Abstract
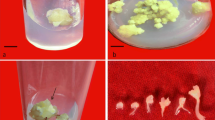
Allium hirtifolium (Amaryllidaceae), called “Mooseer” or Persian shallot in Iran, is a bulbous plant with high medicinal values. It is native and endemic to the North West to central and South West of Iran. In the present study, regeneration plants with chromosomal changes are produced through in vitro mature and immature endosperm culture. In our study, both cultured endosperms formed yellow- green calli induced in the MS medium supplemented with (1.5 or 2 mg·L− 1) 2,4-dichlorophenoxyacetic acid (2,4- D), (0.5 or 1 mg·L− 1) α-naphthalene acetic acid (NAA) plus (0.5 or 1 mg·L− 1) 6-furfurylamino purine (Kin) and 6-benzylaminopurine (BAP). The highest callus induction in mature (87.62%) and immature (62.49%) endosperms was achieved on a medium with 1 mg·L− 1 NAA and 1 mg·L− 1 BAP. Regenerated shoots were transferred for bulblet formation to investigate their potential for bulblet production. Regeneration of plantlets from endosperm tissue shows high chromosome variations and polyploidy. Flow cytometry analysis and chromosome number confirmed that diploid (2n = 2x = 16), triploid (2n = 3x = 24), tetraploid (2n = 4x = 32) and aneuploid plantlets were obtained. The comparison of stomatal density and size in complete plantlets has revealed that with the increase ploidy level, stomatal density decreased because of enlargement in stomatal size.









Similar content being viewed by others
References
Akhavan A, Saeidi H, Zarre S, Rahiminejad MR (2015) Chromosome numbers and karyotype features of selected species of Allium L. (Amaryllidaceae) sect. Acanthoprason in Iran. Iran J Bot 21:158–164
Alexander L (2017) Production of triploid Hydrangea macrophylla via unreduced gamete breeding. HortScience 52:221–224
Antoniazzi CA, de Faria RB, de Carvalho PP et al (2018) In vitro regeneration of triploid plants from mature endosperm culture of commercial passionfruit (Passiflora edulis Sims). Sci Hortic (Amsterdam) 238:408–415. https://doi.org/10.1016/j.scienta.2018.05.001
Asakura I, Hoshino Y (2017) Endosperm-derived triploid plant regeneration in diploid Actinidia Kolomikta, a cold-hardy kiwifruit relative. Sci Hortic (Amsterdam) 219:53–59. https://doi.org/10.1016/j.scienta.2017.02.045
Ascough GD, Van Staden J, Erwin JE (2008) Effectiveness of colchicine and oryzalin at inducing polyploidy in Watsonia lepida NE Brown. HortScience 43:2248–2251
Asha Devi A, Khar A, Lawande KE (2011) Genotypic response of short day garlic (Allium sativum L.) accessions to shoot multiplication. J Spices Aromat Crop 16
Asili A, Behravan J, Naghavi MR, Asili J (2010) Genetic diversity of Persian shallot (Allium hirtifolium) ecotypes based on morphological traits, allicin content and RAPD markers. Open Access J Med Aromat Plants 1:1
Askari-Khorasgani O, Pessarakli M (2020) Evaluation of cultivation methods and sustainable agricultural practices for improving shallot bulb production–a review. J Plant Nutr 43:148–163
Azadi HG, Ghaffari SM, Riazi GH et al (2008) Antiproliferative activity of chloroformic extract of Persian Shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology 56:179–185
Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV (2015) Plant biology and biotechnology: volume II: plant genomics and biotechnology. Plant Biol Biotechnol Vol II Plant Genomics Biotechnol II:1–768. https://doi.org/10.1007/978-81-322-2283-5
Batista RA, Figueiredo DD, Santos-González J, Köhler C (2019) Auxin regulates endosperm cellularization in Arabidopsis. Genes Dev 33:466–476
Beaulieu JM, Leitch IJ, Patel S et al (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179:975–986. https://doi.org/10.1111/j.1469-8137.2008.02528.x
Bednorz L, Krzymińska A, Czarna A (2012) Seed morphology and testa sculptures of some Allium L. species (Alliaceae). Acta Agrobot 64:33–38. https://doi.org/10.5586/aa.2011.015
Bennett MD (2004) Perspectives on polyploidy in plants–ancient and neo. Biol J Linn Soc 82:411–423
Block E (2010) Allium botany and cultivation, ancient and mdern. Garlic Other Alliums lore Sci 1–32
Block E, Dane AJ, Cody RB (2011) Crushing garlic and slicing onions: detection of sulfenic acids and other reactive organosulfur intermediates from garlic and other alliums using direct analysis in real-time mass spectrometry (DART-MS). Phosphorus Sulfur Silicon Relat Elem 186:1085–1093
Celep F, Koyuncu M, Fritsch RM et al (2012a) Taxonomic importance of seed morphology in allium (amaryllidaceae). Syst Bot 37:893–912. https://doi.org/10.1600/036364412X656563
Celep F, Koyuncu M, Fritsch RM et al (2012b) Taxonomic importance of seed morphology in Allium (Amaryllidaceae). Syst Bot 37:893–912
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Costa LM, Gutierrez-Marcos JF, Dickinson HG (2004) More than a yolk: the short life and complex times of the plant endosperm. Trends Plant Sci 9:507–514
Da silva NT, Silva LAS, Reis AC et al (2020) Endosperm culture: a facile and efficient biotechnological tool to generate passion fruit (Passiflora cincinnata mast.) Triploid plants. Plant Cell Tissue Organ Cult 142:613–624. https://doi.org/10.1007/s11240-020-01887-2
Dadpour MR, Movafeghi A, Grigorian W, Omidi Y (2011) Determination of floral initiation in Malus domestica: a novel morphogenetic approach. Biol Plant 55:243–252
Divya UK, Rekha K, Kumari SS (2018) Development of triploid callus of Hevea brasiliensis using endosperm. Asian J Res Agric for 1:1–15. https://doi.org/10.9734/ajraf/2018/42469
Doležel J, Bartoš J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110. https://doi.org/10.1093/aob/mci005
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244. https://doi.org/10.1038/nprot.2007.310
Lampe L, Mills CO (1933) Growth anddevelopment of isolated endosperm and embryo of maize. Abs Pap Bot Soc, Bost
Ebrahimi R, Zamani Z, Kashi A (2009) Genetic diversity evaluation of wild persian shallot (Allium Hirtifolium Boiss.) Using morphological and RAPD markers. Sci Hortic (Amsterdam) 119:345–351
Ebrahimi R, Hassandokht M, Zamani Z et al (2014) Seed morphogenesis and effect of pretreatments on seed germination of Persian shallot (Allium Hirtifolium Boiss.), an endangered medicinal plant. Hortic Environ Biotechnol 55:19–26. https://doi.org/10.1007/s13580-014-0032-7
Farhadi N, Panahandeh J, Azar AM, Salte SA (2017) Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sci Hortic (Amsterdam) 218:80–86. https://doi.org/10.1016/j.scienta.2016.11.056
Farhadi N, Panahandeh J, Motallebi-Azar A, Mokhtarzadeh S (2022) Production of autotetraploid plants by in vitro chromosome engineering in Allium hirtifolium. Hortic Plant J. https://doi.org/10.1016/J.HPJ.2022.12.013
Forster B, Van De Ville D, Berent J et al (2004) Complex wavelets for extended depth-of-field: a new method for the fusion of multichannel microscopy images. Microsc Res Tech 65:33–42. https://doi.org/10.1002/jemt.20092
Friedman WE (2009) Auxin at the evo-devo intersection. Sci (80-) 324:1652–1653
Friesen N, Smirnov SV, Leweke M et al (2021) Taxonomy and phylogenetics of Allium section Decipientia (Amaryllidaceae): morphological characters do not reflect the evolutionary history revealed by molecular markers. Bot J Linn Soc 197:190–228
Fritsch RM (1996) The Iranian species of Allium subg. Melanocrommyum sect. Megaloprason (Alliaceae). Nord J Bot 16:9–17
Fritsch RM, Abbasi M (2013) A taxonomic review of Allium subg. Melanocrommyum in Iran. Leibniz-Institut für Pflanzengenetik und Kulturpflanzenforschung
Fritsch RM, ABBASI M, Keusgen M (2006) Useful wild Allium species in northern Iran
Ghahremani-majd H, Dashti F, Dastan D et al (2012) Antioxidant and antimicrobial activities of Iranian mooseer (Allium Hirtifolium Boiss) populations. Hortic Environ Biotechnol 53:116–122
Gimdil R, Shahgholi H, Ajiorloo AR, Shaban M (2013) Study on phenology stages of cultivated persian shallot (Allium hirtifolium) in Mashhad region. Int J Farm Sci 2:1223–1225
Góralski G, Popielarska-Konieczna M, Ślesak H et al (2005) Organogenesis in endosperm of Actinidia deliciosa cv. Hayward cultured in vitro. Acta Biol Cracoviensia Ser Bot 47
Gui Y, Hong S, Ke S, Skirvin RM (1993) Fruit and vegetative characteristics of endosperm-derived kiwifruit (Actinidia chinensis F) plants. Euphytica 71:57–62
Haider SR, Hossain MR, Rahman S et al (2015) In vitro plantlet regeneration of four local garlic (Allium sativum) accessions of Bangladesh. Br Biotechnol J 8:1–12
Havranek P, Novak FJ (1973) The bud formation in the callus cultures of Allium sativum L. Z für Pflanzenphysiologie 68:308–318
Hehenberger E, Kradolfer D, Köhler C (2012) Endosperm cellularization defines an important developmental transition for embryo development. Development 139:2031–2039
Herben T, Suda J, Klimešová J (2017) Polyploid species rely on vegetative reproduction more than diploids: a re-examination of the old hypothesis. Ann Bot 120:341–349
Hong W, Debergh P (1995) Somatic embryogenesis and plant regeneration in garden leek. Plant Cell Tissue Organ Cult 43:21–28
Hoshino Y, Miyashita T, Thomas TD (2011) In vitro culture of endosperm and its application in plant breeding: approaches to polyploidy breeding. Sci Hortic (Amsterdam) 130:1–8
Hoshino Y, Miyashita T, Thomas TD (2018) In vitro culture of endosperm and its application in plant breeding: Approaches to polyploidy breeding. 1–7
Hourston JE, Pérez M, Gawthrop F et al (2020) The effects of high oxygen partial pressure on vegetable Allium seeds with a short shelf-life. Planta 251:1–9
Husband BC (2004) The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol J Linn Soc 82:537–546
Ismail S, Jalilian FA, Talebpour AH et al (2013) Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium Boiss. Biomed Res Int 2013
Izabel Mikovski A, Tayane da Silva N, Aline Simões Silva L et al (2021) From endosperm to triploid plants: a stepwise characterization of the de novo shoot organogenesis and morpho-agronomic aspects of an ornamental passion fruit (Passiflora foetida L). Plant Cell, Tissue Organ Cult. https://doi.org/10.1007/s11240-021-02120-4
Jinous A, Bahareh G (2012) Pharmacologic and medicinal properties of Allium Hirtifolium Boiss. Afr J Pharm Pharmacol 6:1809–1814
Karthikkumar V, Anbu S, Rajasekar P (2020) Beneficial biological role of Allium hirtifolium on various diseases. Res J Pharm Technol 13:1009–1014
Khorasani M, Mehrvarz SS, Zarre S (2018) Scape anatomy and its systematic importance in the Allium stipitatum species complex (Amaryllidaceae). Nord J Bot 36. https://doi.org/10.1111/njb.02008
Kim K, Jang Y, Nam S et al (2006) Multiple shoot regeneration and bulblet formation through meristem culture of garlic (Allium sativum L.)’Godang’. Korean J Hortic Sci Technol 24:37–42
Kusterer J, Vogt A, Keusgen M (2010) Isolation and identification of a new cysteine sulfoxide and volatile sulfur compounds from Allium Subgenus Melanocrommyum. J Agric Food Chem 58:520–526
Lafon-Placette C, Köhler C (2014) Embryo and endosperm, partners in seed development. Curr Opin Plant Biol 17:64–69. https://doi.org/10.1016/j.pbi.2013.11.008
Lewis DH (1984) Storage carbohydrates in vascular plants: distribution, physiology and metabolism. CUP Archive
Mahboubi M, Kazempour N (2015) The anti-dermatophyte activity of Allium Hirtifolium Boiss aqueous extract. J Mycol Med 25:e10–e14
Marlin M, Handajani M, Yulian Y et al (2021) Induction of Plantlet Regeneration on Shallot (Allium cepa var. Aggregatum). Proc Int Semin Promot Local Resour Sustain Agric Dev (ISPLRSAD 2020) 13:239–244. https://doi.org/10.2991/absr.k.210609.038
Miyashita T, Ohashi T, Shibata F et al (2009) Plant regeneration with maintenance of the endosperm ploidy level by endosperm culture in Lonicera caerulea var. emphyllocalyx. Plant Cell Tissue Organ Cult 98:291–301. https://doi.org/10.1007/s11240-009-9562-6
Muñoz-Concha D (2016) Culture of triploid tissue from the endosperm of an endangered Chilean tree species gomortega keule. J Hortic Sci Biotechnol 91:80–87. https://doi.org/10.1080/14620316.2015.1110995
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Naing AH, Jeon SM, Han JS et al (2014) Factors influencing in vitro shoot regeneration from leaf segments of Chrysanthemum. Comptes Rendus - Biol 337:383–390. https://doi.org/10.1016/j.crvi.2014.03.005
Nakano A, Mii M, Hoshino Y (2021) Simultaneous production of triploid and hexaploid plants by endosperm culture with colchicine treatment in diploid Haemanthus albiflos. Plant Cell Tissue Organ Cult 144:661–669. https://doi.org/10.1007/s11240-020-01974-4
Navarro L, Aleza P, Cuenca J et al (2015) The mandarin triploid breeding program in Spain. Acta Hortic 1065:389–396
Neshati F, Fritsch RM (2009) Seed characters and testa sculptures of some Iranian Allium L. species (Alliaceae). Feddes Repert 120:322–332
O’Donnell G, Poeschl R, Zimhony O et al (2009) Bioactive pyridine-N-oxide disulfides from Allium Stipitatum. J Nat Prod 72:360–365
Ohad NIR, Margossian L, Hsu Y-C et al (1996) A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci 93:5319–5324
Otto SP (2007) The evolutionary consequences of polyploidy. Cell 131:452–462
Panahandeh J, Farhadi N (2020) Haploid induction via in Vitro Gynogenesis in Persian Shallot (Allium hirtifolium). J Hortic Res 27:91–98. https://doi.org/10.2478/johr-2019-0017
Panahandeh J, Mahna N (2011) The karyomorphology of Allium Hirtifolium Bioss., a less known edible species from Iran. J Plant Physiol Breed 1:53–57
Panahandeh J, Farhadi N, Motallebi-Azar AR, Alizadeh S (2015) Improved in vitro culture and multiplication of different Allium hirtifolium Bioss. ecotypes. In: VII International Symposium on Edible Alliaceae 1143. pp 105–110
Peighambardoust SH, Dadpour MR, Dokouhaki M (2010) Application of epifluorescence light microscopy (EFLM) to study the microstructure of wheat dough: a comparison with confocal scanning laser microscopy (CSLM) technique. J Cereal Sci 51:21–27. https://doi.org/10.1016/j.jcs.2009.09.002
Prakash MG, Gurumurthi K (2009) Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 100:13. https://doi.org/10.1007/s11240-009-9611-1
Rangan P (2020) Endosperm variability: from endoreduplication within a seed to higher ploidy across species, and its competence. Seed Sci Res 30:173–185. https://doi.org/10.1017/S0960258520000148
Razdan Tiku A, Razdan MK, Raina SN (2014) Production of triploid plants from endosperm cultures of Phlox drummondii. Biol Plant 58:153–158. https://doi.org/10.1007/s10535-013-0372-7
Rineksane IA, Budiawan R (2017) In vitro sterilization and shoot induction of fig (Ficus carica L.) using MS containing GA3 medium Ssupplemented with BAP and NAA. In: Taufik T, Prabasari I, Rineksane IA, (eds) ICoSI 2014, Proceedings of the 2nd International Conference on Sustainable Innovation. Springer Singapore
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243:281–296
Shigyo M, Khar A, Abdelrahman M (2018) The allium genomes. Springer
Sliwinska E, Bewley JD (2013) Overview of seed development, anatomy and morphology. Seeds Ecol Regen Plant Communities 1–17. https://doi.org/10.1079/9781780641836.0001
Soltis DE, Visger CJ, Soltis PS (2014) The polyploidy revolution then… and now: Stebbins revisited. Am J Bot 101:1057–1078
Sun DQ, Lu XH, Liang GL et al (2011) Production of triploid plants of papaya by endosperm culture. Plant Cell Tissue Organ Cult 104:23–29. https://doi.org/10.1007/s11240-010-9795-4
Tate JA, Soltis DE, Soltis PS (2005) Polyploidy in plants. The evolution of the genome. Elsevier, pp 371–426
Te Beest M, Le Roux JJ, Richardson DM et al (2012) The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot 109:19–45
Thammina C, He M, Lu L et al (2011) In vitro regeneration of triploid plants of euonymus alatus compactus (burning bush) from endosperm tissues. HortScience 46:1141–1147. https://doi.org/10.21273/hortsci.46.8.1141
Thomas, Chaturvedi R (2008) Endosperm culture: a novel method for triploid plant production. Plant Cell Tissue Organ Cult 93:1–14. https://doi.org/10.1007/s11240-008-9336-6
Tomaszewska P, Kosina R (2018) Instability of endosperm development in amphiploids and their parental species in the Genus Avena L. Plant Cell Rep 37:1145–1158
Tubić L, Zdravković-Korać S, MITIĆ N et al (2011) Plant regeneration from transverse stalk sections of chive plants. Rom Biotechnol Lett 16
Van Thang B, Van Viet N, Nam VQ et al (2018) Triploid plant regeneration from immature endosperms of Melia Azedazach. Plant Cell Tissue Organ Cult 133:351–357. https://doi.org/10.1007/s11240-018-1387-8
Wang W, Zhao X, Zhuang G et al (2008) Simple hormonal regulation of somatic embryogenesis and/or shoot organogenesis in caryopsis cultures of Pogonatherum paniceum (Poaceae). Plant Cell Tissue Organ Cult 95:57–67. https://doi.org/10.1007/s11240-008-9414-9
Wang X, Cheng ZMM, Zhi S, Xu F (2016) Breeding triploid plants: a review. Czech J Genet Plant Breed 52:41–54. https://doi.org/10.17221/151/2015-CJGPB
Xu Z, Um Y-C, Kim C-H et al (2008) Effect of plant growth regulators, temperature and sucrose on shoot proliferation from the stem disc of Chinese jiaotou (Allium chinense) and in vitro bulblet formation. Acta Physiol Plant 30:521–528
Yan MM, Xu C, Kim CH et al (2009) Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense). Sci Hortic (Amsterdam) 123:124–128. https://doi.org/10.1016/j.scienta.2009.07.021
ThomasTD, Bhatnagar AK, Bhojwani SS (2000) Production of triploid plants of mulberry(Morus alba L) by endosperm culture. Plant Cell Rep 19:395–399. https://doi.org/10.1007/s002990050746
Acknowledgements
This work is based upon research funded by Iran National Science Foundation (INSF) under project No 99027060. We would like to thanks Jaroslav Dolezel from the Laboratory of Molecular Cytogenetics and Cytometry, Institute of Experimental Botany, Sokolovska´ 6, CZ-77200 Olomouc, Czech Republic, for sending standard references seeds.
Funding
This project was funded by University of Tabriz, as the PhD. thesis of first author.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Communicated by Maria Antonietta Germanà.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jahanian, A., Motallebi-Azar, A., Panahandeh, J. et al. Evidence of the change in ploidy levels in the plantlets established from endosperm culture of Persian shallot (Allium hirtifolium). Plant Cell Tiss Organ Cult 156, 78 (2024). https://doi.org/10.1007/s11240-024-02694-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02694-9