Abstract
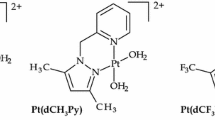
Substitution reactions of the aqua ligands from azine-bridged dinuclear platinum(II) complexes of the type [{cis-Pt(py)2(OH2)} 2(μ-pzn)](ClO4)4 [pzn = pyrazine (Pt-PZN), 2,3-dimethylpyrazine (Pt-2,3PZN), 2,5-dimethylpyrazine (Pt-2,5PZN) or 2,6-dimethylpyrazine (Pt-2,6PZN)] by thiourea nucleophiles were investigated under pseudo first-order conditions as a function of concentration and temperature using the stopped-flow technique. The experimental results are discussed in reference to structures obtained by DFT calculations. The results are in good agreement with the pKa values of the complexes as well as DFT calculations. Compared to [{cis/trans-Pt(NH3)2(OH2)} 2(μ-pzn)](ClO4)4, the complexes in this series react faster by a factor of 10 or 23 respectively due to the presence of pyridine rings, which forces the geometry to allow π-back bonding to take place such that the electrons from the metal centres are accepted to the empty π*-orbitals of the pyridine subunits. The reactivity of the nucleophile is sterically dependent, with N,N,N′,N′-tetramethylthiourea reacting three times slower than thiourea. In all complexes and for both substitution steps, the mode of activation remains associative in nature.
Graphical Abstract
The presence of pyridine rings in [{cis-Pt(py)2(OH2)} 2(μ-pzn)](ClO4)4 where py = pyridine, pzn = pyrazine (Pt-PZN), 2,3-dimethylpyrazine (Pt-2,3PZN), 2,5-dimethylpyrazine (Pt-2,5PZN) and 2,6-dimethylpyrazine (Pt-2,6PZN) allows the substitution reactions by thiourea nucleophiles to proceed faster by a factor of 10 than [{cis-Pt(NH3)2(OH2)} 2(μ-pzn)](ClO4)4 through π-back bonding.











Similar content being viewed by others
References
Mambanda A, Jaganyi D, Hochreuther S, van Eldik R (2010) Dalton Trans 39:3595
Mambanda A, Jaganyi D (2012) Dalton Trans 41:908
Hochreuther S, van Eldik R (2012) Inorg Chem 51:3025
Hochreuther S, Puchta R, van Eldik R (2011) Inorg Chem 50:8984
Soldatović T, Jovanović S, Bugarčić ŽD, van Eldik R (2012) Dalton Trans 41:876
Hochreuther S, Puchta R, van Eldik R (2011) Inorg Chem 50:12747
Reddy D, Jaganyi D (2011) Int J Chem Kinet 43:161
Jaganyi D, Munisamy VM, Reddy D (2006) Int J Chem Kinet 38(3):202
Ertürk H, Hofmann A, Puchta R, van Eldik R (2007) Dalton Trans 2295
Ertürk H, Maigut J, Puchta R, van Eldik R (2008) Dalton Trans 2759
Hofmann A, van Eldik R (2003) Dalton Trans 2979
Ertürk H, Puchta R, van Eldik R (2009) Eur J Inorg Chem 1334
Ongoma P, Jaganyi D (2014) Trans Met Chem 39:407
Jaganyi D, Ongoma P (2013) Dalton Trans 42:2724
Zou Y, Van Houten B, Farrell N (1993) Biochemistry 32:9632
Farrell N, Appleton TG, Qu Y, Roberts JD, Soares Fontes AP, Skov KA, Wu P, Zou Y (1995) Biochemistry 34:15480
Kasparková J, Novaková O, Marini V, Najajreh Y, Gibson D, Perez J-M, Brabec V (2003) J Biol Chem 278(48):47516
Kalinowska-Lis U, Ochocki J, Matlawska-Wasowska K (2008) Coord Chem Rev 252:1328
Farrer NJ, Woods JA, Salassa L, Zhao Y, Robinson KS, Clarkson G, Mackay FS, Sadler PJ (2010) Angew Chem Int Ed 49:8905
Hollis LS, Amundsen AR, Stern EW (1989) J Med Chem 32:128
Qu Y, Farrell N (1992) Inorg Chem 31:930
Jansen BAJ, van der Zwan J, den Dulk H, Brouwer J, Reedijk J (2001) J Med Chem 44:245
Origin7.5™ SRO, v7.5714 (B5714), Origin Lab Corporation, Northampton, One, Northampton, MA, 01060, USA, 2003
Becke AD (1993) J Chem Phys 98:5648
Wadt WR, Hay PJ (1985) J Chem Phys 82:284
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford
Barone V, Cossi M (1995) J Phys Chem A 102
Cossi M, Scalmani G, Rega N, Barone V (2003) J Comput Chem 24:669
Lee G-Y, Jun M-S (2001) Bull Korean Chem Soc 22(1):11
Eyring H (1935) J Chem Phys 3:107
Mebi CA (2011) J Chem Sci 123(5):727
Jaramillo P, Domingo LR, Pérez P (2006) www.captura.uchile.cl/jspui/bitstream/2250/5781/1/Jaramillo_P.pdf
Cedillo A, Contreras R (2012) J Mex Chem Soc 56(3):257
Parr RG, Szentpály LV, Liu S (1922) J Am Chem Soc 1999:121
Parthasarathi R, Subramanian V, Roy DR, Chattaraj PK (2004) Bioorg Med Chem 12:5533
Katritzky AR, Pozharski AF (2000) Handbook of heterocyclic chemistry, 2nd edn. Elsevier, Pergamon, pp 177–178
Hofmann A, Jaganyi D, Munro OQ, Leihr G, van Eldik R (2003) Inorg Chem 42:1688
Jaganyi D, Hofmann A, van Eldik R (2001) Angew Chem Int Ed 40:1680
Jaganyi D, Reddy D, Gertenbach J-A, Hofmann A, van Eldik R (2004) Dalton Trans 299
Schmülling M, Grove DM, van Koten G, van Eldik R, Veldman N, Spek AL (1996) Organometallics 15:1384
Schiessl WC, Summa NK, Weber CF, Gubo S, Dücker-Benfer C, Puchta R, Eikem Hommes NJR, van Eldik R (2005) Z Anorg Allg Chem 631:2812
Field JS, Gertenbach J-A, Haines RJ, Munro OQ, McMillin DR (2007) Z Naturforsch 62:447
Jaganyi D, Pantoja E, Gallipoli A, van Zutphen S, Komeda S, Reddy D, Lutz M, Tooke DM, Spek AL, Navarro-Ranninger C, Reedijk J (1955) J Inorg Biochem 2006:100
Hofmann A, Dahlernburg L, van Eldik R (2003) Inorg Chem 42:6528
Pasini A, Rigamonti L, Forni A, Manassero M, Manassero C (2010) Inorg Chem 49:123
Jaganyi D, Tiba F (2003) Transition Met Chem 28:803
Reddy D, Jaganyi D (2006) Transition Met Chem 31:792
Tobe ML, Burgess J (1999) Inorganic reaction mechanisms, Addison Wiley, Longman, Ltd., Essex, pp 30–33, 70–112
Acknowledgments
The authors thank the University of Dar es Salaam (Tanzania) and University of KwaZulu-Natal (South Africa) for financial support to Grace Kinunda.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kinunda, G., Jaganyi, D. A kinetic study of aqua ligand substitution in dinuclear Pt(II) complexes containing four non-coplanar pyridine ligands. Transition Met Chem 39, 939–949 (2014). https://doi.org/10.1007/s11243-014-9879-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-014-9879-9