Abstract
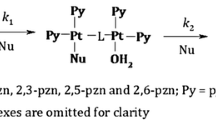
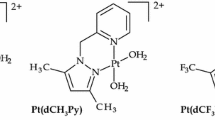
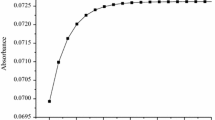
The substitution kinetics of the complexes [Pt(terpy)Cl]Cl·2H2O (PtL1), [Pt(tBu3terpy)Cl]ClO4 (PtL2), [Pt{4′-(2′′′-CH3-Ph)terpy}Cl]BF4 (PtL3), [Pt{4′-(2′′′-CF3-Ph)terpy}Cl]CF3SO3 (PtL4), [Pt{4′-(2′′′-CF3-Ph)-6-Ph-bipy}Cl] (PtL5) and [Pt{4′-(2′′′-CH3-Ph)-6-2′′-pyrazinyl-2,2′-bipy}Cl]CF3SO3 (PtL6) with the nucleophiles imidazole (Im), 1-methylimidazole (MIm), 1,2-dimethylimidazole (DIm), pyrazole (Pyz) and 1,2,4-triazole (Trz) were investigated in a methanolic solution of constant ionic strength. Substitution of the chloride ligand from the metal complexes by the nucleophiles was investigated as a function of nucleophile concentration and temperature under pseudo first-order conditions using UV/Visible and stopped-flow spectrophotometric techniques. The reactions follow the rate law \( k_{\text{obs}} = k_{2} \left[ {\text{Nu}} \right] + k_{ - 2} \). The results indicate that changing the nature or distance of influence of the substituents on the terpy moiety affects the π-back-donation ability of the chelate. This in turn controls the electrophilicity of the metal centre and hence its reactivity. Electron-donating groups decrease the reactivity of the metal centre, while electron-withdrawing groups increase the reactivity. Placing a strong σ-donor cis to the leaving group greatly decreases the reactivity of the complex, while the addition of a good π-acceptor group significantly enhances the reactivity. The results indicate that the metal is activated differently by changing the surrounding atoms even though they are part of a conjugated system. It is also evident that substituents in the cis position activate the metal centre differently to those in the trans position. The kinetic results are supported by DFT calculations, which show that the metal centre is less electrophilic when a strong σ-donor is cis to the leaving group and more electrophilic when a good π-acceptor group is part of the ring moiety. The temperature dependence studies support an associative mode of activation. An X-ray crystal structure of Pyz bound to PtL3 was obtained and confirmed the results of the DFT calculations as to the preferred N-atom as a binding site.









Similar content being viewed by others
References
Lippert B (1999) Cisplatin. chemistry and biochemistry of a leading anticancer drug. Wiley-VCH, Weinheim, pp 3–27
Sigel A, Sigel H (1996) Metal ions in biological systems. Marcel Dekker Inc, New York, p 393
Richens DT (2005) Chem Rev 105:1961
Tobe ML, Burgess J (1999) Inorganic reaction mechanisms. Addison Wesley Longman Ltd., Essex, pp 30–43, 70–112
Atwood JD (1997) Inorganic and organometallic reaction mechanisms, 2nd edn. Wiley-VCH Inc, New York, pp 43–61
Jordan RB (1991) Reaction mechanisms of inorganic and organometallic systems. Oxford University Press Inc, Oxford, pp 29–74
Wilkins RG (1991) Kinetics and mechanism of reactions transition metal complexes, 2nd edn. VCH, Weinheim, pp 199–201, 221, 232–242
Cross RJ (1989) Adv Inorg Chem 34:219
Banerjee P (1999) Coord Chem Rev 190–192:19–28
Romeo R (1990) Comments Inorg Chem 11:21
Faraone G, Ricevuto V, Romeo R, Trozzi M (1971) J Chem Soc A 1877
Basolo F (1996) Coord Chem Rev 154:151
Millard MM, Macquet JP, Theophanides T (1975) Biochim Biophys Acta 402:166
Sigel H, Massoud SS, Corfu NA (1994) J Am Chem Soc 116:2958
Bugarčić ZD, Nandibewoor AT, Hamza MSA, Heinemann F, van Eldik R (2006) Dalton Trans 2984
Kaim W, Schwederski B (1994) Bioinorganic chemistry: inorganic elements in the chemistry of life, 1st edn. Wiley, New York, pp 16–35, 106–123
Pitteri B, Bortoluzzi M (2006) Polyhedron 25:2698
Sundberg RJ, Martin RB (1974) Chem Rev 74:471
Carlsen L, Egsgaard H, Anderson JR (1979) Anal Chem 51:1593
Kröhnke F (1976) Synthesis 1
McDermott JX, White JF, Whitesides GM (1976) J Am Chem Soc 98:6521
Annibale G, Brandolisio M, Pitteri B (1995) Polyhedron 14:451
Lai S-W, Chan MCW, Cheung K-K, Che C-M (1998) Inorg Chem 38:4262
Summerton GC (1997) Solid state structures and photophysical properties of polypyridyl complexes of platinum(II). PhD thesis, University of Natal, Pietermaritzburg, South Africa, p 51, 53, pp 71–77, 94–107, 150–156
Field JS, Haines RJ, McMillin DR, Summerton GC (2002) J Chem Soc Dalton Trans 1369
Gertenbach JA (2002) Synthesis and emission studies of polypyridyl complexes of platinum(II). PhD thesis University of Natal, Pietermaritzburg, South Africa, pp 220–230, 260–262
Jaganyi D, Reddy D, Gertenbach JA, Hofmann A, van Eldik R (2004) Dalton Trans 299–304
Spartan ‘08, Wavefunction, Inc. (2008) 18401 Von Karman Avenue, Suite 370, Irvine, CA, 92612, USA; Q-Chem, Inc., The Design Center, Suite 690, 5001 Baum Blvd., Pittsburgh, PA, 15213, USA. http://www.wavefun.com/
Kong J, White CA, Krylov AI, Sherrill CD, Adamson RD, Furlani TR, Lee MS, Lee AM, Gwaltney SR, Adams TR, Ochsenfeld C, Gilbert ATB, Kedziora GS, Rassolov VA, Maurice DR, Nair N, Shao Y, Besley NA, Maslen PE, Dombroski JP, Daschel H, Zhang W, Korambath PP, Baker J, Byrd EFC, Van Voorhuis T, Oumi M, Hirata S, Hsu C-P, Ishikawa N, Florian J, Warshel A, Johnson BG, Gill PMW, Head-Gordon M, Pople JA (2000) J Comput Chem 21:1532
Becke AD (1993) J Chem Phys 98:5648
Friesner RA (1985) Chem Phys Lett 116:39
Friesner RA (1991) Ann Rev Phys Chem 42:341
Hay PJ, Wadt WR (1985) J Chem Phys 82:299
Reddy D, Jaganyi D (2008) Dalton Trans 6724
Katritzky R, Rees CW (1984) Comprehensive heterocyclic chemistry, vol 5. Pergamon Press, Oxford, pp 35–50, 169–225, 374–386, 734–747
Appleton TG, Hall JR, Ralph SF, Thompson CSM (1984) Inorg Chem 23:3521
CrysAlis CCD and CrysAlis RED (2002) Version 170, Oxford Diffraction Ltd., Abingdon
Sheldrick GM (1997) SHELXS-97, Program for solution of crystal and structures. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, Program for refinement of crystal structures. University of Göttingen, Germany
Blessing R (1995) Acta Cryst A51:33
Farrugia J (1999) WinGX v1.70.01. J Appl Cryst 32:837
Mercury (2008) Version 2.2. Cambridge Crystallographic Data Centre, Cambridge
Microcal™ Origin™ (1991–1997) Version 5.0, Microcal Software, Inc., One Roundhouse Plaza, Northampton, MA, 01060, USA
Rosić J, Petrović B, Djuran MI, Bugarčić ZD (2007) Monatshefte für Chemie 138:1
Roszak AW, Clement O, Buncel E (1996) Acta Cryst C52:1654
Muller J, Freisinger E, Lax P, Megger DA, Polonius F (2007) Inorg Chim Acta 360:255
Jaganyi D, De Boer KL, Gertenbach JA, Perils J (2008) Int J Chem Kinet 808–818
Jaganyi D, Hofmann A, van Eldik R (2001) Angew Chem Int Ed 40:1680
Hofmann A, Jaganyi D, Munro OQ, Liehr G, van Eldik R (2003) Inorg Chem 42:1688
Jaganyi D, Reddy D, Gertenbach JA, Hofmann A, van Eldik R (2004) Dalton Trans 299–304
Hofmann A, Dahlenburg L, van Eldik R (2003) Inorg Chem 42:6528
Pitteri B, Marangoni G, Viseutim FV, Cattalini L, Bobbo T (1998) Polyhedron 17:475–482
Acknowledgments
The authors gratefully acknowledge financial support from the University of KwaZulu-Natal and the National Research Foundation (NRF, Pretoria). We thank Mr C. Grimmer for the NMR analysis of the samples.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, D., Akerman, K.J., Akerman, M.P. et al. A kinetic investigation into the rate of chloride substitution from chloro terpyridine platinum(II) and analogous complexes by a series of azole nucleophiles. Transition Met Chem 36, 593–602 (2011). https://doi.org/10.1007/s11243-011-9507-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-011-9507-x