco (Nassik et al. 2019), and many Asian counties, i.e., Japan (Suzuki et al. 1996; Huangfu et al. 2012; Zhang et al. 2017), Taiwan (Lin et al. 2001), China (Chuan-qing et al. 2008; Guo, 2011), India (Singh, 2016; Singh et al. 2016, 2018), Iran (Ataei et al. 2017, 2019; Allahghadry et al. 2021), Turkey (Yaman and Sahan 2019), and Syria (Janetschke and Risk 1970). The incidence of G. anatis infection in various parts of the world is depicted in Table 1.
Susceptibility
Numerous domestic and wild avian species, including chickens, ducks, geese, turkeys, pigeons, pheasants, guinea fowls, budgerigars, peacocks, parrots, partridges, cattle egrets, web-footed galliformes, psittacine birds, and owls, may harbor G. anatis (Mushin et al. 1980; Bisgaard, 1982; Bojesen et al. 2003a; Zellner et al. 2004; Bojesen and Shivaprasad 2007; Christensen et al. 2007a; Rzewuska et al. 2007; Bojesen et al. 2008; Persson and Bojesen, 2015; Sorour et al. 2015; Singh, 2016; Singh et al. 2016, 2018). Infection was reported even in non-avian species, namely, cattle, horses, pigs, sheep, and rabbits (Kristensen et al. 2011; Lawal et al. 2017). Furthermore, some G. anatis infections in humans have been detected (Aubin et al. 2013; Driessche et al. 2020). Regarding age susceptibility, older research studies have reported that young birds are less susceptible to G. anatis than adults (Bisgaard 1977; Mushin et al. 1980), whereas Huangfu et al. (2012) informed an extreme rate of detection and isolation of G. anatis from chickens aged 5–6, 12, 18, and 55–58 weeks. However, G. anatis was isolated from broiler chickens (Abd El-Hamid et al. 2016).
Infection and dissemination
The primary route of G. anatis infection is the horizontal one through the respiratory tract (Bisgaard 1977). Therefore, this bacterium is primarily prevalent in flocks with low biosecurity measures (Bojesen et al. 2003a). Isolation of G. anatis from the different body organs indicates the systemic circulation and spread of the bacterium from its natural habitat (Zepeda et al. 2010). Affection of the reproductive organs may be associated with ascending infections from the cloaca (Bojesen et al. 2003a; Neubauer et al. 2009). There is experimental evidence of vertical transmission in embryonated eggs through the trans-ovarian/oviduct route or the trans-eggshell route (Matthes and Hanschke 1977; Persson and Bojesen, 2015; Wang et al. 2018).
G. anatis was isolated from the egg yolk and ovaries of hens 10 days after the experimental infection (Shapiro et al. 2013; Paudel et al. 2014a). In embryonated eggs, G. anatis may penetrate the eggshell pores and infect the growing embryo causing mortality (Wang et al. 2018). Besides, venereal transmission has been suggested since G. anatis damages semen quality and causes epididymitis (Paudel et al. 2014b). Semen of G. anatis infected cockerels may function in transmission among adult birds and probably to their offspring.
Intranasal inoculation of cockerels with G. anatis resulted in the presence of the pathogen in the testis and epididymis within a week post-infection (pi). In addition, the sperm density was lower, total and progressive motility were lower, and membrane integrity was worse in the semen, all indicating a detrimental effect on fertility. The study of Neubauer et al. (2009) revealed that G. anatis affected 13-layer flocks, and the birds suffered from cannibalism (extensive wound pecking around the cloaca).
Zoonotic importance
It is presumed that G. anatis is a food contaminant that transmits to humans. In 2013, in France, the first case of bacteremia initiated by G. anatis was reported in an immunocompromised woman infected through consuming contaminated food. The predominant symptoms include fever, severe abdominal pain, anemia, diarrhea, and neutropenia (Gautier et al. 2005; Aubin et al. 2013). Driessche et al. (2020) reported that poultry and cattle have a likely risk for zoonotic transmission of G. anatis, and further research should be conducted to establish their zoonotic potential. Recently, Wang et al. (2023) reported a case of acute watery diarrhea (7–8 times daily) brought on by G. anatis in a 62-year-old man with type 2 diabetes and hypertension. There was no urgency, no bloating or pain in the abdomen, no dread of a cold or fever, and only nausea and vomiting were the symptoms of the illness. The bacteria were identified using 16S rRNA sequencing and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS).
Virulence factors
The different virulence factors of G. anatis are illustrated in Fig. 1.
Gallibacterium toxin A
Gallibacterium toxin A (GtxA) is a vital virulence protein that is responsible for the hemolytic property of G. anatis biovar hemolytica (Kristensen et al. 2011). It has a leukotoxic impact on chicken macrophage cell line HD11 and lyses red blood cells (RBCs) (Kristensen et al. 2010; Persson and Bojesen 2015), and stimulates the immune response (Bager et al. 2014). This toxin has two domains, the C- and N-; both are needed for hemolytic activity. The C-terminus is similar to repeat in toxins (RTX) of the Pasteurellaceae family, while the N-terminal has RBCs lytic and leukocidal actions (Yang et al. 2020). The GtxA knockout mutant bacterium was exposed to a reduction of pathogenicity. It is comparable to the cytoskeletal protein talin, which binds integrins to the actin and vacuolar cytoskeleton and is crucial for interactions between the host and the bacteria (Kristensen et al. 2010). Actin can support cell motility, recognition, adhesion, phagocytosis, signal modulation of immune cells, and free radical generation. When GtxA binds to actin in host immune cells, the cell shape can be altered, and cellular signal transmission is impeded, and as a result, the bacteria can elude the host immune system (Aktories et al. 2011). Nassik et al. (2019) demonstrated that all the molecularly identified strains of G. anatis expressed the virulent GtxA. Tang et al. (2020) implied that GtxA plays a crucial function in an acute cytokine-mediated Th2-like response versus G. anatis infection in the ovarian tissue, helping in the pathogenesis of G. anatis infections in laying hens. Additionally, Tang and Bojesen (2020) attempted to explain the immunosuppressive effect of G. anatis GtxA during the interaction with chicken macrophage-like HD11 cells by encouraging cell adhesion and invasion, reducing the host inflammatory response based on an initial over-expression of interleukin (IL)-10 and a corresponding low-level expression of tumor necrotizing factor (TNF)-α, and concluded that GtxA induces cell death (apoptosis) without revealing clear causes. Fimbriae.
Strains of G. anatis can adhere to the host’s mucosal surface via hair-like structures, fimbriae, that belong to the F17-like family that contains 1–3 different fimbrial clusters (Johnson et al. 2013; Kudirkiene et al. 2014; Persson and Bojesen 2015). The F17-like fimbria (GalF-A) is encoded by 4 genes: flfD, flfC, flfG, and flfA (Bager et al. 2013b). These fimbriae can bind to N-acetyl-D-glucosamine receptors of the host (Klemm and Schembri, 2000; Vaca et al. 2011; Lucio et al. 2012; Kudirkiene et al. 2014), and the adhesion protein assists in interacting to receptors (Lintermans et al. 1991). In vivo, the fimbria protein (flfA) is crucial for virulence (Bager et al. 2013b). Accordingly, fimbrial expression can control G. anatis tissue tropism (Bager et al. 2013b). It has been found that G. anatis strains can adhere to chicken epithelial cells in vitro and inert surfaces via their short fimbria-like structures (Vaca et al. 2011; Lucio et al. 2012).
Haemagglutinin
Some strains of Gallibacterium can agglutinate RBCS of avian and mammalian species (Zepeda et al. 2009; Ramirez-Apolinar et al. 2012). Though most strains of G. anatis can agglutinate rabbits’ RBCs, few strains may agglutinate the RBCs of chickens and quails due to the expression of haemagglutinins or adhesins binding receptors on the cells’ surface (Bager et al. 2013a; Johnson et al. 2013). Moreover, hemagglutinin protein was detected in biofilms and outer membrane vesicles (OMVs) liberated from G. anatis (Montes-Garcia et al. 2016).
Outer membrane vesicles
Proteins, lipopolysaccharides, and DNA are found in the budding regions of the outer cell membrane, OMVs, of several Gram-negative bacteria (Mashburn-Warren and Whiteley 2006; Kulp and Kuehn 2010; MacDonald and Kuehn 2012). The enormous collection of core proteins found in the OMVs of G. anatis strains is mainly unaffected by the various in vitro growth conditions, but certain environmental stimuli greatly influence their expression. They facilitate bacterial adhesion and colonization, the formation of biofilms, and the removal of several antibiotic compounds (Bager et al. 2013a). Additionally, G. anatis releases hemagglutinin in the OMVs to agglutinate avian RBCs (Zepeda et al. 2009; Bager et al. 2013a, 2014; Johnson et al. 2013) and trigger a robust immunological response (Pors et al. 2016b).
Capsule
Bojesen et al. (2007a) identified and characterized the genetic elements responsible for capsule biosynthesis in Gallibacterium for the first time. The capsule biosynthetic locus of Gallibacterium resembles the Escherichia coli (E. coli) group 2 capsule in structure and is considered a critical virulence factor for the pathogenesis of G. anatis infection. Some Gallibacterium strains have a polysaccharide capsule that adds virulence (Persson and Bojesen 2015). Capsule in G. anatis has an essential role in the adhesion and interaction of the bacterium with the surface of the host and immune evasion (Bojesen et al. 2011b; Singh et al. 2011; Harper et al. 2012). Although this capsule can be seen using electron microscopy (Bojesen et al. 2011c), it disappears following sub-cultures (Kjos-Hansen 1950). A capsule-knockout mutant (ΔgexD) became more virulent than its wild-type equivalent (Bojesen et al. 2011c).
Biofilm formation
Biofilms mainly comprise proteins, polysaccharides, nucleic acids, and amyloid proteins (Costerton et al. 1999; Larsen et al. 2007; Lopez-Ochoa et al. 2017). Biofilm formation begins through the bacterium’s capacity to bind the cell’s inert surface. The adhesive capacity of G. anatis as a tool for colonization of tissue surfaces and for allowing infection to persist inside the host was studied by scanning electron microscopy, and the findings implied that all the used isolates had formed robust biofilms on polystyrene and glass within the first 3 h of exposure (Vaca et al. 2011). Strains of G. anatis are classified into 3 categories: weak, moderate, and strong groups according to the biofilm formation capability (Johnson et al. 2013). This may indicate that the development of biofilms is significant for particular bacterial clades. Additionally, the biofilm is crucial for the chronicity and duration of infections and for reducing their sensitivity to antibiotics (Costerton et al. 1999; Donlan and Costerton 2002; Persson and Bojesen 2015). It has been found that proteins in biofilms are capable of interacting with several host proteins, including fibronectin, fibrinogen, laminin, and plasminogen, and consequently alter the host's homeostasis (Epstein and Chapman 2008; Lopez-Ochoa et al. 2017).
Metalloproteases
Metalloprotease enzymes are essential in G. anatis bacterium for proteolysis, increasing virulence, colonization, nutrient acquisition, and degradation of immunoglobulin (Ig) (G), along with bacterial invasion into the systemic circulation (Garcia-Gomez et al. 2005; Chavez et al. 2017b). It was found that these enzymes can down-regulate the immune response by acting on antibodies and complement system (Miyoshi and Shinoda 2000) that helps in colony establishment and bacterial transmission to blood circulation. In addition, proteases may be accountable for the host-specific pathogenicity of G. anatis strains (Zepeda et al. 2010). Metalloproteases such as metal-dependent endonuclease domain, zinc, and ATP-dependent metalloprotease are encoded in the G. anatis genome (Johnson et al. 2013).
Elongation factor Tu
Elongation factor Tu is a protein released through vesicle formation, possesses amyloid characters, and is consequently included in the pathogenesis of G. anatis (Lopez-Ochoa et al. 2017).
Clustered regularly interspaced short palindromic repeats (CRISPR)
According to Johnson et al. (2013), the bacterial innate defense system known as clustered regularly interspaced short palindromic repeats (CRISPR) breaks down invasive foreign and phage and plasmid nucleic acids. It can obstruct transformation, indicating that different strains of G. anatis have different levels of innate ability (Kristensen et al. 2012).
Integrative conjugative elements
Integrative conjugative elements have specific distinguishing characteristics, such as a site-specific integrase, transfer genes, and genes that control excision and transfer. Genes encoded inside these elements have been used to identify them in the G. anatis genome (Wozniak et al. 2009; Johnson et al. 2013). They have genes for antibiotic resistance, which spreads resistance to other bacteria (Bojesen et al. 2011b).
Small colony variants
Small colony variants are detected in the cultures of G. anatis as they have hemolytic activity (Greenham and Hill 1962). Moreover, they might aid in bacterial survival, recurrent infections, and the development of antibiotic resistance (Proctor et al. 2006).
Other virulence genes
G. anatis pathogenicity has been linked to the genes cps16A, 16B, and 16F that encode the enzymes glycosyltransferase, hyaluronidase, and UDP-glucose 6-dehydrogenase, respectively (Bosse et al. 2017), through the cloning and characterization of the gene encoding G. anatis fnrG (a homolog of the global regulator gene conferring a hemolytic phenotype in E. coli) and its function in the production of the E. coli silent hemolysin. Bojesen (2003) investigated the relationship between G. anatis and E. coli. sheA was studied by creating an E. coli-sheA, a null mutant of the silent hemolysin, from a 4.2 kilobase pair hind segment harboring fnrG. The fact that fnrG activates sheA and produces a non-hemolytic transformant led him to conclude that fnrG is probably a member of the fnr global regulatory protein family.
Pathogenicity
The bacterium was isolated from apparent healthy chickens’ nasal and tracheal passages and cloaca as a part of their normal microbiome (Bojesen et al. 2003a). Several studies verified the existence of G. anatis as a key bacterial pathogen in both natural and experimental infections. The association of G. anatis with septicemia, oophoritis, salpingitis, egg abnormalities, pericarditis, hepatitis, tracheitis, and elevated mortality in pullets signifies that at least some strains of Gallibacterium possess pathogenic possibility in chickens (Bojesen et al. 2004; Neubauer et al. 2009; Jones et al. 2013; Paudel et al. 2013, 2014a, b, 2015; Elbestawy 2014; Persson and Bojesen 2015). The differentiation between pathogenic and non-pathogenic isolates of G. anatis using embryo lethality assay failed to obtain any significant results because both G. anatis isolates from healthy and sick chickens caused hemorrhagic lesions and death of embryos in 70 to 100% of inoculated eggs (Trampel and Nalon, 2008).
The virulence of the G. anatis strain, the infection’s route, and the immune status and age of the host are factors that influence and exacerbate the pathogenicity of the bacterium in chickens (Bisgaard, 1977; Bojesen et al. 2004, 2008). Concomitant infection with other viruses or bacteria (Matthes et al. 1969; Shaw et al. 1990), malnutrition, hormonal disturbance (Kohlert 1968; Gerlach, 1977), and environmental stressors such as seasonal variations (Mirle et al. 1991), cold (Matthes and Loliger, 1976; Rzewuska et al. 2007), inadequate ventilation, and overcrowding, as well as poor biosecurity, increase the severity of G. anatis infection (Verbrugghe et al. 2012; Paudel et al. 2015, 2017a, b; Persson and Bojesen, 2015). For instance, a co-infection of G. anatis with E. coli, Avibacterium paragallinarum (A. paragallinarum), and Mycoplasma gallisepticum causes increased morbidity and mortality in chickens (Neubauer et al. 2009; Paudel et al. 2017a, b; Abd El-Hamid et al. 2018). Furthermore, the systemic infection was worsened by a bacterial infection that included the infectious bronchitis virus (He-ping et al. 2012; Mataried, 2016).
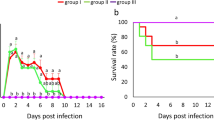
Experimental infection of naturally immunocompromised layer chickens with G. anatis led to 8–10% lowered egg production and a 73% mortality rate (Jordan et al. 2005; Shapiro et al. 2013). Infection of layer hens with G. anatis induced hemorrhagic oophoritis and rupture of ovarian follicles (Neubauer et al. 2009; Jones et al. 2013; Paudel et al. 2014a), while infection in cockerels resulted in epididymitis, decreased semen quality, decreased sperm density, altered overall motility, and loss of membrane integrity (Paudel et al. 2014b). The disease affects broiler chickens on a systemic level (Zepeda et al. 2010; Paudel et al. 2013; Zhang et al. 2019). Generally, G. anatis biovar hemolytica caused septicemia, oophoritis, salpingitis, peritonitis, liver necrosis, perihepatitis, pericarditis, airsacculitis, tracheitis, and enteritis in infected chickens (Bojesen et al. 2004, 2007a; Neubauer et al. 2009; Paudel et al. 2013).
Despite the previous pathological findings of G. anatis, it was found that this pathogen may colonize the upper respiratory and reproductive tracts without initiating substantial signs or lesions (Paudel et al. 2013, 2014a, b). Many virulence factors enable G. anatis to invade, adhere, and colonize the host’s surface epithelium. After oropharyngeal and oviduct epithelial cells infection with G. anatis, the pathogen adheres firmly via the reaction between the adhesin and the host’s cell surface receptor, followed by rampant multiplication, colonization, and syntheses of virulence factors (Vaca et al. 2011; Lucio et al. 2012; Bager et al. 2013a,b). G. anatis F149T can express fimbriae responsible for mucosal attachment and colonization to the epithelium of the oropharynx (Lucio et al. 2012). The in vitro study of Zhang et al. (2017) showed that higher adhesion to primary chicken oviduct epithelial cells and increased generation of inflammatory cytokines have been observed in the highly pathogenic strains of G. anatis [IL-6, TNF-α, and interferon (IFN-c)], resulting in inflammation and tissue injury. Many other specific virulence factors can influence the pathogenicity of G. anatis, such as IgG-degrading proteases, biofilm, hemagglutinin, fimbriae, capsule, GtxA, OMVs, metalloproteases, elongation factor Tu, and clustered regulatory short palindromic repeats (Bojesen et al. 2004; Garcia-Gomez et al. 2005; Christensen et al. 2007b; Zepeda et al. 2009; Kristensen et al. 2010; Lopez-Ochoa et al. 2017).
The primary target for G. anatis colonization is the respiratory tract, where the bacterium persists for 4 weeks pi, then spreads to the reproductive organs such as ovaries, oviducts, or even testicles, causing drop in egg production, inflammatory lesions, and mortality, and these pictures support the claim for its systemic infection (Shaw et al. 1990; Neubauer et al. 2009; Paudel et al. 2013; Abd El-Hamid et al. 2016). Systemic infection with G. anatis may occur, resulting in septicemia, which sets off an inflammatory cascade in several organs that causes inflammation of the upper respiratory tract, oophoritis, follicular hemorrhage, rupture, and degeneration, salpingitis, peritonitis, pericarditis, and perihepatitis (Harbourne, 1962; Kohlert, 1968; Janetschke and Risk, 1970; Hacking and Pettit 1974; Bisgaard, 1977; Gerlach 1977; Addo and Mohan, 1985; Majid et al. 1986; Shaw et al. 1990; Mirle et al. 1991; Suzuki et al. 1996; Neubauer et al. 2009).
Three G. anatis isolates from Mexico, China, and Austria were evaluated for their differences in pathogenicity in a specific pathogen-free Lohmann layer chicken population collected from various geographic locations. The results indicated that the Mexican isolate had a slightly higher pathogenicity than the two other strains, suggesting a geographical pathogenicity difference (Paudel et al. 2013). Regarding the genetic variety of isolates of G. anatis, Bojesen et al. (2003b) obtained 114 G. anatis isolates from tracheal and cloacal swab samples of organic flocks, egg-producing flocks, and layer parent chicken flocks. The findings showed higher genetic similarity (more than 94%) in the organic flock isolates. On the contrary, the layer parent flock isolates were divided into two subclusters containing each tracheal or cloacal isolated, with similarity above 90%. This genetic diversity, indicating clonal lineages, may have altered to several spots inside the same bird, granting them additional capability to initiate a disease (Bojesen and Shivaprasad 2007; Johnson et al. 2008).
Clinical manifestations
Infection with G. anatis is associated with multiple and variable clinical pictures but is often non-specific. In broilers, the pathogen causes respiratory manifestations in the form of nasal discharge, swelling and shaking of the head, cough, rales, dyspnea, diarrhea, and emaciation (Bojesen et al. 2008; El-Adawy et al. 2018; Elbestawy et al. 2018). In addition, G. anatis infection may induce a 3–18% reduction in egg production in layers (Jones et al. 2013), while in breeders, it causes an increasing mortality rate of 0.06–4.9% (Elbestawy et al. 2018). Mixed respiratory and reproductive disorders could be noticed in layers and breeder chicken flocks (Ataei et al. 2017; Chavez et al. 2017a; Abd El-Hamid et al. 2018). Elewa (2021) diagnosed G. anatis in layer chickens suffering from sneezing, rales, gasping, coughing, head swelling, and a 5–10% drop in egg production. Moreover, Bojesen et al. (2004) investigated the pathology of G. anatis virulent strain 12656–12 by intravenous or intraperitoneal inoculation in normal or immunosuppressed commercial brown laying chickens aged 15 weeks old. The author found that intravenously infected birds had severe septicemic lesions in both the normal and immunosuppressed birds. Mortality (73%) was recorded with severe depression and reluctance to move chickens. While Hui-min et al. (2009) reported the relationship between G. anatis and layer oviduct cysts, they did not indicate whether these lesions were co-associated with other viral diseases such as infectious bronchitis and low pathogenic avian influenza (LPAI-H9N2) or not. The clinical signs and gross lesions of 42 naturally infected layers with G. anatis were observed and recorded. Furthermore, a combined infection among G. anatis and other pathogens was also investigated. Hyperemia, swelling, and epithelium cell degeneration or necrosis were found in the mucosa of the respiratory tract and mucosa of the oviduct that presented many neutrophilic granulocytes and ovarian cysts. Most importantly, the severity of the clinical manifestations induced by G. anatis is exaggerated by co-infection with other pathogens, such as A. paragallinarum in chickens. Paudel et al. (2017a) observed that chickens infected with both G. anatis and A. paragallinarum had more severe nasal secretions and swelling of infraorbital sinuses than those with a single infection. In addition, in the presence of E. coli infection, G. anatis caused higher egg embryonic mortality than eggs with a single infection (Wang et al. 2018).
Under experimental infection conditions, G. anatis induced typical respiratory signs and weight losses in broiler chickens (Abd El-Hamid et al. 2018). However, layer chickens exhibited whitish diarrhea with a 66 to 47% drop in egg production (Paudel et al. 2014a). Specific pathogen-free cockerels showed no signs following experimental infection with G. anatis, but there was an alteration in the semen quality (Paudel et al. 2014b). Intranasal inoculation of G anatis showed an extensive and regular bacterial distribution in the respiratory and reproductive tract till 28 days pi (dpi) vs. one dpi in intravenously inoculated birds (Jones et al. 2013; Paudel et al. 2013).
Post-mortem lesions
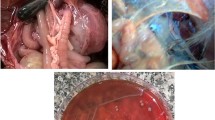
The post-mortem lesions of G. anatis are represented in Fig. 2. Respiratory, reproductive, intestinal, and septicemic lesions could be observed in infected cases with G. anatis (Ataei et al. 2019). Degeneration of the ovarian follicles, salpingitis, peritonitis, enteritis, and respiratory tract lesions have been associated with G. anatis infection (Bojesen et al. 2004). Furthermore, other G. anatis can induce other lesions in joints, heart, wattles, abdomen, and brain (El-Adawy et al. 2018). In the research of Neubauer et al. (2009), hemorrhagic oophoritis, damaged or malformed follicles, ovarian regression, hemorrhagic or dysfunctional oviducts, peritonitis, fibrinous perihepatitis, and pericarditis were all lesions of G. anatis infection in layer hens. Elewa (2021) demonstrated mild tracheitis, peritonitis, oophoritis, and salpingitis in layers with G. anatis infection.
Following experimental induction with G. anatis, layer chickens exhibited variable gross lesions such as hemorrhagic or ruptured ovarian follicles, pericarditis, multifocal hepatic necrosis, egg deformities in the oviduct, and fibrinous peritonitis (Paudel et al. 2014a). It has been noted that G. anatis biovar 3 caused purulent oophoritis, swollen blood vessels in the ovary, oviduct, and peritoneum, as well as purulent or fibrinous exudates in the peritoneum. Further experimental studies of G. anatis in chickens represented regression and deformity of ovaries, focal or diffuse salpingitis, catarrhal to purulent tracheitis, congestion of lungs, air sacculitis, pericarditis, liver congestion, and ascites (Paudel et al. 2013; Pors et al. 2016a; Abd El-Hamid et al. 2018).
G. anatis biovar haemolytica had been isolated from the nasal sinuses of infected cases with a nephropathogenic strain of infectious bronchitis and had respiratory signs (Franca et al. 2011). As they cause more severe peritonitis, salpingitis, and oophoritis, concurrent E. coli and G. anatis infections might worsen lesions compared to a single infection (Pors and Bojesen 2011). Besides, another study confirmed the localization of Gallibacterium mainly in the trachea and ovaries (Huangfu et al. 2012). G. anatis was isolated at a meager rate (2.7%) from pododermatitis cases in numerous layer flocks in Denmark in 2015 (Olsena et al. 2018).
Diagnosis
The different diagnostic tools of G. anatis are shown in Fig. 3.
Isolation and phenotypic identification
The absence of pathognomonic respiratory and reproductive signs and lesions caused by G. anatis necessitates a specific diagnosis to confirm the infection. Isolation and phenotypic identification of G. anatis are the basis for diagnosing G. anatis infections in almost all cases (Christensen et al. 2003). However, overgrowth by other bacteria, mainly E. coli, significantly hinders the isolation process (Wang et al. 2016). Furthermore, phenotypic characterization techniques can produce heterogeneous, time- and labor-intensive findings. Consequently, molecular diagnostic methods that depend on identifying 16S–23S rRNA sequences are growing (Bojesen et al. 2007b; Alispahic et al. 2012; Huangfu et al. 2012).
Species of Gallibacterium are usually grown at 37°C for 24–48 h on blood agar medium (with 5–10% citrated bovine blood) under facultative anaerobic/microaerophilic conditions (Christensen et al. 2003). Most G. anatis isolates produce a wide β-hemolytic zone with butyrous, circular, smooth, greyish, non-pigmented, and shiny colonies (El-Adawy et al. 2018). After 24–48 h of incubation, G. anatis develops with a modest granular deposit and weak turbidity in a nutrient broth supplemented with 5% horse serum and 3% glucose. Better bacterial growth is observed in brain heart infusion broth when 5% horse serum is added.
Biochemically, several tests are used to identify G. anatis, as detailed in Table 2 (Christensen et al. 2003; Bisgaard et al. 2009).
Other automated identification methods like VITEK2, Phoenix100, and MALDI-TOF–MS could be used for Gallibacterium identification (Alispahic et al. 2012; Allahghadry et al. 2021).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF–MS)
MALDI-TOF–MS is an effective technique for identifying G. anatis biomarkers (El-Adawy et al. 2018). This test has an impressive prospective for a routine laboratory diagnosis of the bacteria as it is rapid, can proceed with many samples concurrently, and needs small sample sizes. This test identified one clonal lineage of G. anatis in various flocks (Alispahic et al. 2011, 2012; Allahghadry et al. 2021). The genetic diversity of Gallibacterium isolated from 13 farms with various biosecurity measures and management techniques was examined using phylogenetic analysis of incomplete rpoB sequences and biotyping using MALDI-TOF–MS. The findings showed significant variability among isolates from farms with poor biosecurity standards and those with greater biosecurity standards (more closely linked and grouped). Low biosecurity standards allow viruses to spread horizontally, and gene transfer creates genetic diversity (Lozica et al. 2020).
Molecular detection
Molecular diagnosis of G. anatis has been recently developed, being fast, easy, highly specific, sensitive, and reliable (Ataei et al. 2017). Molecular assays such as polymerase chain reaction (PCR) are confirmatory to both phenotypically detected G. anatis strains and the negative samples following initial isolation (Bisgaard et al. 2009; Elewa 2021). Primers, including 16S to 23S rRNA internal transcribed spacer sequence, are specific to Gallibacterium (Bojesen et al. 2007a) and used for its differentiation from other Pasteurellaceae generas (Christensen et al. 2003). In addition, the primer 1133fgal is specific for Gallibacterium, not for other members of Pasteurellaceae. The 23S rRNA gene sequence primer 114r is utilized as a reverse primer (Lane 1991). Three specific amplicons of around 789, 985, and 1032 base pair can be identified in G. anatis infection (Neubauer et al. 2009; Singh, 2016; Singh et al. 2016; Ataei et al. 2017; Wang et al. 2018).
When phenotypic identification of Gallibacterium is challenging, classification based on the gene sequence of the DNA-dependent RNA-polymerase (rpoB) subunit might be utilized (Korczak et al. 2004; Christensen et al. 2007b). The gtxA-encoding gene, the fimbrial gene, and the gyrase subunit B gene may all be found using the PCR approach (Sorour et al. 2015; Krishnegowda et al. 2020).
Real-time quantitative PCR (qPCR) is used as a species-specific identification and quantifying method for G. anatis (Huangfu et al. 2012; Wang et al. 2016, 2018). The protein gyrase subunit B gene is essential for the function of the DNA replication enzyme because it encodes the ATPase domain of this enzyme. The qPCR is more rapid, highly specific, sensitive, reproducible, and cost-effective, and it needs a lower DNA concentration than conventional PCR or phenotypic characterization methods (Wang et al. 2016; Huangfu et al. 2012).
Real-time loop-mediated isothermal amplification PCR assay has recently been used to detect G. anatis. This assay targets the sodA gene. It has been described as sensitive, rapid, and specific for G. anatis identification. Moreover, it is quicker and cheaper than quantitative PCR (Stępień-Pyśniak et al. 2018).
Genotypic characterization methods of G. anatis have been used, such as DNA–DNA hybridization, pulsed-field gel electrophoresis, amplified fragment length polymorphism, and sequencing genes like infB, recN, and rpoB (Christensen et al. 2003; Bisgaard et al. 2009). Moreover, Allahghadry et al. (2021) detected 84 (70%) of G. anatis out of 120 layers and broiler chicken tracheal samples, and after genotyping by pulsed-field gel electrophoresis and genome sequencing revealed a total of 24 pulsotypes for 71 G. anatis strains (87% similarity level) and 7 genome clusters including 21 strains (97% similarity level), respectively.
Other diagnostic techniques
Agar gel precipitation test
Galaz-Luna et al. (2009) used an agar gel precipitation test for serotyping and cross-reactivity and found no relationship between biovar and serovar among the species of Gallibacterium.
Enzyme-linked immunosorbent assay
Serological testing is helpful for flock monitoring or diagnosing Gallibacterium infection. Wang (2012) developed an indirect enzyme-linked immunosorbent assay (ELISA) to recognize all antibodies against G. anatis in chickens. The response curve was established when the optical density 450 nm values varied along with the time. The antibody level peaked in 2-month pi, but for a short period, and then gradually dropped. Nowadays, ovotransferrin can be detected in chicken serum using ELISA as an acute phase protein marker in experimental G. anatis infections (Roy et al. 2014). Acute-phase proteins are good markers for diagnosis and prognosis.
Haemagglutination test
As G. anatis is a haemagglutinating bacterium for avian and mammalian RBCs [chickens, turkeys, pigeons, quails, ducks, Harris’s hawks (Parabuteo unicinctus), house finches (Carpodacus mexicanus), cows, sheep, horses, dogs, rabbits, pigs, and humans (groups A, B, AB, and O; Rh +)]; thus, haemagglutination test is used for the rapid detection of the organism using microdilution method or microtiter plates (Zepeda et al. 2009; Montes-Garcia et al. 2016).
Hemolysis and cytotoxicity assay
The hemolysis assay of G. anatis can be adopted using washed bovine blood RBCs and detected using ELISA, while the cytotoxicity assay can be applied on HD11 cells in 96-well tissue culture plates (Kristensen et al. 2010). The GtxA is the cause of cytotoxicity and hemolysis (Kristensen et al. 2011).
Immunofluorescence microscopy
The culture of G. anatis is fixed with paraformaldehyde on glass slides and labeled with anti-fimbriae immune serum. The conjugated goat anti-rabbit secondary antibodies are added, the slides are mounted, and the images are captured using laser scanning microscopy (Bager et al. 2013b).
In situ hybridization
The 16S rRNA of Gallibacterium is the target of an in situ hybridization probe dyed cyanine. The pathogenic changes in the spleen and liver tissues of experimentally infected hens have been studied using this hybridization approach (Bojesen et al. 2003a, 2004). This technique is vital for the detection of G. anatis pathogenies and dissemination.
Immuno-gold electron microscopy
This method is processed as an immunofluorescence assay, but the secondary antibodies are gold particles on nickel grids coated with Formvar carbon (Bager et al. 2013b). Electron microscopy is used for the detection of G. anatis.
Histopathology and immunohistochemistry
Several studies reported the histopathological examinations. Following the intravenous or intraperitoneal inoculation of virulent G. anatis strains in normal or immunosuppressed 15-weeks-old brown laying chickens, the liver lesions in the intravenously infected birds included basophilic aggregates (Gallibacterium microcolonies) bordered by necrotic hepatocytes, non-identifiable necrotic cells, proteinaceous fluid, and eosinophilic and basophilic aggregates in the ellipsoids in the spleens. After 12 and 24 h pi, the histopathological changes included forming multinucleated giant cells around some of the ellipsoid lesions in the spleen and liver. At the same time, the intraperitoneally infected chickens with a normal immune status showed diffuse purulent peritonitis and fibrinous perihepatitis 12 h pi (Bojesen et al. 2004; Zepeda et al. 2010). A combined infection of G. anatis and other pathogens was studied, and the results indicated changes in the lung, trachea, oviduct, and ovary regarding hyperemia, swelling, epithelium cell degeneration, and necrosis in the mucosa of the respiratory tract. The mucosa of the oviduct presented several neutrophilic granulocytes and ovarian cysts (Hui-min et al. 2009). The different histopathological severity degrees were due to the differences in virulence among the used G. anatis biovar hemolytica in chickens (Zepeda et al. 2010; Abd El-Hamid et al. 2016; Mataried 2016).
Immunochemistry was used to detect the ability of G. anatis isolates to adhere to or invade the chicken oviduct epithelial cells using polyclonal antibodies. The results of this assay revealed that G. anatis could attach epithelial cells without invasion (Zhang et al. 2017).
Prevention and control strategies
Adopting effective biosecurity measures, “all in-all out,” in poultry farms prevents the horizontal transmission of G. anatis, the gene transfer, and consequently, the heterogeneity or the diversity of this pathogen (Lozica et al. 2020). Treatment of a mixed infection with other bacteria using a specific drug, controlling of other immunosuppressive agents, and amelioration of stress conditions are the must to prevent or control G. anatis infection (Mataried 2016; Paudel et al. 2017a, b; Abd El-Hamid et al. 2018). Eggs or hatchery cleanliness is crucial to prevent trans-eggshell transmission caused by fecal contamination with G. anatis (Wang et al. 2018).
Antibiotic treatment
The administration of effective antimicrobials is needed to treat G. anatis infections. As of now, the antigenic diversity among G. anatis strains and MDR causes treatment failure and hinders vaccination-based prophylaxis (Bojesen et al. 2003b, 2007c; Christensen et al. 2003; Bojesen et al. 2011a, b; Johnson et al. 2013; Jones et al. 2013; Chavez et al. 2017b; Hess et al. 2019). In this regard, Elbestawy (2014) detected absolute resistance of 20 isolates of G. anatis to oxytetracycline and lincospectin and owed this resistance to the extensive exposure of layer chicken flocks in Egypt to long courses of these antibiotics in feed as a rational prophylactic program. A similar resistant pattern was obtained in Morocco by Nassik et al. (2019), who found that all G. anatis strains were resistant to ampicillin, erythromycin, oxytetracycline, and sulfamethoxazole-trimethoprim.
Besides, Bojesen et al. (2011b) reported that field strains of G. anatis were resistant to sulfamethoxazole and tetracycline at rates of 97% and 92%, respectively, while reference strains were resistant to the previous antimicrobials at rates of 42% and 67%, respectively. Alarmingly, MDR has markedly increased worldwide in the last decade, which is deemed a public health threat. Numerous recent epidemiological studies established the occurrence of extensive drug resistance and MDR bacterial pathogens from distinct origins, including humans, fish, food products, and poultry (Abd El-Ghany, 2021; Algammal et al. 2021b, c; Hetta et al. 2021; Algammal et al. 2022b).
The antimicrobial resistance (AMR) of G. anatis strains isolated from chickens in different localities were studied using PCR and cloning and sequencing of plasmid-mediated resistant gene of streptomycin, sulfamethoxazole, and fluoroquinolones (Bojesen et al. 2011a, b; Guo, 2011; Gao, 2012; Monita et al. 2011). The antimicrobial susceptibility for 84 chicken isolates of G. anatis from broiler and breeder flocks was studied in the USA compared to P. multocida isolates. Amoxicillin, neomycin, and sulfonamide-trimethoprim resistance levels were superior in G. anatis than in P. multocida (Jones et al. 2013). Resistance to tetracycline could be due to the genes tetB, tetH, and tetL, which have been detected in strains of G. anatis (Abd El Tawab et al. 2018).
El-Adawy et al. (2018) reported that 93% of the field G. anatis strains were resistant to sulfamethoxine, 93% to spectinomycin, 87% to tylosin, and 80% to oxytetracycline, but they were sensitive to apramycin, florfenicol, and neomycin. Chavez et al. (2017b) found resistance of G. anatis isolates to penicillin, tylosin, lincomycin, ampicillin, enrofloxacin, oxytetracycline, norfloxacin, and cephalexin, but sensitivity to ceftiofur (73%) and florfenicol (68%). Moreover, Elbestawy et al. (2018) revealed antibiotic sensitivity differences among G. anatis isolates obtained during 2013 and 2015. The highest antibiotic sensitivity results for 2013 G. anatis isolates were to florfenicol, ciprofloxacin, and norfloxacin, respectively, while those isolates of 2015 were susceptible to cefotaxime, followed by florfenicol, norfloxacin, and ciprofloxacin. In Nigeria, the sensitivity of non-hemolytic and hemolytic biovars of G. anatis isolated from Muscovy ducks was high to cefotaxime, ciprofloxacin, doxycycline, and florfenicol (Lawal et al. 2017). In layer flocks, 96% of G. anatis isolates showed MDR to tylosin (95%), tetracycline (89%), nalidixic acid or sulfamethoxazole (77%), and enrofloxacin (58%) (Hess et al. 2019).
In Egypt, a recent study by Elewa (2021) showed that all the isolated layer strains of G. anatis were resistant to kanamycin and neomycin (98%), penicillin and ampicillin (96%), oxytetracycline (95%), and sulfamethoxazole-trimethoprim and norfloxacin (27.5%), but susceptible to erythromycin and azithromycin (96%), florfenicol (90%), sulphamethaxole trimethoprim (57.3%), and norfloxacin (44%). Another recent Romanian study revealed that all G. anatis strains showed a resistant profile to tetracyclines, fluoroquinolones, ampicillin, trimethoprim, nalidixic acid, and clindamycin (Sorescu et al. 2021). Furthermore, Allahghadry et al. (2021) showed a lower sensitivity of 2 G. anatis strains to tetracycline (76.2%) and enrofloxacin (90.5%), suggesting the widespread occurrence of MDR G. anatis isolates and announced calls for non-antibiotic prophylactics.
Owing to the emergence of widespread MDR strains of G. anatis, traditional antimicrobials are not suggested for treating G. anatis infection. In the study by Prasai et al. (2017), feed supplementation with zeolites (aluminosilicate minerals) showed an effective reduction of G. anatis in chickens. In addition, Zhang et al. (2019) reported that specific chicken egg yolk IgY produced against recombinant N-terminal of GtxA revealed significant protection against G. anatis infection and decreased the severity of the lesion in the liver and intestine. Moreover, a recent study by Zhang et al. (2021) showed that a strain of Leuconostoc mesenteroides (QZ1178) exerts potential in vitro antibacterial effects against G. anatis through lactic acid formation.
Vaccines
Prior to now, the effectiveness of conventional vaccinations in protection against G. anatis infection was limited due to the antigenic diversity reported by Krishnegowda et al. (2020). A bacterin including 3 different biovars accounting for 95% of the outbreaks in Mexico has been developed by Boehringer Ingelheim, Mexico. The serological response was monitored from week 9 to week 40 using a custom-made ELISA test for G. anatis. The results indicated that the birds vaccinated with G. anatis bacterin were high in antibody titers and egg production (Gonzalez et al. 2003; Vazquez et al. 2003). The prepared autogenous bacterin was very effective in protecting against clinical disease and decreasing egg production and performance in layers (Castellanos et al. 2007) and in broiler breeder flocks (Calderon and Thomas 2009).
The vaccination potential of many immunogenic antigens such as GtxA, OMVs, fimbriae, capsules, elongation factor Tu, CRISPR, and recombinant proteins (GtxA and fimbriae) has been studied (Bager et al. 2013a, 2014; Pedersen et al. 2015; Pors et al. 2016a, b). Moreover, the efficacy of various immunogenic proteins such as GtxA, fimbriae, Gab_1309, and Gab_2348 for epitope recognition and prediction was investigated. The results revealed that these immunogenic proteins could stimulate the immune response (Ataei et al. 2019). Vaccination of layer chickens with OMVs of G. anatis decreased the severity of lesions and increased the serum titers of specific immunoglobulin (Y) (Pors et al. 2016b). Immunization with OMVs and the fimbrial protein of G. anatis could protect against the disease (Persson et al. 2018). A killed bivalent commercial vaccine (bacterin), including A. paragallinarum and G. anatis antigens, can induce complete protection in immunized chickens (Paudel et al. 2017a,b). Finally, Allahghadry et al. (2021) detected three major immunogen genes, gtxA, Gab_1309, and Gab_2312, in the examined G. anatis isolates, which possibly exemplify efficient vaccine targets, and the authors suggested the use of the gtxA gene (the most conserved sequence in all G. anatis strains) for the future vaccine development purposes (recombinant vaccines).
In conclusion, periodic studies regarding Gallibacterium infections should be applied to control their economic losses and improve poultry production. These studies should investigate the current epidemiological situation of the disease in the locality, the sources of infection in terms of public health, the antibiogram profile of the isolated strains, and the development of future vaccines. In addition, it is crucial to discuss the precise biology and pathophysiology of Gallibacterium infection and how this bacterium switches from being a normal inhabitant flora to a pathogen producing a disease condition in the host.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Abd El Tawab, A.A., Hassan, W.M. and El Shemy, M.M., 2018. Prevalence, phenotypic and genotypic characterization of Gallibacterium anatis isolated from layer. International Journal of Advanced Research, 6, 998–1003. https://doi.org/10.21474/IJAR01/8226
Abd El-Ghany, W.A., 2021. Staphylococcus aureus in poultry with special emphasis on methicillin-resistant strain infection: A comprehensive review from one health perspective. International Journal of One Health, 7, 257–267. https://doi.org/10.14202/IJOH.2021.257-267
Abd El-Hamid, H.S., Ellakany, H.F., Bekheet, A.A., Elbestawy, A.R. and Mataried, N.E., 2016. Pathogenicity of ten Gallibacterium anatis isolates in commercial broiler chickens. Alexandria Journal of Veterinary Sciences, 49, 42–49. https://doi.org/10.5455/ajvs.224857
Abd El-Hamid, H.S., Ellakany, H.F., Bekhit, A.A., Elbestawy, A.R. and Elshafey, M.S., 2018. Effect of mixed experimental infection with Gallibacterium anatis and Mycoplasma gallisepticum on performance of broiler chickens. Alexandria Journal of Veterinary Sciences, 57, 87–97. https://doi.org/10.5455/ajvs.286407
Addo, P.B. and Mohan, K., 1985. Atypical Pasteurella hemolytica type A from poultry. Avian Diseases, 29, 214–217. https://doi.org/10.2307/1590710
Aktories, K., Lang, A.E., Schwan, C. and Mannherz, H.G., 2011. Actin as target for modification by bacterial protein toxins. FEBS Journal, 278, 4526–4543. https://doi.org/10.1111/j.1742-4658.2011.08113.x
Algammal, A., Abd El-Ghany, W., Elewa, A. and Hamouda, A., 2021a. Preliminary study for investigation of G. anatis in broilers. Suez Canal Veterinary Medical Journal, 26, 355–365. DOI: https://dx.doi.org/https://doi.org/10.21608/scvmj.2021.217921
Algammal, A.M., Hashem, H.R., Alfifi, K.J., Hetta, H.F., Sheraba, N.S., Ramadan, H. and El‐Tarabili, R.M., 2021b. atpD gene sequencing, multi-drug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Scientific Reports, 11, 9476. https://doi.org/10.1038/s41598-021-88861-
Algammal, A.M., Hashem, H.R., Al-Otaibi, A.S., Alfifi, K.J., El-Dawody, E.M., Mahrous, E., Hetta, H.F., El-Kholy, A.W., Ramadan, H. and El-Tarabili, R.M., 2021c. Emerging MDR-Mycobacterium avium subsp. avium in house-reared domestic birds as the first report in Egypt. BMC Microbiology, 21, 237. https://doi.org/10.1186/s12866-021-02287-y
Algammal, A.M., Abo Hashem, M.E., Alfifi, K.J., Al-Otaibi, A.S., Alatawy, M., ElTarabili, R.M., Abd El-Ghany, W.A., Hetta, H.F., Hamouda, A.M., Elewa, A.A. and Azab, M.M., 2022a. Sequence analysis, antibiogram profile, virulence and antibiotic resistance genes of XDR and MDR Gallibacterium anatis isolated from layer chickens in Egypt. Infectious Drug Resistance, 15, 4321-4334. https://doi.org/10.2147/2FIDR.S377797
Algammal, A.M., Mabrok, M., Ezzat, M., Alfifi, K.J., Esawy, A.M., Elmasry, N. and El-Tarabili, R.M., 2022b. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture, 548, 737643. https://doi.org/10.1016/j.aquaculture.2021.737643
Alispahic, M., Christensen, H., Hess, C., Razzazi-Fazeli, E., Bisgaard, M. and Hess M., 2011. Identification of Gallibacterium species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry evaluated by multilocus sequence analysis. International Journal of Medical Microbiology, 301, 513–522. https://doi.org/10.1016/j.ijmm.2011.03.001
Alispahic, M., Christensen, H., Hess, C., Razzazi-Fazeli, E., Bisgaard, M. and Hess, M., 2012. MALDI-TOF mass spectrometry confirms clonal lineages of Gallibacterium anatis between chicken flocks. Veterinary Microbiology, 160, 269–273. https://doi.org/10.1016/j.vetmic.2012.05.032
Allahghadry, T., Ng, D.Y.K., Dibaei, A. and Bojesen, A.M., 2021. Clonal spread of multi-resistant Gallibacterium anatis isolates among Iranian broilers and layers. Veterinary Research, 52, 27. https://doi.org/10.1186/s13567-021-00894-1
Antenucci, F., Arak, H., Gao, J., Allahgadry, T., Thøfner, I. and Bojesen, A.M., 2020. Hydrostatic filtration enables large-scale production of outer membrane vesicles that effectively protect chickens against Gallibacterium anatis. Vaccines (Basel), 8, 40. https://doi.org/10.3390/vaccines8010040
Ataei, S., Bojesen, A.M., Amininajafi, F., Ranjbar, M.M., Banani, M., Afkhamnia, M., Abtin, A. and Goodarzi, H., 2017. First report of Gallibacterium isolation from layer chickens in Iran. Archives of Razi Institute, 72, 125–130. https://doi.org/10.22092/ari.2017.109842
Ataei, S., Ranjbar, M.M., Motamed, N., Ataei, S. and Amini Njafi, F., 2019. Designing a polytopic complex vaccine candidate against Gallibacterium anatis: an In-silico study. Archives of Razi Institute, 74, 7–20. https://doi.org/10.22092/ari.2017.109804.1118
Aubin, G.G., Haloun, A., Treilhaud, M., Reynaud, A. and Corvec, S., 2013. Gallibacterium anatis bacteremia in a human. Journal of Clinical Microbiology, 51, 3897–3899. https://doi.org/10.1128/2FJCM.01638-13
Bager, R.J., Persson, G., Nesta, B., Soriani, M., Serino, L., Jeppsson, M., Nielsen, T.K. and Bojesen, A.M., 2013a. Outer membrane vesicles reflect environmental cues in Gallibacterium anatis. Veterinary Microbiology, 167, 565–572. https://doi.org/10.1016/j.vetmic.2013.09.005
Bager, R.J., Nesta, B., Pors, S.E., Soriani, M., Serino, L., Boyce, J.D., Adler, B. and Bojesen, A.M., 2013b. The fimbrial protein flfA from Gallibacterium anatis is a virulence factor and vaccine candidate. Infection and Immunity, 81, 1964–1973. https://doi.org/10.1128/2FIAI.00059-13
Bager, R.J., Kudirkiene, E., Da Piedade, I., Seemann, T., Nielsen, T.K., Pors, S.E., Mattsson, A.H., Boyce, J.D., Adler, B. and Bojesen, A.M., 2014. In silico prediction of Gallibacterium anatis pan-immunogens. Veterinary Research, 45, 80–91. https://doi.org/10.1186/s13567-014-0080-0
Bisgaard, M., 1977. Incidence of Pasteurella hemolytica in the respiratory tract of apparently healthy chickens and chickens with infectious bronchitis. Characterization of 213 strains. Avian Pathology, 6, 285–292. https://doi.org/10.1080/03079457708418238
Bisgaard, M., 1982. Isolation and characterization of some previously unreported taxa from poultry with phenotypical characters related to Actinobacillus-and Pasteurella species. Acta Pathologica Microbiologica Scandinavica Series B Microbiology Immunology, 90, 59–67. https://doi.org/10.1111/j.1699-0463.1982.tb00081.x
Bisgaard, M., Korczak, B.M., Busse, H.J., Kuhnert, P., Bojesen, A.M. and Christensen, H., 2009. Classification of the taxon 2 and taxon 3 complex of Bisgaard within Gallibacterium and description of Gallibacterium melopsittaci sp. nov., Gallibacterium trehalosifermentans sp. nov. and Gallibacterium salpingitidis sp. nov. International Journal of Systematic Evolutionary Microbiology, 59, 735–744. https://doi.org/10.1099/ijs.0.005694-0
Bojesen, A.M. and Shivaprasad, H.L., 2007. Genetic diversity of Gallibacterium isolates from California turkeys. Avian Pathology, 36, 227–230. https://doi.org/10.1080/03079450701332352
Bojesen, A.M., Nielsen, S.R. and Bisgaard, M., 2003a. Prevalence and transmission of hemolytic Gallibacterium species in chicken production systems with different biosecurity levels. Avian Pathology, 32, 503–510. https://doi.org/10.1080/0307945031000154107
Bojesen, A.M., Torpdahl, M., Christensen, H., Olsen, J.E. and Bisgaard, M.J., 2003b. Genetic diversity of Gallibacterium anatis isolates from different chicken flocks. Journal of Clinical Microbiology, 41, 2737–2740. https://doi.org/10.1128/2FJCM.41.6.2737-2740.2003
Bojesen, A.M., Nielsen, O.L., Christensen, J.P. and Bisgaard, M., 2004. In vivo studies of Gallibacterium anatis infection in chickens. Avian Pathology, 33, 145–152. https://doi.org/10.1080/03079450310001652059
Bojesen, A.M., Vazquez, M.E., Robles, F., Gonzalez, C., Soriano, E.V., Olsen, J.E. and Christensen, H., 2007b. Specific identification of Gallibacterium by a PCR using primers targeting the 16S rRNA and 23S rRNA genes. Veterianry Microbiology, 123, 262–268. https://doi.org/10.1016/j.vetmic.2007.02.013
Bojesen, A.M., Christensen, J.P. and Bisgaard, M., 2008. Gallibacterium infections and other avian Pasteurellaceae. Pattison, Mark. In: McMullin PF., Bradbury JM, Alexander DJ, editors. Poultry Diseases 6th edn (pp.160–163). Edinburgh: W.B. Saunders.
Bojesen, A.M., Vazquez, M.E., Bager, R.J., Ifrah, D., Gonzalez, C. and Aarestrup, F.M., 2011a. Antimicrobial susceptibility and tetracycline resistance determinant genotyping of Gallibacterium anatis. Veterinary Microbiology, 148, 105–110. https://doi.org/10.1016/j.vetmic.2010.08.011
Bojesen, A.M., Bager, R.J., Ifrah, D. and Aarestrup, F.M., 2011b. The rarely reported tet (31) tetracycline resistance determinant is common in Gallibacterium anatis. Veterinary Microbiology, 149, 497–499. https://doi.org/10.1016/j.vetmic.2010.11.015
Bojesen, A.M., Soriano, E.V. and Kristensen, B.M., 2007a. Identification and characterization of the capsule biosynthetic locus of Gallibacterium anatis biovar haemolytica. Proceedings of the XVth World Veterinary Poultry Association Congress (p. 245). Beijing, China.
Bojesen, A.M., Vazquez, M.E., Gonzalez, C. and Aarestrup, F.M., 2007c. Antimicrobial susceptibility of Gallibacterium from chickens in Denmark and Mexico. Proceedings of the XVth World Veterinary Poultry Association Congress (oral contribution). Beijing, China.
Bojesen, A.M., Kristensen, B.M. and Pors, S.E., 2011c. The role of the capsule in the pathogenesis of Gallibacterium anatis in chickens. In: International Pasteurellaceae Conference (IPC), Elsinore.
Bojesen, A.M., 2003. Gallibacterium infection in chickens [PhD thesis]. [Frederiksberg (Denmark)]: University Frederiksberg, Denmark.
Bosse, J.T., Li, Y., Sarkozi, R., Gottschalk, M., Angen, Ø., Nedbalcova, K., Rycroft, A.N., Fodor, L. and Langford, P.R., 2017. A unique capsule locus in the newly designated Actinobacillus pleuropneumoniae serovar 16 and development of a diagnostic PCR assay. Journal of Clinical Microbiology, 55, 902–907. https://doi.org/10.1128/2FJCM.02166-16
Calderon, E.N. and Thomas, K., 2009. Gallibacterium anatis in broiler breeder flocks in the Republic of Panama: Clinical diagnosis and control measures. Proceedings of American Veterinary Medical Association/American Association of Avian Pathologists Annual Meeting (p. 99). Seattle, USA.
Castellanos, L.N., Vázquez, M.E., Carrillo, A.G. and Campogarrido, R., 2006. Protección conferida por una vacuna comercial contra Gallibacterium anatis en aves de postura comercial. Boehringer Ingelheim Vetmédica, 5, 1–3.
Castellanos, L.N., Vázquez, M.E., Carrillo, A.G. and Gonzalez, C.G., 2007. Evaluation of production and economic performance of two layer flocks vaccinated against Gallibacterium anatis with different bacterins. Proceedings of the XVth World Veterinary Poultry Association Congress (p. 241). Beijing, China.
Chavez, R.F.O., Barrios, R.M.M., Chavez, J.F.H., Mascareno, J.R., Escalante, J.G.A.I. and Yanes, M.A., 2017a. First report of biovar 6 in birds immunized against Gallibacterium anatis in poultry farms located in Sonora, Mexico. Veterinaria Mexico, 4, 2–9. https://doi.org/10.21753/vmoa.4.3.389
Chavez, R.F.O., Barrios, R.M.M., Xochihua, J.A.M., Chavez, J.F.H., Leon, J.B.L., Yanes, M.A., Martınez, V.A.F., Mascareno, J.R. and Escalante, J.G.A.I., 2017b. Antimicrobial resistance of Gallibacterium anatis isolates from breeding and laying commercial hens in Sonora, Mexico. Revista Mexicana de Ciencias Pecuarias, 8, 305–312. https://doi.org/10.22319/rmcp.v8i3.4506
Christensen, H.G., Bisgaard, M., Bojesen, A.M., Mutters, R. and Olsen, J.E., 2003. Genetic relationships among avian isolates classified as Pasteurella hemolytica, 'Actinobacillus salpingitidis' or Pasteurella anatis with proposal of Gallibacterium anatis gen. nov., comb. nov. and description of additional genomo species within Gallibacterium gen. nov. International Journal of Systematic Evolutionary Microbiology, 53, 275–287. https://doi.org/10.1099/ijs.0.02330-0
Christensen, H.G., Kuhnert, P., Busse, H.J., Frederiksen, W.C. and Bisgaard, M., 2007b. Proposed minimal standards for the description of genera, species and subspecies of the Pasteurellaceae. International Journal of Systematic Evolutionary Microbiology, 57, 166–178. https://doi.org/10.1099/ijs.0.64838-0
Christensen, H.G., Korczak, B.M., Kuhnert, P., Busse, J., Bojesen, A.M. and Bisgaard, M., 2007a. Five new species of Gallibacterium- a diagnostic enigma. Proceedings of the XVth World Veterinary Poultry Association Congress (p. 240). Beijing, China.
Chuan-qing, W., Lu, C., Xia, Y., He-ping, H., Hong-ying, L., Zhi-feng, P., Lu-ping, Z., Xue, X., Hiu-min, L., Ren-yi, F. and Lun-tao, G., 2008. A Premilinary study of Gallibacterium anantis infection in laying hens. Journal of Henan Agriculutral Science, P-3.
Costerton, J.W., Stewart, P.S. and Greenberg, E.P., 1999. Bacterial biofilms: a common cause of persistent infections. Science, 284, 1318–1322. https://doi.org/10.1126/science.284.5418.1318
Donlan, R.M. and Costerton, J.W., 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews, 15, 167–193. https://doi.org/10.1128/cmr.15.2.167-193.2002
Driessche, L., Vanneste, K., Bogaerts, B., De Keersmaecker, S.C., Roosens, N.H., Haesebrouck, F. and Boyen, F., 2020. Isolation of drug-resistant Gallibacterium anatis from calves with unresponsive bronchopneumonia, Belgium. Emerging Infectious Diseases, 26, 721–730. https://doi.org/10.3201/2Feid2604.190962
El-Adawy, H., Bocklisch, H., Neubauer, H., Hafez, H.M. and Hotzel, H., 2018. Identification, differentiation and antibiotic susceptibility of Gallibacterium isolates from diseased poultry. Irish Veterinary Journal, 71, 5. https://doi.org/10.1186/s13620-018-0116-2
Elbestawy, A.R., Ellakany, H.F., Abd El-Hamid, H.S., Bekheet, A.A., Mataried, N.E., Nasr, S.M. and Amarin, N.M., 2018. Isolation, characterization, and antibiotic sensitivity assessment of Gallibacterium anatis biovar hemolytica, from diseased Egyptian chicken flocks during the years 2013 and 2015. Poultry Science, 97, 1519–1525. https://doi.org/10.3382/ps/pey007
Elbestawy, A.R., 2014. Studies on Gallibacterium anatis infection in chickens (Ph.D. thesis in Poultry Diseases), Faculty of Veterinary Medicine, Alexandria University, Egypt.
Elewa, A.A., 2021. Molecular typing of Gallibacterium anatis strains isolated from chickens. MVSc thesis, Faculty of Veterinary Medicine, Suez canal University, Egypt.
Epstein, E.A. and Chapman, M.R., 2008. Polymerizing the fibre between bacteria and host cells: the biogenesis of functional amyloid fibres. Cell Microbiology, 10, 1413–1420. https://doi.org/10.1111/j.1462-5822.2008.01148.x
Franca, M., Woolcock, P.R., Yu, M., Jackwood, M.W. and Shivaprasad, H.L., 2011. Nephritis associated with infectious bronchitis virus cal99 variant in game chickens. Avian Diseases, 55, 422–428. https://doi.org/10.1637/9417-060510-reg.1
Galaz-Luna, G.A., Schumacher, T., Balzarettic, C., Vazquez, M.F., Islas-Figueroa, A. and Leon-Kempis, M.R., 2009. Serotyping of Gallibacterium anatis strains by agar gel precipitation and determination of cross reactivity by ELISA. Proceedings of the XVIth International Congress of the World Veterinary Poultry Association (p. 81). Morocco.
Gao, D.S., 2012. The correlation between drug resistance and some drug resistance genes in sixty-one Gallibacterium anatis isolates. Master Thesis in Veterinary Medicine, China (abstract).
Garcia-Gomez, E., Vaca, S., Perez-Mendez, A., Ibarra-Caballero, J., Perez-Marquez, V., Tenorio, V.R. and Negrete-Abascal, E., 2005. Gallibacterium anatis-secreted metalloproteases degrade chicken IgG. Avian Pathology, 34, 426–429. https://doi.org/10.1080/03079450500267866
Gautier, A-L., Dubois, D., Escande, F., Avril, J-L., Trieu-Cuot, P. and Gaillot, O., 2005. Rapid and accurate identification of human isolates of Pasteurella and related species by sequencing the sodA gene. Journal of Clinical Microbiology, 43, 2307–2314. https://doi.org/10.1128/2FJCM.43.5.2307-2314.2005
Gerlach, H., 1977. The significance of Pasteurella hemolytica in poultry. Praktische Tierarzt, 58, 324–328.
Gilchrist, P., 1963. A survey of avian respiratory diseases. Australian Veterinary Journal, 39, 140–144.
Gonzalez, C.H., Vazquez, M.E., Campogarrido, R.M., Humberto, R. and Sivanandan, V., 2003. A new and emerging disease of chickens caused by Pasteurella haemolytica-like organisms: disease and control strategy. Proceedings of the XIIIth International Congress of the World Veterinary Poultry Association (p. 60). Denver, USA.
Greenham, L. and Hill, T., 1962. Observations on an avian strain of Pasteurella haemolytica. Veterinary Record, 74, 861–863.
Guo, L.T., 2011. Studies on drug resistance and resistant genes of Gallibacterium anatis strains isolated from chickens in different localities. Master Thesis in Veterinary Medicine, China.
Hacking, W.C. and Pettit, J.R., 1974. Case report: Pasteurella hemolytica in pullets and laying hens. Avian Diseases, 18, 483–486. https://doi.org/10.2307/1589119
Harbourne, J.F., 1962. A hemolytic cocco-bacillus recovered from poultry. Veterinary Record, 74, 566–567.
Harper, M., Boyce, J.D. and Adler, B., 2012. The key surface components of Pasteurella multocida: capsule and lipopolysaccharide. Part of the Current Topics in Microbiology and Immunology, book series (CT Microbiology, volume 361), Pasteurella multocida, pp, 39–51.
He-Ping, H., Jun, Z. and Xia, Y., 2012. Tissue distribution of Gallibacterium anatis in chickens co-infected with infectious bronchitis virus. Journal of Acta Veterinaria Et Zootechnica Sinica, 43, 1623–1629.
Hess, C., Grafl, B., Bagheri, S., Kaesbohrer, A., Zloch, A. and Hess, M., 2019. Antimicrobial resistance profiling of Gallibacterium anatis from layers reveals high number of multiresistant strains and substantial variability even between isolates from the same organ. Microbial Drug Resistance, 26, 169–177. https://doi.org/10.1089/mdr.2019.0056
Hetta, H.F., Al-Kadmy, I.M.S., Khazaal, S.S., Abbas, S., Suhail, S., El-Mokhtar, M.A., Abd Ellah, N.H., Ahmed, E.A., Abd-ellatief, R.B., El-Masry, E.A., Batiha, G.E., Elkady, A.A., Mohamed N.A. and Algammal, A.M., 2021. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Scientific Reports, 11, 10751. https://doi.org/10.1038/s41598-021-90208-4
Huangfu, H., Zhao, J., Yang, X., Chen, L., Chang, H., Wang, X., Li, Q., Yao, H. and Wang, C., 2012. Development and preliminary application of a quantitative PCR assay for detecting gtxA-containing Gallibacterium species in chickens. Avian Diseases, 56, 315–320. https://doi.org/10.1637/9907-082511-reg.1
Hui-min, L., Kuang, L.H., Wang, A.L., Yang, C., Huangfu, L., Zheng, H., Xue-fu, X. and Ren-yi, S., 2009. Histopathological observation of layer oviduct cysts and primary detection for correlated pathogens. Journal of Henan Agriculutral Science, P-6.
Janda, W.M., 2011. Update on family Pasteurellaceae and the status of genus Pasteurella and genus Actinobacillus. Clinical Microbiological Newsletter, 33, 135–140.
Janetschke, P. and Risk, G., 1970. Frequent occurrence of Pasteurella hemolytica in the domestic chicken in Syria. Monatshefte für Veterinärmedizin, 25, 23–27.
Johnson, T.J., Fernandez-Alarcon, C., Bojesen, A.M., Nolan, L.K., Trampel, D.W. and Seemann, T., 2011. Complete genome sequence of Gallibacterium anatis strain UMN179, isolated from a laying hen with peritonitis. Journal Of Bacteriology, 193, 3676–3677. https://doi.org/10.1128/2FJB.05177-11
Johnson, T.J., Danzeisen, J.L., Trampel, D.W., Nolan, L.K., Seemann, T., Bager, R.J. and Bojesen, A.M., 2013. Genome analysis and phylogenetic relatedness of Gallibacterium anatis strains from poultry. PLoS One, 8, e54844. https://doi.org/10.1371/journal.pone.0054844
Johnson, T.J., Nolan, L.K. and Wannemuehler, Y.M. , 2008. Genome sequencing of Gallibacterium anatis causing peritonitis in laying hens. Proceedings of American Veterinary Medical Association/American Association of Avian Pathologists Annual Meeting (p. 93). New Orleans, LA, USA.
Jones, K.H., Thornton, J.K., Zhang, Y. and Mauel, M.J., 2013. A 5-year retrospective report of Gallibacterium anatis and Pasteurella multocida isolates from chickens in Mississippi. Poultry Science, 92, 3166–3171. https://doi.org/10.3382/ps.2013-03321
Jordan, F.T., Williams, N.J., Wattret, A. and Jones, T., 2005. Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. Veterinary Record, 157, 573–577. https://doi.org/10.1136/vr.157.19.573
Kjos-Hansen, B., 1950. Egg peritonitis in hens caused by pathogenic cloacal bacteria. Nordisc Veterinaermedicin, 2, 523–531.
Klemm, P. and Schembri, M.A., 2000. Bacterial adhesins: function and structure. International Journal of Medical Microbiology, 290, 27–35. https://doi.org/10.1016/s1438-4221(00)80102-2
Kohlert, R., 1968. Studies on the etiology of inflammation of the oviduct in the hen. Monatsh Veterinarmedicin, 23, 392–395.
Korczak, B., Christensen, H., Emler, S., Frey, J. and Kuhnert, P., 2004. Phylogeny of the family Pasteurellaceae based on rpoB sequences. International Journal of Systematic and Evolutionary Microbiology, 54, 1393–1399. https://doi.org/10.1099/ijs.0.03043-0
Krishnegowda, D.N., Dhama, K., Mariappan, A.K., Munuswamy, P., Yatoo M.I., Tiwari, R., Karthik, K., Bhatt, P.P. and Reddy, M.R., 2020. Etiology, epidemiology, pathology, and advances in diagnosis, vaccine development, and treatment of Gallibacterium anatis infection in poultry: a review. Veterinary Quarterly, 40, 16–34. https://doi.org/10.1080/2F01652176.2020.1712495
Kristensen, B.M., Frees, D. and Bojesen, A.M., 2010. GtxA from Gallibacterium anatis, a cytolytic RTX-toxin with a novel domain organization. Veterinary Research, 41, 25. https://doi.org/10.1051/vetres/2009073
Kristensen, B.M., Frees, D. and Bojesen, A.M., 2011. Expression and secretion of the rtx-toxin gtxA among members of the genus Gallibacterium. Veterinary Microbiology, 153, 116–123. https://doi.org/10.1016/j.vetmic.2011.05.019
Kristensen, B.M., Sinha, S., Boyce, J.D., Bojesen, A.M., Mell, J.C. and Redfield, R.J., 2012. Natural transformation of Gallibacterium anatis. Applied and Environmental Microbiology, 78, 4914–4922. https://doi.org/10.1128/2FAEM.00412-12
Kudirkiene, E., Bager, R.J., Johnson, T.J. and Bojesen, A.M., 2014. Chaperone-usher fimbriae in a diverse selection of Gallibacterium genomes. BMC Genomics, 15, 1093. https://doi.org/10.1186/1471-2164-15-1093
Kulp, A. and Kuehn, M.J., 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Review of Microbiology, 64, 163–184. https://doi.org/10.1146/annurev.micro.091208.073413
Lane, D.J., 1991. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acids techniques in bacterial systematics (pp. 115–147). Chichester: John Wiley & Sons.
Larsen, P., Nielsen, J.L., Dueholm, M.S,, Wetzel, R., Otzen, D. and Nielsen, P.H., 2007. Amyloid adhesins are abundant in natural biofilms. Environmental Microbiology, 9, 3077–3090. https://doi.org/10.1111/j.1462-2920.2007.01418.x
Lawal, J.R., Ndahi, J.J., Majama, Y.B., Gazali, Y.A., Dauda, J. and Ibrahim, U.I., 2017. Isolation, antimicrobial susceptibility pattern and prevalence of Gallibacterium anatis in local breed of female Muscovy ducks (Cairina moschata) in Gombe Northeastern Nigeria. Direct Research Journal of Veterinary Medicine and Animal Science, 2, 93–105. DOI: https://doi.org/10.26765
Lin, M.Y., Lin, K.J., Lan, Y.C., Liaw, M.F. and Tung, M.C., 2001. Pathogenicity and drug susceptibility of the Pasteurella anatis isolated in chickens in Taiwan. Avian Diseases, 45, 655–658. https://doi.org/10.2307/1592907
Lintermans, P.F., Bertels, A., Schlicker, C., Deboeck, F., Charlier, G., Pohl, P., Norgren, M., Normark, S., Van Montagu, M. and De Greve, H., 1991. Identification, characterization, and nucleotide sequence of the F17-G gene, which determines receptor binding of Escherichia coli F17 fimbriae. Journal of Bacteriology, 173, 3366–3373. https://doi.org/10.1128/2Fjb.173.11.3366-3373.1991
Lopez-Ochoa, J., Montes-Garcia, J.F., Vazquez, C., Sanchez-Alonso, P., Perez-Marquez, V.M., Blackall, P.J., Vaca, S. and Negrete-Abascal, E., 2017. Gallibacterium elongation factor-Tu possesses amyloid-like protein characteristics, participates in cell adhesion, and is present in biofilms. Journal of Microbiology, 55, 745–752. https://doi.org/10.1007/s12275-017-7077-0
Lozica, L., Kazazić, S.P. and Gottstein, Ž., 2020. High phylogenetic diversity of Gallibacterium anatis is correlated with low biosecurity measures and management practice on poultry farms. Avian Pathology, 49, 467–475. https://doi.org/10.1080/03079457.2020.1765970
Lucio, M.L., Vaca, S., Vazquez, C., Zenteno, E., Rea, I., Perez-Marquez, V.M. and Negrete-Abascal, E., 2012. Adhesion of Gallibacterium anatis to chicken oropharyngeal epithelial cells and the identification of putative fimbriae. Advanced Microbiology, 2, 505–510. https://doi.org/10.4236/aim.2012.24064
MacDonald, I.A. and Kuehn, M.J., 2012. Offense and defense: microbial membrane vesicles play both ways. Research in Microbiology, 163, 607–618. https://doi.org/10.1016/j.resmic.2012.10.020
Majid, M.S., Ideris, A. and Aziz, A.R., 1986. Isolation of Pasteurella hemolytica from the spleen of chickens. Pertanika (Malaysia), 9, 265–266.
Mashburn-Warren, L.M. and Whiteley, M., 2006. Special delivery: vesicle trafficking in prokaryotes. Molecular Microbiology, 61, 839–846. https://doi.org/10.1111/j.1365-2958.2006.05272.x
Mataried, N., 2016. Interaction between infectious bronchitis virus and Gallibacterium anatis in chickens. (M.V.Sc. Thesis in Poultry Diseases), Faculty of Veterinary Medicine, Damanhour University, Egypt.
Matthes, S. and Hanschke, J., 1977. Experimentelle Untersuchungen zür Übertragung von Bakterien über das Hühnerei. Berliner und Muenchener Tieraerztliche Wochenschrift, 90, 200–203.
Matthes, S. and Loliger, H.C., 1976. Kinetics of bacterial infections in hens. Berliner und Muenchener Tieraerztliche Wochenschrift, 89, 98–102.
Matthes, S., Loliger, H.C. and Schubert, H.J., 1969. Enzootic in chicken due to Pasteurella hemolytica. Deutschetierärztliche Wochenschrift, 76, 88–95.
Mendoza, K.A., Zavaleta, A.I., Koga, Y., Rodrıguez, J.B., Alvarado, A.S. and Tinoco, R.R., 2014. Genetic variability of Gallibacterium anatis strains isolated from Peruvian commercial birds with respiratory infections. Revista de Investigaciones Veterinarias del Perú, 25, 233–244. https://doi.org/10.15381/rivep.v25i2.8496
Mirle, C., Schongarth, M., Meinhart, H. and Olm, U., 1991. Studies on the incidence and importance of Pasteurella hemolytica infections in hens, especially taking into account diseases of the laying apparatus. Monatshefte für Veterinärmedizin, 46, 545–549.
Miyoshi, S.I. and Shinoda, S., 2000. Microbial metalloproteases and pathogenesis. Microbes and Infection, 2, 91–98. https://doi.org/10.1016/s1286-4579(00)00280-x
Monita, V., Filip, B. and Koen, D.G., 2011. Determination of the MIC values of Ornithobacterium rhinotracheale, Enterococcus cecorum and Gallibacterium sp for tiamulin and tilmicosin using a novel MIC-Strip. Proceedings of the XVIIth International Congress of the World Veterinary Poultry Association (p. 1006). Mexico.
Montes-Garcia, J.F., Vaca, S., Vazquez-Cruz, C., Soriano-Vargas, E., Aguilar-Romero, F., Blackall, P.J. and Negrete-Abascal, E., 2016. Identification of a hemagglutinin from Gallibacterium anatis. Current Microbiology, 72, 450–456. https://doi.org/10.1007/s00284-015-0969-5
Mraz, O., Vladık, P. and Bohacek, J., 1976. Actinobacilli in domestic owl. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene A, 236, 294–307.
Mushin, R., Weisman, Y. and Singer, N., 1980. Pasteurella haemolytica found in the respiratory tract of fowl. Avian Diseases, 24, 162–168. https://doi.org/10.2307/1589775
Nassik, S., Tallouzt, S., Karbach, N., Touzani, C., Bidoudan, Y., Aamarine, N. and Hess, C., 2019. First report of isolation of Gallibacterium anatis from layer chickens in Morocco with decrease in laying performance. Avian Diseases, 63, 727–730. https://doi.org/10.1637/aviandiseases-d-19-00119
Neubauer, C., De Souza-Pilz, M., Bojesen, A.M., Bisgaard, M. and Hess, M., 2009. Tissue distribution of hemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology, 38, 1–7. https://doi.org/10.1080/03079450802577848
Neubauer, C., De Souza, M.P. and Hess, M., 2007. Monitoring the prevalence and significance of Gallibacterium anatis in Austrian layer flocks. Proceedings of the XVth World Veterinary Poultry Association Congress (p. 242). Beijing, China.
Olsena, R.H., Christensen, H., Kabell, S. and Bisgaard, M., 2018. Characterization of prevalent bacterial pathogens associated with pododermatitis in table egg layers. Avian Pathology, 47, 281–285. https://doi.org/10.1080/03079457.2018.1440066
Paudel, S., Alispahic, M., Liebhart, D., Hess, M. and Hess, C., 2013. Assessing pathogenicity of Gallibacterium anatis in a natural infection model: the respiratory and reproductive tracts of chickens are targets for bacterial colonization. Avian Pathology, 42, 527–535. https://doi.org/10.1080/03079457.2013.843160
Paudel, S., Liebhart, D., Hess, M. and Hess, C., 2014a. Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch's postulates: 1. Folliculitis and drop in egg production are the predominant effects in specific pathogen free layers. Avian Pathology, 43, 443–449. https://doi.org/10.1080/03079457.2014.955782
Paudel, S., Liebhart, D., Aurich, C., Hess, M. and Hess, C., 2014b. Pathogenesis of Gallibacterium anatis in a natural infection model fulfils Koch's postulates: 2. Epididymitis and decreased semen quality are the predominant effects in specific pathogen free cockerels. Avian Pathology, 43, 529–534. https://doi.org/10.1080/03079457.2014.967176
Paudel, S., Hess, C., Wernsdorf, P., K€aser, T., Meitz, S., Jensen-Jarolim, E., Hess, M. and Liebhart D., 2015. The systemic multiplication of Gallibacterium anatis in experimentally infected chickens is promoted by immunosuppressive drugs which have a less specific effect on the depletion of leukocytes. Veterinary Immunology and Immunopathology, 166, 22–32. https://doi.org/10.1016/j.vetimm.2015.05.001
Paudel, S., Hess, M. and Hess, C., 2017a. Coinfection of Avibacterium paragallinarum and Gallibacterium anatis in specific-pathogen-free chickens complicates clinical signs of infectious coryza, which can be prevented by vaccination. Avian Diseases, 61, 55–63. https://doi.org/10.1637/11481-081016-reg
Paudel, S., Ruhnau, D., Wernsdorf, P., Liebhart, D., Hess, M. and Hess, C., 2017b. Presence of Avibacterium paragallinarum and histopathologic lesions corresponds with clinical signs in a co-infection model with Gallibacterium anatis. Avian Diseases, 61, 335–340. https://doi.org/10.1637/11609-021317-regr
Pedersen, I.J., Pors, S.E., Skjerning, R.J.B., Nielsen, S.S. and Bojesen, A.M., 2015. Immunogenic and protective efficacy of recombinant protein GtxA-N against Gallibacterium anatis challenge in chickens. Avian Pathology, 44, 386–391. https://doi.org/10.1080/03079457.2015.1069259
Persson, G. and Bojesen, A.M., 2015. Bacterial determinants of importance in the virulence of Gallibacterium anatis in poultry. Veterinary Research, 46, 57–67. https://doi.org/10.1186/2Fs13567-015-0206-z
Persson, G., Pors, S.E., Thøfner, I.C.N., and Bojesen, A.M., 2018. Vaccination with outer membrane vesicles and the fimbrial protein FlfA offers improved protection against lesions following challenge with Gallibacterium anatis. Veterinary Microbiology, 217, 104–111. https://doi.org/10.1016/j.vetmic.2018.03.010
Pors, S.E., Skjerning, R.B., Flachs, E.M. and Bojesen, A.M., 2016a. Recombinant proteins from Gallibacterium anatis induces partial protection against heterologous challenge in egg-laying hens. Veterinary Research, 47, 36–43.
Pors, S.E., Pedersen, I.J., Skjerning, R.B., Thøfner, I.C.N., Persson, G. and Bojesen, A.M., 2016b. Outer membrane vesicles of Gallibacterium anatis induce protective immunity in egg-laying hens. Veterinary Microbiology, 195, 123–127. https://doi.org/10.1016/j.vetmic.2016.09.021
Pors, S.E. and Bojesen, A.M., 2011. Gallibacterium antis: an improved intraperitoneal infection model. Proceedings of the XVIIth International Congress of the World Veterinary Poultry Association (p. 778–780). Mexico.
Prasai, T.P., Walsh, K.B., Bhattarai, S.P., Midmore, D.J., Van, T.T., Moore, R.J. and Stanley, D., 2017. Zeolite food supplementation reduces abundance of enterobacteria. Microbiological Research, 195, 24–30. https://doi.org/10.1016/j.micres.2016.11.006
Proctor, R.A., Von Eiff, C., Kahl, B.C., Becker, K., McNamara, P., Herrmann, M. and Peters, G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nature Reviews Microbiology, 4, 295–305. https://doi.org/10.1038/nrmicro1384
Ramirez-Apolinar, S., Guerra-Infante, F.M., MdJd, H-C., Salgado-Miranda, C., Madrid-Morales, E., Kristensen, B.M., Bojesen, A.M., Negrete-Abascal, E. and Soriano-Vargas, E., 2012. Characterization of a Gallibacterium genomospecies 2 hemagglutinin. Journal of Animal and Veterinary Advances, 11, 556–560. https://doi.org/10.3923/javaa.2012.556.560
Roy, K., Kjelgaard-Hansen, M., Pors, S.E., Christensen, J.P., Biswas, P.K. and Bojesen, A.M., 2014. Performance of a commercial Chicken-Ovo-transferrin-ELISA on the serum of brown layer chickens infected with Gallibacterium anatis and Streptococcus zooepidemicus. Avian Pathology, 43, 57–61. https://doi.org/10.1080/03079457.2013.867011
Rzewuska, M.A., Karpinska, E., Szeleszczuk, P.I. and Binek, M.A., 2007. Isolation of Gallibacterium spp. from peacocks with respiratory tract infections. Medycyna Weterynaryjna, 63, 1431–1433.
Shapiro, J., Brash, M.M.E., Brooks, A., Slavic, D. and McEwen, B., 2013. Gallibacterium anatis-a review of culture-positive cases from commercial poultry submitted to the AHL in 2011 and 2012. AHL Newsletter Guelph, Ontario. Animal Health Services, Laboratory Services Division, University of Guelph, 17, 6.
Shaw, D.P., Cook, D.B., Maheswaran, S.K., Lindeman, C.J. and Halvorson, D.A., 1990. Pasteurella haemolytica as a co-pathogen in pullets and laying hens. Avian Diseases, 34, 1005–1008. https://doi.org/10.2307/1591397
Shini, S., Shini, A. and Blackall, P.J., 2013. The potential for probiotics to prevent reproductive tract lesions in free-range laying hens. Animal Production Science, 53, 1298–1308. https://doi.org/10.1071/AN12337
Singh, K., Ritchey, J.W. and Confer, A.W., 2011. Mannheimia hemolytica: bacterial–host interactions in bovine pneumonia. Veterinary Pathology, 48, 338–348. https://doi.org/10.1177/0300985810377182
Singh, S.V., Singh, B.R., Sinha, D.K., Vinodh, K.O., Prasanna, V.A., Monika, B. and Sakshi, D., 2016. Gallibacterium anatis: an emerging pathogen of poultry birds and domiciled birds. Journal of Veterinary Science and Technology, 7, 1–7. https://doi.org/10.4172/2157-7579.1000324
Singh, B.R., Singh, S.V., Palanivelu, M., Kumar, M.A., Sinha, D.K. and Kumar, O.R., 2018. Gallibacterium anatis outbreaks in domestic birds in North India: Antimicrobial and herbal drug sensitivity of Avibacterium and Gallibacterium isolates. Indian Journal of Poultry Science, 53, 225–233. https://doi.org/10.5958/0974-8180.2018.00043.0
Singh, S.V., 2016. Studies on growth kinetics of Gallibacterium anatis in presence of deuterium oxide (D2O, heavy water). (M.V.Sc). Thesis submitted to Deemed University), Izatnagar, Uttar Pradesh, Indian Veterinary Research Institute, Idia.
Sorescu, I., Romascu, L.M., Ionescu, M., Popovici, A., Stoica, C. and Barbuceanu, F., 2021. Isolation and phenotypic characterization of Gallibacterium anatis biovar haemolytica from a hen with hemorrhagic ooforitis. Romanian Biotechnological Letters, 26, 3090–3094. DOI: https://dx.doi.org/https://doi.org/10.25083/rbl/26.6/3090-3094.
Sorour, H.K., Atfeehy, N.M.A. and Shalaby, A.G., 2015. Gallibacterium anatis infection in chickens and ducks. Assiut Veterinary Medical Journal, 61, 80–86. DOI: https://dx.doi.org/https://doi.org/10.21608/avmj.2015.170239
Stępień-Pyśniak, D., Kosikowska, U., Hauschild, T., Burzynski, A., Wilczynski, J., Kolinska, A., Nowaczek, A. and Marek, A., 2018. A loop-mediated isothermal amplification procedure targeting the sodA gene for rapid and specific identification of Gallibacterium anatis. Poultry Science, 97, 1141–1147. https://doi.org/10.3382/ps/pex420
Suzuki, T., Ikeda, A., Shimada, J., Yanagawa, Y., Nakazawa, M. and Sawada, T., 1996. Isolation of Actinobacillus salpingitidis/ avian Pasteurella hemolytica-like organisms group from diseased chickens. Journal of the Japanese Veterinary Medical Association, 49, 800–804.
Tang, B. and Bojesen, A.M., 2020. Immune suppression induced by Gallibacterium anatis GtxA during interaction with chicken macrophage-like HD11 cells. Toxins, 12, 536. https://doi.org/10.3390/toxins12090536
Tang, B., Pors, S.P., Kristensen, B.M., Skjerning, R.B.J., Olsen, R.H. and Bojesen, A.M., 2020. GtxA is a virulence factor that promotes a Th2‑like response during Gallibacterium anatis infection in laying hens. Veterinary Research, 51, 40. https://doi.org/10.1186/s13567-020-00764-2
Trampel, D. and Nalon, L.K., 2008. Embryo Lethality Assay for Gallibacterium anatis. Project of College ofVeterinary Medicine Iowa State University.
Vaca, S., Monroy, E., Rojas, L., Vazquez, C., Sanchez, P., Soriano-Vargas, E., Bojesen, A.M. and Negrete-Abascal, E., 2011. Adherence of Gallibacterium anatis to inert surfaces. Journal of Animal and Veterinary Advances, 10, 1688–1693. DOI: http://dx.doi.org/https://doi.org/10.3923/javaa.2011.1688.1693
Vazquez, M.E., Campogarrido, R., Rodriguez, H., Sivanandan, V. and Gonzalez, C. 2003. A new emerging disease of chickens caused by Pasteurella haemolytica-like organisms: prevalence, isolation and characterization. Proceedings of the XIIIth International Congress of the World Veterinary Poultry Association (p. 121–122). Denver, USA.
Verbrugghe, E., Boyen, F., Gaastra, W., Bekhuis, L., Leyman, B., Van Parys, A., Haesebrouck, F. and Pasmans, F., 2012. The complex interplay between stress and bacterial infections in animals. Veterinary Microbiology, 155, 115–127. https://doi.org/10.1016/j.vetmic.2011.09.012
Wang, C., Robles, F., Ramirez, S., Riber, A. and Bojesen, A., 2016. Culture-independent identification and quantification of Gallibacterium anatis (G. anatis) by real-time quantitative PCR. Avian Pathology, 45, 538–544. https://doi.org/10.1080/03079457.2016.1184743
Wang, C., Pors, S.E., Olsen, R.H. and Bojesen, A.M., 2018. Transmission and pathogenicity of Gallibacterium anatis and Escherichia coli in embryonated eggs. Veterinary Microbiology, 217, 76–81. https://doi.org/10.1016/j.vetmic.2018.03.005
Wang, H., Wu, F., Han, H., Zhao, J., and Mao, L. 2023. A case of human diarrhea caused by Gallibacterium anatis: a case report. Under publication, research square, DOI: https://doi.org/10.21203/rs.3.rs-3134681/v1.
Wang, S., 2012. Studies on dynamic regularity of antibody against G. anatis and development of hybridoma cell lines secreting monoclonal antibodies against G. anatis LPS. Master Thesis in Veterinary Medicine, China (abstract).
Wozniak, R.A., Fouts, D.E., Spagnoletti, M., Colombo, M.M., Ceccarelli, D., Garriss, G., Dery, C., Burrus, V. and Waldor, M.K., 2009. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genetics, 5, e1000786. https://doi.org/10.1371/journal.pgen.1000786
Yaman, S. and Sahan, O.Y., 2019. Diagnosis of Gallibacterium anatis in layers: First report in Turkey. Brazilian Journal of Poultry Science, 21, 1806–9061. https://doi.org/10.1590/1806-9061-2019-1019
Yang, X., Xia, Y.H., Wang, J.Y., Li, Y.T., Chang, Y.F., Chang, H.T. and Wang, C.Q., 2020. The role of gtxA during Gallibacterium anatis infection of primary chicken oviduct epithelial cells. Molecular and Cellular Probes, 53, 101641. DOI: https://doi.org/https://doi.org/10.1016/j.mcp.2020.101641
Zellner, D.E., Charlton, B.R., Cooper, G. and Bickford, A.A., 2004. Prevalence of Gallibacterium species in California poultry over 10 years (1994–2003) from diagnostic specimens. In Proceedings of the Annual Conference of the American Association of Avian Pathologists (p. 64). Philadelphia, PA, USA.
Zepeda, A., Ramırez, S., Vega, V., Morales, V., Talavera, M., Salgado-Miranda, C., Simon-Martınez, J., Bojesen, A.M. and Soriano-Vargas, E., 2009. Hemagglutinating activity of Gallibacterium strains. Avian Diseases, 53, 115–118. https://doi.org/10.1637/8375-060908-ResNote.1
Zepeda, V.A., Calderon-Apodaca, N.L., Paasch, M.L., Martın, P.G., Paredes, D.A., Ramırez-Apolinar, S. and Soriano-Vargas, E., 2010. Histopathologic findings in chickens experimentally infected with Gallibacterium anatis by nasal instillation. Avian Diseases, 54, 1306–1309. https://doi.org/10.1637/9423-061410-ResNote.1
Zhang, X.P., Lu, C.J., Li, Y.T., Yang, X., Wang, X.W., Chang, H.T., Liu, H.Y., Chen, L., Zhao, J., Wang, C.Q. and Chang, Y.F., 2017. In vitro adherence and invasion of primary chicken oviduct epithelial cells by Gallibacterium anatis. Veterinary Microbiology, 203, 136–142. https://doi.org/10.1016/j.vetmic.2017.02.009
Zhang, J.J., Kang, T.Y., Kwon, T., Koh, H., Chandimali, N., Wang, X.Z., Kim, N.and Jeong, D.K., 2019. Specific chicken egg yolk antibody improves the protective response against Gallibacterium anatis infection. Infection and Immunity, 87, e00619–18. https://doi.org/10.1128/2FIAI.00619-18
Zhang, H., HuangFu, H., Wang, X., Zhao, S., Liu, Y., Lv, H., Qin, G. and Tan, Z., 2021. Antibacterial activity of lactic acid producing Leuconostoc mesenteroides QZ1178 against pathogenic Gallibacterium anatis. Frontiers in Veterinary Science, 8, 630294. https://doi.org/10.3389/fvets.2021.630294
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The idea for the article, collecting papers, and writing the draft were conceived by W.A.A. and A.R.E. Revision of the article was performed by A.M.A, H.F.H., and A.R.E. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Consent for publication
All authors declare that they participated in developing the manuscript titled “Gallibacterium anatis infection in poultry: A comprehensive review.” They have read the final version and consented to the article being published in Tropical Animal Health and Production.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Camelids
Guest Editor: Bernard Faye
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Ghany, W.A., Algammal, A.M., Hetta, H.F. et al. Gallibacterium anatis infection in poultry: a comprehensive review. Trop Anim Health Prod 55, 383 (2023). https://doi.org/10.1007/s11250-023-03796-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-023-03796-w