Abstract
MicroRNAs are now known to have an important role in regulating gene expression of eukaryotic organisms. miRNA research in plants gained importance after the discovery that several stress factors alter certain miRNA expressions, which subsequently regulate their target gene expressions and affect development and growth of plants. In this study, two of the widely studied abiotic stress conditions for plants, nitrogen deficiency and drought were used individually and as a combined stress treatment on Arabidopsis thaliana callus tissues to observe the change of expressions in certain miRNAs, when multiple stressors are encountered. Combined stress strongly inhibited callus growth compared to other conditions, while strongly altering certain miRNA expressions. Compared to control, in 7-day stress treated callus, miR165a-3p,b, miR319a,b, miR396b-5p, miR399d and miR827 showed significant downregulation for all stress treatments, while 7-day N deficiency caused miR167 upregulation. Stress treatments for 7 days mostly downregulated miR167c-5p, miR319c, miR399a, miR399e expressions except for the N deficient samples. After 14 days of stress, miR165a-3p,b, miR396a-5p, miR399b, miR399d were downregulated. During 14-day drought and combined stress, miR399a and miR396b-5p expressions were upregulated, respectively. The differences observed in this study between stress responses of 7 and 14-day-long treatments are believed to be valuable to further elucidate the associated molecular mechanisms of miRNAs, determination and validation of miRNA targets, and how plants respond to stress conditions via various genetic regulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants in wild are constantly face to face with several stress factors: Biotic and abiotic stresses, such as bacteria, fungi, temperature fluctuations, salt, drought, heavy metals, nutrient deficiencies, radiation etc. (Mahajan and Tuteja 2005; Fedoroff et al. 2010; Hacquard et al. 2017; Hussain et al. 2018). Nitrogen deficiency and drought stresses are extremely important factors that affect development and crop growth (Clarkson 1996), also causing morphological and physiological disturbances in plants (Wang et al. 2004; Anithakumari et al. 2012).
Plants respond to stress conditions by utilizing antioxidant defense mechanisms (Duan et al. 2007; Praba et al. 2009; Anjum et al. 2011). Antioxidant defense systems are closely related to Reactive Oxygen Species (ROS) and the production of ROS is the first response of the cell against drought stress (Miller et al. 2010). Although drought causes morphological, physiological, and molecular changes in plants, it also causes a decrease in plant biomass and crop yield due to the limited availability of water in soil (Anithakumari et al. 2012). Above all these effects, photosynthesis, pigment content, water uptake, respiration, and membrane integrity are all severely affected by drought, which in return triggers other plant defense mechanisms (Praba et al. 2009; Anjum et al. 2016). When plants are faced with limited water, they slow down their growth rates, adjust water balance in cells and tissues, activate antioxidant defense systems to survive (Duan et al. 2007; Praba et al. 2009; Anjum et al. 2011). During drought stress, shoot development is also further obstructed compared to root development (Anithakumari et al. 2012). The tolerance to drought stress in plants depends on species, amount of time and severity of the stress they are exposed to and the development phase in which they are found in (Demirevska et al. 2009).
Relative Water Content (RWC) is a characteristic feature such as stomatal conductivity, transpiration rate, leaf temperature and leaf water potential to understand the relationship between plant and water. Majority of plants respond to drought stress with a decline in RWC (Ings et al. 2013). RWC is also a parameter using osmotic potential for measuring the severity of the drought stress treatment and used in drought stress treatment methods in vitro. Drought stress is characterized with the low availability of water, which is quantified as a low water potential (Ψw). By reducing the water potential of the plant medium in vitro using PEG8000, drought stress can be mimicked (Verslues 2006).
Aside from nitrogen (N) being the most important macronutrient for plant development and growth, it is also required in critical processes such as protein, nucleic acid, and chlorophyll synthesis (Clarkson 1996). Nitrate is both an important plant nutrient and has a valuable role as a signal molecule in promoting gene expressions associated with intracellular transport and assimilation (Medici and Krouk 2014). Gene expressions related to mechanisms of plant viability, especially organic acid and glucose metabolism are affected by nitrate (Marín et al. 2011). Nitrate Reductase (NR) activity is reduced under nitrogen starvation, while NLA (NITROGEN LIMITATION ADAPTATION) genes are activated whose expression is shown to be regulated by miR827 in Arabidopsis thaliana (Wang et al. 2004; Kant et al. 2011).
MicroRNAs (miRNAs) are non-coding, 20–24 nucleotides long, endogenous small RNAs that regulate multiple biological pathways in multicellular organisms (Llave et al. 2002; Dinger et al. 2008). Most miRNAs are evolutionarily well conserved and responsible for regulating transcription factors which have important roles in plant survival. Therefore, any mutation that may occur in the miRNA biogenesis mechanism may cause a significant defect in plant growth and even have a lethal effect (Yu et al. 2017). Plant miRNAs target transcription factors or stress-regulated genes by recognizing and degreading target gene mRNAs, therefore repressing gene expression (Sunkar and Zhu 2004; Sunkar et al. 2006).
The evaluated miRNAs in this study and their potential roles in chosen stress conditions were summarized in Supplementary Table 1. In this study, miRNA expression changes related to drought and nitrogen deficiency were observed according to the exposure time to stressors in Arabidopsis thaliana Col-0 callus tissues. Combination of these two conditions were also used to observe changes, compared to individual treatments of these abiotic stresses.
Material and methods
Plant material, growth conditions and stress treatments
Arabidopsis thaliana Columbia (Col-0) seeds were provided by Prof. Dr. Neslihan Turgut Kara, Istanbul University, Department of Molecular Biology and Genetics who acquired the seeds from Dr. Ralf Stracke, Bielfeld University, Biotechnology Center, Genome Research Department. To sterilize A. thaliana Col-0 seeds, 70% ethanol was added above the seeds and centrifuged at 600 rpm for 2 min in column tubes, 3 times. 1 mL 100% ethanol was added, and the alcohol was removed by centrifugation at 600 rpm for 2 min. Sterile seeds were sown on basal MS agar plates and were left to germinate in plant growth chamber [25 ± 2 °C, 16 h light/8 h dark cycle (Nüve, TK 252)]. In vitro cultured 3-week-old A. thaliana plant roots were selected as explants for callus induction. Explants were transferred to MS agar plates (Murashige and Skoog 1962) (Sigma, M2909) containing 1 mg/L 2,4-D (2,4-dichlorophenoxyacetic acid) (Sigma, D7299) and incubated in the growth chamber for a month. After 1 month, callus tissues were obtained from the medium, and transferred to fresh media which have the exact composition of initiation media. Callus subculturing was continued monthly during the study. For stress treatments, 1-month-old calluses were transferred to control, nitrogen deficiency, drought and combination MS medium containing 1 mg/L 2,4-D. Calli were treated for 7 and 14 days, then collected for each condition after the treatment, as a total of 8 samples, for each biological replicate. To proceed with RNA isolations for the following qRT-PCR analysis, calli were portioned into 0.1 g samples, snap-frozen by liquid nitrogen and stored at -80 °C until further use. These steps were repeated for three biological repeats.
Murashige and Skoog (MS) medium was used for germination, plant growth and callus induction of Arabidopsis thaliana Col-0 seeds. Plant growth regulator 2,4-D was applied in 1 mg/L concentration to MS media used in callus induction. Control condition for both 7- and 14- day treatments, had the same medium content as the callus induction media. Nitrogen deficiency in the media was created using MS modified basal salt mixture (without NH4NO3, Sigma®, Lot: 103K2354). To prepare nitrogen deficiency stress medium, 3% sucrose and 2.68 g/L NH4NO3-free MS powder were dissolved in distilled water. Drought stress medium was prepared according to the protocol of Verslues et al. (2006) and Van Der Weele et al. (2000), at -0.7 MPa (Osmotic Potential). Polyethylene Glycol 8000 (PEG 8000) (Sigma, P2139) was used to create drought stress in the medium by overnight incubation of PEG8000 overlay solution on half-strength MS-agar plates. While preparing the combined stress medium, NH4NO3-free MS salt mixture and PEG 8000 were used in accordance with the drought medium and N deficiency medium protocol. Therefore, PEG-infused plates for combined treatment were prepared using modified MS basal salt mixture (without NH4NO3). Prepared medium plates were stored at 4 °C.
Morphological and growth index analysis
Callus tissues were analyzed for morphological differences after 7-day and 14-day long treatments. Plates were photographed to observe varying colors and textures between treatments. To conduct growth index analysis, callus tissues were weighed equally as 0.5 g. Change in fresh callus weight was measured after 7- and 14- day treatments. Growth percentages were calculated according to Sahraroo et al. (2014).
RNA isolation and qRT-PCR analysis
Equal amounts of 0.1 g frozen callus tissues were grinded by mortar and pestle using liquid nitrogen. Isolations were performed manually by phenol:chloroform extraction using Hibrizol (Hibrigen™). After the RNA pellets were dissolved in DEPC (Diethyl Pyrocarbonate)-treated water, absorbances were measured on Nanodrop (Thermo Fisher Scientific, Nanodrop 2000 Spectrophotometer). A260/A280 ratio was used to determine the purity of RNA samples. RNAs with A260/A280 ratio ≈2.0 were accepted as pure and were stored for further use at -80 °C.
cDNA synthesis of isolated RNAs was performed in a Thermal Cycler (BIORAD, T100) using TaqMan™ MicroRNA Reverse Transcription Kit (Applied Biosystem, Lot: 00575529) according to the instructions of manufacturers. For the cDNA synthesis of respective selected miRNAs, specifically designed stem-loop primers were used per 1000 ng total RNA (Suppl. Table 2). Using the synthesized cDNA, qPCR analyses are conducted to determine the changes in miRNA expressions between 7-days and 14-days stress treated and control samples. Cq values of all miRNAs and the reference gene Actin were detected using real-time thermal cycler, BIORAD CFX96. For 20 µL of qPCR reaction, 1X SYBR Green Mix (Hibrigen), 10 µM specifically designed forward miRNA primer, 10 µM Universal Reverse Primer, 2 µL cDNA and 6 µL nuclease-free water were used as reaction components. Reaction conditions entail: Initial denaturation at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s and annealing at 62 °C for 1 min. Following melting curve analysis validated the specificity of the designed primers. Fold change expressions of all microRNAs were calculated using the delta-delta ct method (Livak and Schmittgen 2001).
Additionally, to have a broader idea on the implications of the detected expression changes of our miRNAs, we performed target candidate analysis via psRNATarget, plant small RNA target analysis server (Dai et al. 2018).
Statistical analysis
miRNA expression assays were conducted for three biological replicates. For each set of the experiment, qPCR reactions were set up using three technical repeats and NTC. Statistical analyzes were performed using GraphPad 9.1.2 demo version. Growth index analysis were statistically tested by two-way ANOVA and Tukey tests for significance. For statistical analysis of miRNA expressions, one Way ANOVA and Tukey test were applied. For all analysis, values of p < 0.05 were considered significant.
Results
Plant growth and morphological analysis
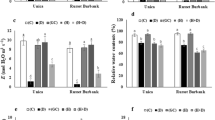
Control and stress-treated callus tissues were collected after 7- and 14-days. All treatments had their own control conditions as shown in Fig. 1. Callus tissues showed visible changes in their colors and morphology, especially in drought and combined stress treatments, both in 7-day and 14-day old samples. Callus tissues were also analyzed for their growth percentages. Callus weight was measured and the amount of growth after the corresponding treatments were calculated according to the growth index formula. Growth percentages of all conditions are shown as a graph with multiple comparison analysis in Fig. 2.
Growth index results show significantly reduced callus weight after 7-days of drought and combined treatments with no change in weight for 7-day N deficiency and control samples. Callus weight of 14-day control sample was highly significantly increased compared to all other conditions, reaching twice the weight of initial stage, indicating normal growth without stress treatment. Even though the weight of callus tissue was highly decreased after 7 days of drought, after 14 days, 20% growth compared to day-0 was observed. Initial drop in weight (7-d) could potentially be due to the lowered water content of the agar plate causing water-loss from the plant, whereas after 14-day the growth of callus tissue overcompensated for the water-loss showing a significant increased growth percentage in the results. Callus weight decreased by 39% after 7-day combined stress treatment, after 14 days growth percentage was still negative at -11%.
RNA isolation and qRT-PCR analysis
Isolated RNAs from all samples were checked for RNA quantity and quality by Nanodrop and agarose gel electrophoresis, respectively. All RNA samples were deemed suitable for use as templates in cDNA synthesis by miRNA-specific stem loop primers. Expression analysis by qRT-PCR were performed for all samples using Actin gene as positive control. Fold changes for all miRNA expressions compared to control are shown as a heatmap in Fig. 3.
Statistical analysis resulted in significantly changed expressions of miRNA transcripts under different treatment conditions in this study. 7-day treatment results are given in Fig. 4, while 14-day treatment results are shown in Fig. 5. According to the expression analysis, miR165a-3p and miR165b were significantly downregulated in all 7-day treatments compared to the control. Treatment of 14 days caused downregulation for these miRNAs in all conditions. miR167a-5p and miR167b showed significant downregulation for all stress conditions after 7 days of treatment, whereas no meaningful change occurred in 14 days treatment samples. These miRNAs had no significant variance in between treatments for both 7-day and 14-day samples. For miR167c-5p, 7-day drought and combined stresses had lower expression compared to control. 14-day samples of this miRNA were upregulated under N deficiency, while downregulated for both drought and combination. miR167d was highly significantly upregulated during 7-day N deficiency stress and downregulated during combined stress treatment. Upregulation of miR167d occurred significantly on 14-day N deficiency, and it was also downregulated on 14-day combined stress treatment. miR169b-5p and miR169c, under 7-day combined stress showed significant downregulation compared to control. 14-day drought and combined stress resulted in the downregulated expression of miR169b-5p and miR169c compared to control samples. miR319a and b were downregulated for all three treatments in 7-day samples, on 14-day samples, only drought treatment had significantly downregulated miRNA expressions. miR319c, on the other hand, showed highly significant downregulation in 7-day drought and combined stress, similar to the 14-day treatments. On the contrary, 14-day N deficiency upregulated the expression of miR319c significantly.
Only significant change of miR396a-5p is in 14-day samples, all three treatments had lower expression of this miRNA. miR396b expression decreased on all 7-day treatment samples, whereas 14-day combined treatment had increased expression of this miRNA compared to control. 7-day drought and combined stress lowered the expression of miR399a, while 14-day drought increased the miR399a expression in comparison with the control. 7-day combined stress also lowered the expression of this miRNA compared to drought stress, even though no significant change was observed in N deficiency treatment. miR399b was downregulated significantly for all treatments in 14-day samples compared to control. Downregulation of miR399d was highly significant in all 7-day and 14-day stress treatments, whereas miR399e expression only decreased in 7-day drought and combined stress while increasing in 14-day N deficiency. miR399e was significantly downregulated in 14-day drought and combined stress conditions. miR399f was downregulated in 7-day N deficiency and combined stress compared to control. Finally, the miR827 only showed significant upregulation under N deficiency on 14-day samples, though in 7-day samples, it was downregulated significantly under all stress treatments compared to control.
Determination of potential miRNA targets
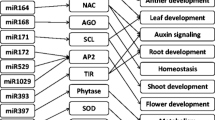
Certain potential target genes of studied miRNAs have been detected using psRNATarget tool, all gene ID and expectation values are given in Table 1. How these miRNA targets could have been affected by the expression changes observed in this study are analyzed.
Discussion
Nitrate, besides being an important nutrient, is a valuable component of the cell promoting expressions of genes with various functions. Scarcity of nutrients have been a widely studied stress condition because of its detrimental effects on plant productivity (Marín et al. 2011; Medici and Krouk 2014). When plants face drought, processes such as photosynthesis and respiration are affected, while antioxidant defense systems are activated. Plants create a stress response to cope with both drought and nutrient deficiencies via physiological, morphological, and molecular changes (Anithakumari et al. 2012). Micronutrients such as nitrogen (as inorganic nitrate (NO3−) and ammonium (NH4+)) are absorbed from the soil via water uptake (Lambers et al. 2008). Drought conditions therefore strongly inhibit the nutrient uptake capability of the plant making it vulnerable to nutrient deficiencies, as validated previously where the N and P concentrations of plant tissues decreased significantly after drought stress (Cramer et al. 2009; Sardans and Peñuelas 2012; He and Dijkstra 2014). Reason for this lower N uptake under drought has been found to be related to conditions such as mineralization-caused decreased soluble nutrient source or reduced diffusion (Fierer and Schimel 2002; Bista et al. 2018). Plant miRNAs were first discovered in Arabidopsis, and it has been repeatedly shown that these small regulatory RNA molecules have key roles in controlling expressions of genes responsible for plant growth and stress response (Chen 2004; Pegler et al. 2019).
In this study, expressions of various stress-related microRNAs in Arabidopsis thaliana were studied to evaluate their responses to a combination of these two widely studied stress conditions, drought, and nitrogen deficiency. Combined treatment of these stresses strongly downregulated the expressions of miR165a-3p, miR165b, miR167c-5p, miR167d, miR319c, miR399d and miR399e both on day 7 and day 14. Among these, there are miRNAs previously associated with stress conditions in other studies, HD-ZIP transcription factor family, specifically HD-ZIP III, being one of the most prominent target genes of miR165 were detected to be ABA regulated and triggered under abiotic stress conditions due to their roles in signal perception and transduction (Zhong and Ye 2007; Li et al. 2022). During stress, due to the miR165/HD-ZIP regulation, miR165 are expected to be downregulated, as we have observed for all conditions in our study.
Effect of drought, heat and salinity stresses on certain miRNAs and their targets in Arabidopsis thaliana Col-0 ecotype were studied previously, in which drought stress upregulated 111 miRNAs and downregulated 2 miRNAs. Salinity decreased miR169g expression 8.7 times, while all three stresses decreased miR169f and miR169h expressions. Our study also demonstrated a downregulation during both 7-day drought and combined stress for miR169f and g, compared to N-deficient callus. Downregulation of miR169 was also demonstrated in other studies of Arabidopsis thaliana (Li et al. 2008; Zhao et al. 2011).
Adding to the previously reported studies, our psRNATarget results also pointed out significant targets of the studied miRNAs (Table 1). Drought and salinity stresses have been associated with miR319 expression in Arabidopsis thaliana plants, which targets and regulates the expressions of TCP transcription factors (Liu et al. 2008; Zhou and Luo 2014). Drought conditions are known to cause disturbances in growth and development due to altered metabolic activity and functions, to tolerate these conditions, plants activate certain signal mechanisms and differentially express transcriptional regulators (Mahmood et al. 2019). Results of our study showed that after 7-days of N deficiency and drought stress, miR319a and b expressions were downregulated for both stresses and their combination, signaling a potential regulation of transcription factors which are responsible in plant growth and development (Li 2015). The downregulation was observed only in drought conditions for 14-day stress treated callus, potentially due to the stress response of the plant to battle deteriorated growth by preventing the negative regulation of TCP factors by miR319. Increasing the number of growth and development related factors and compensating for the effect of stress, as a result. Our growth index analysis supported this idea, where we could observe the lowered growth during 7 days of drought and combination stress. After 14-days, callus weight was significantly higher than 7-day samples, showing that the growth was slower but persistent.
In a previous study, drought and salinity stresses changed miR396 expression which is known to target GRF (“GROWTH REGULATION FACTOR”) genes (Covarrubias and Reyes 2010). Pegler et al. (2019), also showed that drought stress treatment resulted in upregulated miR396 expression in Arabidopsis thaliana (Pegler et al. 2019). In our study, after 7-day stress treatment, miR396a-5p had no significant change, though miR396b was downregulated for all conditions. 14-day stress treated callus had downregulated expression of miR396a-5p for all conditions, whereas miR396b expression was upregulated during combined stress. Previously, it’s been shown that miR396/GRF regulatory module regulates growth-related processes. Increased number of miR396 inhibits GRF expressions resulting in the disruption or deceleration of growth in plants (Liu et al. 2009; Omidbakhshfard et al. 2015). A combination of drought and N deficiency is demonstrated to possibly regulate miR396 expression, as a result of this study. Similar to miR319/TCP, miR396/GRF regulation is correlated to our growth and expression analysis. Lowered miR396 expression increases the number of GRF factors, mending negative effects of stress on plant growth. Being the negative regulator of growth-regulating factors (GRF), downregulation of miR396 increases the number of GRF in cells, which could be seen as an indication of battling disrupted growth-related processes after 7 days.
N deficiency is expected to affect miR827 expressions, 7-day stress downregulated miR827 for all conditions, though in 14-days, there was a significant upregulation. This could be due to the miR827/NLA (nitrogen limitation adaptation) regulation of stress adaptation during prolonged N starvation (Kant et al. 2011). Higher NLA expression during downregulated miR827 would potentially provide an adaptation to the stress, which would explain the downregulation in our 7-day samples. For miR399e, 14-day stress caused an upregulation. Being responsible for phosphate homeostasis in Arabidopsis (Fujii et al. 2005), this miRNA could have been induced by nutrient deficiency. miR399e specifically targets and downregulates a phosphate transporter coding gene (PHO2), which occurs expectedly in higher frequency in response to various abiotic stress conditions due to its upregulation (Pegler et al. 2019). PHO2 is responsible from the uptake and translocation of phosphate, lowered function of this gene results in higher accumulation of phosphate species in plant shoots, making its downregulation meaningful under phosphate deficient conditions (Dong et al. 1998). Though, how this applies to our nitrogen deficient media can be explained by another observation in Arabidopsis, where the N and P availability responses seem to interfere. Phosphate starvation response (PSR) was found to be actively regulated by N signaling pathways, with PHO2 acting as the messenger of N presence while also interacting with NRT1.1 (NITRATE TRANSPORTER1.1) and controlling its transcript levels, all leading to the PSR being highly influenced by N signaling, therefore the N content of the environment (Medici et al. 2019).
As inferred from both the previous studies and the target analysis, many factors associated with stress responses, growth and nutrient metabolisms in plants were linked with the examined miRNAs. A summary of how and when these changes occurred are given in Fig. 6.
Naturally, wild plants encounter multiple stress conditions at the same time, as a result, miRNA expressions change to adapt to the environment by regulating plant responses. In this study, by combining drought and N deficiency stressors, the differences in plants’ molecular response to a combination of stress and individual stress conditions were pointed out. Potential targets of these miRNAs were taken into consideration and their possible impact on the plant stress response was conversed, depending on the changes of expression detected. Additionally, the amount of time plant faces the stressor has also been shown to have impact on the plant microRNAs’ earlier and later responses, while effecting the plant growth as well. Findings of this study could help further studies using combined stressors and envisioning the use of miRNAs to combat multiple stress factors effecting plant growth and development.
Data availability
Data will be made available on reasonable request.
References
Anithakumari AM, Nataraja KN, Visser RGF, van der Linden CG (2012) Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol Breeding 30(3):1413–1429. https://doi.org/10.1007/s11032-012-9728-5
Anjum SA, Xie X, Wang L, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6(9):2026–2032. https://doi.org/10.5897/AJAR10.027
Anjum SA, Tanveer M, Ashraf U, Hussain S, Shahzad B, Khan I, Wang L (2016) Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ Sci Pollut Res 23(17):17132–17141. https://doi.org/10.1007/s11356-016-6894-8
Bista DR, Heckathorn SA, Jayawardena DM, Mishra S, Boldt JK (2018) Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 7(2):28. https://doi.org/10.3390/plants7020028
Chen X (2004) A MicroRNA as a translational repressor of APETALA2 in arabidopsis flower development. Science 303(5666):2022–2025. https://doi.org/10.1126/science.1088060
Clarkson D (1996) Marschner H. 1995. Mineral nutrition of higher plants. 2nd ed. London: Academic Press, Annals of Botany, 78(4):527–528. https://doi.org/10.1006/anbo.1996.0155
Covarrubias AA, Reyes JL (2010) Post-transcriptional gene regulation of salinity and drought responses by plant microRNAs. Plant Cell Environ 33(4):481–489. https://doi.org/10.1111/j.1365-3040.2009.02048.x
Cramer MD, Hawkins HJ, Verboom GA (2009) The importance of nutritional regulation of plant water flux. Oecologia 161(1):15–24. https://doi.org/10.1007/s00442-009-1364-3
Dai X, Zhuang Z, Zhao PX (2018) psRNATarget: a plant small RNA target analysis server (2017 release). Nucleic Acids Res 46(W1):W49–W54. https://doi.org/10.1093/nar/gky316
Demirevska K, Zasheva D, Dimitrov R, Simova-Stoilova L, Stamenova M, Feller U (2009) Drought stress effects on rubisco in wheat: changes in the rubisco large subunit. Acta Physiol Plant 31(6):1129–1138. https://doi.org/10.1007/s11738-009-0331-2
Dinger ME, Pang KC, Mercer TR, Mattick JS (2008) Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput Biol 4(11):e1000176. https://doi.org/10.1371/journal.pcbi.1000176
Dong B, Rengel Z, Delhaize E (1998) Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta 205(5):251–256. https://doi.org/10.1007/s004250050318
Duan B, Yang Y, Lu Y, Korpelainen H, Berninger F, Li C (2007) Interactions between water deficit, ABA, and provenances in Picea asperata. J Exp Bot 58(11):3025–3036. https://doi.org/10.1093/jxb/erm160
Fedoroff NV, Battisti DS, Beachy RN, Cooper PJM, Fischhoff DA, Hodges CN, Zhu JK (2010) Radically rethinking agriculture for the 21st century. Science 327:833–834. https://doi.org/10.1126/science.1186834
Fierer N, Schimel JP (2002) Effects of drying–rewetting frequency on soil carbon and nitrogen transformations. Soil Biol Biochem 34:777–787. https://doi.org/10.1016/S0038-0717(02)00007-X
Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15(22):2038–2043. https://doi.org/10.1016/j.cub.2005.10.016
Hacquard S, Spaepen S, Garrido-Oter R, Schulze-Lefert P (2017) Interplay between Innate Immunity and the Plant Microbiota. Annu Rev Phytopathol 55:565–589. https://doi.org/10.1146/annurev-phyto-080516-035623
He M, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol 204(4):924–931. https://doi.org/10.1111/nph.12952
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front Plant Sci 9:393. https://doi.org/10.3389/fpls.2018.00393
Ings J, Mur LAJ, Robson PRH, Bosch M (2013) Physiological and growth responses to water deficit in the bioenergy crop Miscanthus x giganteus. Front Plant Sci 4:468. https://doi.org/10.3389/fpls.2013.0046
Kant S, Peng M, Rothstein SJ (2011) Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet 7(3):e1002021. https://doi.org/10.1371/journal.pgen.1002021
Lambers H, Chapin FS, Pons TL (2008) Plant water relations. In: Plant Physiological Ecology (pp 163–223). Springer. New York, NY, USA. https://doi.org/10.1007/978-0-387-78341-3_5
Li S (2015) The Arabidopsis thaliana TCP transcription factors: A broadening horizon beyond development. Plant Signal Behav 10(7):e1044192. https://doi.org/10.1080/15592324.2015.1044192
Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Zhu JK (2008) The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20(8):2238–2251. https://doi.org/10.1105/tpc.108.059444
Li Y, Yang Z, Zhang Y, Guo J, Liu L, Wang C, Wang B, Han G (2022) The roles of HD-ZIP proteins in plant abiotic stress tolerance. Front Plant Sci 13:1027071. https://doi.org/10.3389/FPLS.2022.1027071
Liu HH, Tian X, Li YJ, Wu CA, Zheng CC (2008) Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14(5):836–843. https://doi.org/10.1261/rna.895308
Liu D, Song Y, Chen Z, Yu D (2009) Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant 136(2):223–236. https://doi.org/10.1111/j.1399-3054.2009.01229.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Llave C, Kasschau KD, Rector MA, Carrington JC (2002) Endogenous and silencing-associated small RNAs in plants. Plant Cell 14(7):1605–1619. https://doi.org/10.1105/tpc.003210
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444(2):139–158. https://doi.org/10.1016/j.abb.2005.10.018
Mahmood T, Khalid S, Abdullah M, Ahmed Z, Shah MKN, Ghafoor A, Du X (2019) Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 9(1):105. https://doi.org/10.3390/cells9010105
Marín IC, Loef I, Bartetzko L, Searle I, Coupland G, Stitt M, Osuna D (2011) Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 233(3):539–552. https://doi.org/10.1007/s00425-010-1316-5
Medici A, Krouk G (2014) The Primary Nitrate Response: a multifaceted signalling pathway. J Exp Bot 65(19):5567–5576. https://doi.org/10.1093/jxb/eru245
Medici A, Szponarski W, Dangeville P, Safi A, Dissanayake IM, Saenchai C, Emanuel A, Rubio V, Lacombe B, Ruffel S, Tanurdzic M, Rouached H, Krouk G (2019) Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. Plant Cell 31(5):1171–1184. https://doi.org/10.1105/tpc.18.00656
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Omidbakhshfard MA, Proost S, Fujikura U, Mueller-Roeber B (2015) Growth-regulating factors (GRFs): a small transcription factor family with important functions in plant biology. Mol Plant 8(7):998–1010. https://doi.org/10.1016/j.molp.2015.01.013
Pegler JL, Oultram JMJ, Grof CPL, Eamens AL (2019) Profiling the abiotic stress responsive microRNA landscape of Arabidopsis thaliana. Plants 8(3):58. https://doi.org/10.3390/plants8030058
Praba ML, Cairns JE, Babu RC, Lafitte HR (2009) Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J Agron Crop Sci 195(1):30–46. https://doi.org/10.1111/j.1439-037X.2008.00341.x
Sahraroo A, Babalar M, Mirjalili MH, Fattahi Moghaddam MR, Nejad Ebrahimi S (2014) In-vitro callus induction and rosmarinic acid quantification in callus culture of Satureja khuzistanica Jamzad (Lamiaceae). Iran J Pharm Res 13(4):1447–1456
Sardans J, Peñuelas J (2012) The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol 160(4):1741–1761. https://doi.org/10.1104/pp.112.208785
Sunkar R, Zhu JK (2004) Novel and stress regulated microRNAs and other small RNAs from Arabidopsis w inside box sign. Plant Cell 16(8):2001–2019. https://doi.org/10.1105/tpc.104.022830
Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18(8):2051–2065. https://doi.org/10.1105/tpc.106.041673
Van Der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51(350):1555–1562. https://doi.org/10.1093/jexbot/51.350.1555
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu J-K (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J: Cell Molec Biol 45(4):523–539. https://doi.org/10.1111/j.1365-313X.2005.02593.x
Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, Crawford NM (2004) Genomic analysis of the nitrate response using a nitrate reductase-null mutant of arabidopsis. Plant Physiol 136(1):2512–2522. https://doi.org/10.1104/pp.104.044610
Yu Y, Jia T, Chen X (2017) The 'how' and 'where' of plant microRNAs. New Phytol 216:1002–1017. https://doi.org/10.1111/nph.14834
Zhao M, Ding H, Zhu JK, Zhang F, Li WX (2011) Involvement of miR169 in the nitrogen-starvation responses in Arabidopsis. New Phytol 190(4):906–915. https://doi.org/10.1111/j.1469-8137.2011.03647.x
Zhong R, Ye ZH (2007) Regulation of HD-ZIP III Genes by MicroRNA 165. Plant Signal Behav 2(5):351–353. https://doi.org/10.4161/psb.2.5.4119
Zhou M, Luo H (2014) Role of microRNA319 in creeping bentgrass salinity and drought stress response. Plant Signal Behav 9(4):e28700. https://doi.org/10.4161/psb.28700
Acknowledgements
This study was supported financially by Istanbul University Scientific Research Projects Coordination Unit (BAP), under the project ID number, 37185.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Istanbul University Scientific Research Projects Coordination Unit (BAP), project ID number, 37185.
Author information
Authors and Affiliations
Contributions
Seda Yaşar: Conceptualization, Methodology, Investigation. Elif Pulat: Conceptualization & Writing- Original draft preparation, Investigation. Özgür Çakır: Writing- Reviewing and Editing, Supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare. All authors have seen and agree with the content of the manuscript and there is no financial interest to report.
Additional information
Communicated by Yi Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaşar, S., Pulat, E. & Çakır, Ö. Effects of nitrogen deficiency and drought stresses on miRNA expressions in Arabidopsis thaliana. Plant Cell Tiss Organ Cult 157, 42 (2024). https://doi.org/10.1007/s11240-024-02754-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02754-0