Abstract
Plantago sinaica is a rare perennial shrub near-endemic to Egypt and found in Saint Katherine Protectorate in Sinai. The first successful in vitro propagation protocol was conducted to protect the plant outside its natural reserves. Shoot tip, stem node section, cotyledonary node, and root explants separated from in vitro germinated seedlings were cultured in vitro on Murashige and Skoog (MS) medium enriched with different concentrations and types of cytokinins. It was found that 6-benzyl adenine (BA) is the most efficient cytokinin. MS medium containing 3.33 µM BA and 0.54 µM α-naphthalene acetic acids (NAA) produced 10.25 and 11.30 shoots/explant using shoot tip and stem node section, respectively. Conversely, MS medium + 2.22 µM BA + 0.54 µM NAA produced 13.25 shoots from root explants. Surprisingly, the cotyledonary node explants favored MS medium free from plant growth regulators (PGRs), which produced only 4.25 shoots/explant. The multiplied shoots were rooted successfully with a 100% rooting percentage on half MS medium containing 1.23 or 2.46 µM indole-3-butyric acid (IBA). In vitro, rooted plantlets were efficiently transferred to the greenhouse with a 90% survivability. Finally, the plant was identified using three DNA barcodes; 1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL), plastid photosystem II protein D1 intergenic spacer region (psbA–trnH), and Internal Transcribed Spacer (ITS) barcodes. Additionally, psbA–trnH and ITS were novel and submitted to the GenBank databases for the first time for Plantago sinaica. Our study supports the United Nations Sustainable Development Goal number 15, which is to preserve, restore and reinstate sustainable usage of terrestrial ecosystems and to stop biodiversity loss.
Key message
This is the first report in in vitro propagation of the rare Plantago sinaica (Barnéoud) and molecular identification of the plant by three DNA barcodes with two newly published barcodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Sinai Peninsula is a triangle-shaped peninsula that serves as a land bridge connecting Asia and Africa (Grainger 2003; Rabei et al. 2021). It comprises approximately 6.0% of Egypt's surface, as it is abundant in both the species number and high proportion of endemics, as it has 1,262 taxa (Boulos 2009; Rabei et al. 2021). Saint Katherine Protectorate (SKP), with its unique mountainous nature in the Sinai Peninsula, is home to the most distinctive flora in Sinai due to its abundance of various Middle Eastern floristic sites (Boulos 2009; Shaltout et al. 2021). It hosts a significant number of endemic species as it has 17 endemic taxa (Ghaly 2015). Six are categorized as endangered or critically endangered (Ghaly 2015; Hosni et al. 2013). The term endemic refers to species that are restricted to a particular geographical region and are typically rare; therefore, they require special conservation efforts (Fattorini 2017).
In Egypt, the Plantaginaceae family consists only of the genus Plantago, which includes twenty species, including Plantago sinaica (Barnéoud) Decne. Approximately 275 species of the Plantago genus are dispersed worldwide (Piyaviriyakul et al. 2017). Plantago species are widely used in modern and traditional medicine due to their pharmacological properties, which include immune enhancing, hepatoprotective, antiulcerogenic, antidiarrheal, free radical scavenging properties, anticancer, cytotoxic activity, hematopoietic, wound healing, anti-inflammatory and anti-fatigue effects (Nazarizadeh et al. 2013; Alsaraf et al. 2019; Soliman et al. 2022). They are also highly valued in the pharmaceutical and cosmetic industries due to their ability to effectively lower high cholesterol and diabetes. Moreover, they are used to improve intestinal performance, prevent colon cancer, and as vegetables in health-promoting dietary ingredients for salads, teas, yogurts, soups, and infant food (Goncalves and Romano 2015; Farcaș et al. 2019; Mohsenzadeh et al. 2020). Plantago sinaica extract inhibits the development of Colletotrichum gloeosporioides in vitro and aids in the anthracnose development of mango fruits inoculated artificially (Baka and Mousa 2020).
Genus Plantago contains phenols, flavonoids, tannins, polysaccharides, amino acids, lipids, terpenoids, iridoid glycosides, caffeic acid derivatives, verbascoside, isoverbascoside, orobanchoside, aucubin, majoroside, geniposidic acid and sorbitol (Golec and Makowczynska 2008; Nazarizadeh et al. 2013; Haddadian et al. 2014).
Plantago sinaica is a perennial rare, densely glandular shrublet with opposite leaves on a well-developed stem, abundantly branched, leaves 1–5 × 0.1–0.3 cm with united, clasping bases, linear, subacute; peduncles (1-) 2–6 (-10) cm. Sepals are 3.5–5 × 1.5–3 mm, ± obliquely ovate, with broad scarious margins; anterior flat; posterior unequal, one lateral, cymbiform, one behind the flower, flat. The corolla is glabrous, its lobes measure 2.2–2.5 × 0.9–1.3 mm, and the capsule contains two seeds (Boulos 2002).
Plantago sinaica plant is near-endemic to Egypt, found in Saint Katherine Protectorate in southern Sinai. It is prevalent as plantain (differs from edible plantain). Although the International Union for Conservation of Nature (IUCN) assessment of Plantago sinaica is unavailable, Boulos (2009) listed it as a scarce plant. However, the survey by Rabei et al. (2021) reported this species is uncommon. Concerning the plant endemism in Egypt, Boulos (2009) categorized the plant as endemic to Egypt, whereas many researchers recorded it in Jordan and Palestine and excluded it from endemics (Hosni et al. 2013; Abdelaal et al. 2018; EL-Khalafy et al. 2021; Rabei et al. 2021). Therefore, Plantago sinaica is categorized now as near-endemic to Egypt. Plantago sinaica is endangered by biotic and abiotic factors, which contribute to its rarity and endemism, and its conservation must be given higher priority (Rabei et al. 2021). Abundantly, the endemic species in Sinai have been subjected to drought and the detrimental effects of overgrazing, overharvesting, tourism, and settlement expansion. Consequently, their risk of extinction ranges from vulnerable to critically endangered (Moustafa et al. 2015). In situ (within the ecosystem) and ex-situ (outside the ecosystem) conservation efforts for medicinal, rare, and endemic plants must vary (the ecosystem) or ex-situ (outside their habitat). In vitro propagation, botanical gardens, artificial seed propagation, and seed storage are examples of ex-situ conservation ways (Heywood 2014).
Biotechnological methods using plant micropropagation are a valuable and effective tool for rapidly propagating and producing large numbers of true-to-type endangered plant copies (Choudhary et al. 2020).
Some Plantago species have succeeded in vitro culture, such as P. major (Mederos et al. 1997), P. asiatica (Makowczynska and Golec 2003), P. lanceolata (Budzianowska et al. 2004), P. camtschatica (Golec and Makowczynska 2008), P. maritima (Makowczynska and Golec 2009), P. ovata (Sharma et al. 2017) and P. lanceolata (Rahamooz-Haghighi et al. 2020).
DNA barcoding is another tool for the ex-situ conservation of endemic and rare plant species. It is identified as utilizing short DNA sequences, also known as the DNA barcode, to identify species by assigning individuals to known taxa by comparing their barcode sequences with a reference library. It has emerged as a powerful tool for identifying traditional medicine, differentiating between species, discovering ambiguous species, as well as protecting and conserving endangered species (Hebert et al. 2003; Kress et al. 2005; Chen et al. 2010; Techen et al. 2014; Hashim et al. 2021). The Consortium for the Barcode of Life (CBOL) has proposed two plastid genes, ribulose-1, 5-bisphosphate carboxylase/oxygenase large subunit (rbcL), and maturase K (matK) as the core universal barcode for land plants (CBOL Plant Working Group 2009). More plant DNA barcodes have been suggested, some of which belong to the nucleus and plastid genome as non-coding spacers such as the nuclear Internal Transcribed Spacer (ITS) and the plastid photosystem II protein D1 intergenic spacer region (psbA–trnH), which are two of the leading candidates (Li et al. 2011; Tripathi et al. 2013; Loera-Sánchez et al. 2020). It was proposed that the ITS2 barcode, in addition to psbA–trnH as a complementary barcode, may serve as a universal barcode for the identification of medicinal plants (Chen et al. 2010; Sun and Chen 2013).
This work aims to optimize a protocol for the in vitro propagation of the rare and near-endemic Plantago sinaica plant for the first time. In addition, add novel barcodes to the universal databases for plant identification, which will increase the ability to differentiate between species as well as different landraces for the same species.
Materials and methods
Samples collection
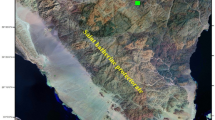
Leaf samples and seeds of Plantago sinaica plants have been collected from shrubs grown in their natural habitats at Elgabal Elahmar, Saint Katherine, Southern Sinai (N: 28.53063, E: 33.95937, Alt: 2032), as shown in Figs. 1and 2. They were identified by Dr. Ibrahim Abdelrafee El Gamal, Nature Conservation Sector, Egyptian Environmental Affairs Agency, Southern Sinai, Egypt, and Dr. Omran Ghaly, Plant Taxonomy Unit head, Desert Research Center, Egypt. Target plant species was identified based on Boulos (2002), then collected samples were compared with well-identified herbarium specimens kept in Saint Katherine Protectorate and Desert Research Center herbaria. Specimens were placed in the Desert Research Center Herbarium with the voucher number CAIH-1008-R. The taxonomic name was compared to Kew's Plants of the World Online (POWO 2023) and International Plant Names Index (https://www.ipni.org/).
In vitro propagation of Plantago sinaica
In vitro seed germination and explant culture of Plantago sinaica
Collected seeds of Plantago sinaica were cleaned with running tap water and detergent. Then they were disinfected by soaking in commercial bleach containing sodium hypochlorite (NaOCl) (5.25%), at different concentrations (0.8, 1.0, and 1.5% sodium hypochlorite solution) with two drops of Tween-20 per 100 mL for 15 min under a laminar airflow cabinet (Holten LaminAir HVR 2448, USA), then washed four times in distilled sterilized water. Sterilized seeds were aseptically transferred to half-strength Murashige and Skoog (½ MS) (Murashige and Skoog 1962) medium containing 3% (w/v) sucrose. The medium pH was adjusted at 5.7 ± 0.1, then 0.3% (w/v) phytagel (Duchefa, Haarlem, the Netherlands) was used for medium solidification before autoclaving at a pressure of 1.06 kg/cm, and 121 ºC for 20 min. The medium was transferred to jars. Each jar holds approximately 50 mL of the medium.
For a day-night period of 16-h photoperiod, cultures were saved under cool fluorescent light with the light intensity of 2500–3000 lx (F140t9d/38, Toshiba) at a stable temperature of 26 ± 2 °C and 60–70% relative humidity. The germination percentage was determined as the number of germinated seeds divided by the total number of inoculated seeds. Germinated seedlings were divided into shoot tips, stem node sections, cotyledonary node explants (hypocotyl with cotyledons), and roots. All of them were used as explants for Plantago sinaica plant propagation.
Effect of different types and concentrations of cytokinins on the in vitro propagation of Plantago sinaica using different types of explants
For the in vitro propagation of the Plantago sinaica plant, shoot tips, stem node sections, cotyledonary nodes, and roots were harvested from four-week-old in vitro germinated sterile seedlings. The explants were inoculated aseptically onto MS basal medium enriched with 3% (w /v) sucrose and solidified with 0.3% phytagel (w /v) and Kinetin (Kin) (1.16, 2.32, and 3.48 µM), 6–benzyl adenine (BA) (1.11, 2.22 and 3.33 µM) or N6-(2-isopentenyl) adenine (2iP) (1.23, 2.46 and 3.69 µM) added individually to the media. Full-strength MS medium without PGRs was used as a control.
The pH of the medium was adjusted, autoclaving and all cultures were incubated as described in the previous experiment. After eight weeks of culture, the percentage (%) of explants forming growth, the average number, and the length of produced shoots per explant were calculated.
Effect of cytokinin and auxin combination on the in vitro propagation of Plantago sinaica using different types of explants
Explants of the same types (shoot tip, stem node section, cotyledonary node, and root) were taken from four-week-old in vitro germinated seedlings of Plantago sinaica to examine the effect of cytokinin and auxin combination on the in vitro propagation of Plantago sinaica plant. The explants were cultured aseptically onto MS basal medium enriched with 3% (w/v) sucrose and solidified with 0.3% (w/v) phytagel and the same concentrations of BA (1.11, 2.22, and 3.33 µM) in addition to different concentrations of α-naphthalene acetic acid (NAA) (0.54 and 2.69 µM). Basal MS medium without plant growth regulators (PGRs) was used as control. The pH of the medium was adjusted at 5.7 ± 0.1. All media were autoclaved at a pressure of 1.06 kg/cm and 121 ºC for 15 min. Cultures were incubated under cool fluorescent light at a day-night period of 16-h photoperiod with a light intensity of 2500–3000 lx.
After eight weeks of culture, the percentage (%) of explants producing growth, the average number of shoots, and the length (cm) of the shoots were measured. Each jar containing these explants was inoculated with 50 ml of medium.
In vitro root induction
Individually excised shoots (7–8 cm) and transferred to half-strength MS media enriched with two forms of auxins, indole-3-butyric acid (IBA) at 1.23, 2.46, 4.96, and 9.80 µM or NAA at 1.35, 2.69, 5.37 and 10.74 µM, each individually. Half-strength MS medium without PGRs was used as control. After six weeks, the rooting percentage, the mean number of roots per shoot, and the length of root induced from the shoot were measured.
Acclimatization of plantlets
Rooted plantlets were removed gently from culture vessels and washed with water to remove the adhered gelling agent, then placed on pots containing sand, vermiculite, and peat moss (1: 1:1) under greenhouse conditions. The pots were covered with transparent plastic bags for three weeks to provide humidity before exposure to natural light to ensure hardening. The plantlets were watered twice a week for three months, then transplanted into soil.
Experimental design and statistical data analysis
The design of all experiments was completely randomized and included 15 jars (with four explants) per treatment. The experiments were repeated three times.
Analysis of variance (ANOVA) and Duncan's multiple range test (Duncan 1955), as modified by Snedecor and Cochran (1990), were used to evaluate the recorded data. Means followed by the same letter are not significantly different at P ≤ 0.05.
Molecular identification of Plantago sinaica by DNA barcode analysis
Genomic DNA isolation and PCR amplification
DNA extraction
The genomic DNA of Plantago sinaica was extracted from silica-gel dried leaves (100 mg) using CTAB protocol with minor modifications. The DNA concentration was quantified using NanoDrop Spectrophotometer (Thermo Fisher Scientific Inc.). For DNA barcoding PCR amplification, the DNA concentration was increased to 10 ng/µl.
DNA barcodes PCR analysis
PCR amplification was carried out for the three barcode loci; two belong to the plastid genome, the (rbcL and psbA–trnH) barcodes, and one belongs to the nuclear genome for the rDNA (ITS). The PCR was carried out in a Thermal Cycler TC-TE BOE 8,089,602 (BOECO, Germany). Table 1 shows the three primer pairs that were used. The PCR reactions were adjusted to 50 µl total reaction volume, containing approximately 50 ng genomic DNA, ThermoScientific DreamTaq Green PCR Master Mix (2X), 200 Reactions (Catalog No: K1081), 1.5 mL of each primer (2.5 mM), and deionized distilled water. The PCR reaction conditions were set as follows: initial denaturation at 95 °C for 4 min, 35 cycles at 94 °C for 30 s, 57 °C for 30 s, 72 °C for 50 s, and 72 °C for 10 min.
The obtained PCR amplicons were resolved on 0.5 mg/mL EB containing agarose gels of 1.5%, and gel electrophoresis was performed in 1X TAE buffer. GeneRuler 1 Kb plus DNA ladder (Thermo Scientific Catalog number: SM1322) was utilized to determine molecular size. The PCR-produced products were examined using a UV transilluminator. Gel purification of the bands with the appropriate molecular size from agarose gel was carried out using Gene JET Gel Extraction Kit, Thermo Scientific Catalog number: K0691. The purified products were sent directly for sequencing to the Macrogen lab (Seoul, South Korea).
Sequence data analysis
The sequences of the three barcodes (rbcL, psbA–trnH, and ITS) were subjected to the removal of the peripheral noisy parts at 3' and 5', then aligned using Basic Local Alignment Search Tool nucleotide (BLASTn) available in BLAST of the National Centre of Biotechnology Information (NCBI) database against other previously submitted sequences using default parameters. Finally, the sequences were assigned accession numbers after being submitted to the GenBank databases. The Phylogenetic tree was constructed using the free online tool Clust Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Results
In vitro propagation of Plantago sinaica
Seed germination and in vitro culture
In this study, eighty five percent of of Plantago sinaica seeds was survived when sterilized with 0.8% (w/v) sodium hypochlorite solution for a period of 15 min and successfully germinated on ½ MS medium without PGRs. Seeds were germinated after 7–10 days of culture (Fig. 3a). The shoot tip, stem node section, cotyledonary node, and root of 4-week-old seedlings were excised and cultured on MS medium enriched with different concentrations and combinations of auxin and cytokinins.
In vitro propagation of Plantago sinaica plant using four types of explants obtained from 4 weeks old in vitro germinated seedlings. (a) In vitro seed germination on ½ MS medium. (b) Proliferation from shoot tip explants on MS medium containing 3.33 µM BA + 0.54 µM NAA. (c) Proliferation from stem node section explants on MS medium containing 3.33 µM BA + 0.54 µM NAA. (d) Proliferation from cotyledonary node explants on MS medium (control). (e) Proliferation from root explants on MS medium containing 2.22 µM BA + 0.54 µM NAA
Impact of different types of cytokinins on the success of the in vitro propagation of Plantago sinaica using different explant types
In the present study, the effect of supplementing the culture media with various cytokinins (Kin, BA, and 2iP) at low concentrations was studied on the in vitro proliferation of Plantago sinaica from seedling parts (shoot tip, stem node section, cotyledonary node, and root).
Tables 2 and 3 show that the growth induction percentage increased with increasing Kin or 2iP concentrations in the shoot tip, stem node section, and root explants. The highest growth percentage (100%) was recorded for the shoot tip (Table 2) and root explant (Table 3) with all BA concentrations (The growth rate exceeded that of the control by 25% for shoot tip and 70% for root explants). With concentrations of 2.22 and 3.33 µM of BA, the same percentage (100%) was also observed in the stem node section, and this rate is higher than the control by approximately 17%. Regarding the cotyledonary node explants, the growth induction percentage showed 100% in all tested media.
Concerning the mean number and length of shoots, both increased with the increase in the concentration of each cytokinin with three types of explants; shoot tip, stem node section (Table 2), and root explants (Table 3). However, it is not applicable for the cotyledonary node explants, which favors the control medium to produce 3.55 shoots with a 2.0 cm length of the shoot (Table 3). Additionally, BA was the most promising cytokinin in Plantago sinaica proliferation using shoot tips, stem node sections, and root explants compared to Kin and 2iP. The most effective concentration of BA among the three concentrations tested was 3.33 µM. It displayed the highest mean number and length of shoots for the shoot tip, stem node section, and root explants. There are 9.33, 9.75, and 8 shoots per explant, with shoot lengths of 5.52, 6.7, and 3.2 cm for shoot tip, stem node section, and root explants, respectively.
It was found that BA at 3.33 µM was the most effective cytokinin than Kin and 2iP for shoot proliferation of Plantago sinaica plant with all tested explants (shoot tip, stem node section, and root explants) excised from plant seedlings, with the exception of the cotyledonary node explants which prefer the medium without PGRs.
Impact of BA and NAA combinations on in vitro propagation of Plantago sinaica using different types of explants
In this study, shoot tip, stem node section, cotyledonary node explants, and roots excised from the plant seedlings were inoculated in full MS medium enriched with different concentrations of BA (1.11, 2.22, and 3.33 µM) in combinations with 0.54 or 2.69 µM NAA to study the effect of cytokinin-auxin combinations on shoot proliferation of Plantago sinaica. According to the results in Table 4 and 5, all tested explants showed a variance response on the in vitro propagation of the Plantago sinaica plant. For shoot tip and stem node section explants, all tested MS media enriched with different concentrations and combinations of BA, and NAA gave a 100% growth induction percentage except the control medium, which gave the lowest growth induction percentage (25%) for shoot tip and 35% for stem node section (Table 4). It means that, the growth induction percentage with the control medium is lower than the the other tested media by 75% for shoot tip explants and 65% for stem node sections. Based on the results in Table 4, MS medium enriched with 3.33 µM BA + 0.54 µM NAA was optimal for shoot proliferation in Plantago sinaica using the shoot tip (Fig. 3b) and stem node section (Fig. 3c). It yielded the highest mean number of shoots (10.25) with 6.9 cm length and 11.3 shoots with 7.3 cm length for shoot tip and stem node sections, respectively. Moreover, the mean number of shoots and the mean length of shoots increased with the increase in BA concentrations for the shoot tip and stem node section when 0.54 µM NAA was used. While using 2.69 µM, NAA with the same concentrations of BA showed decreasing in both the number and length of shoots as the concentration of BA increased. Consequently, MS media enriched with 3.33 µM of BA plus low concentrations of NAA (0.54 µM) was more effective in stimulating shoot formation from shoot tip and stem node section explants than media containing a higher concentration of NAA (2.69 µM) (Table 4).
Table 5 and Fig. 3d show that all tested media yielded a 100% growth induction percentage for cotyledonary node explants. However, MS medium without any PGRs proved optimum for shoot proliferation, producing 4.25 shoots per explant and giving the greatest mean shoot length per explant (2.5 cm). However, the mean shoot length did not differ significantly among the all-tested media.
When fresh and viable roots excised from Plantago sinaica seedlings were used as explant for producing shoots, only the concentration of 0.54 µM NAA in combination with different concentrations of BA (1.11, 2.22, and 3.33 µM) stimulated 100% growth induction (more than control medium by 75%). The concentration of 2.22 µM BA with 0.54 µM NAA was superior in shoot formation (13.75) with a mean length of shoots of 6.40 cm (Table 5 and Fig. 3e).
It was found that the best explant for Plantago sinaica proliferation is the root explants obtained from seed-derived plantlets, which gave 13.25 shoots per explant, then stem node section, which produce 11.3 shoots per explant, then shoot tip explants which produce 10.25 shoots and finally, the cotyledonary nodes were in the last with 4.25 shoots per explant. Moreover, the low concentration of NAA (0.54 µM) was more effective in Plantago sinaica proliferation than 2.69 µM in combination with BA as a cytokinin.
In vitro root induction and acclimatization of plantlets
The multiplied shoots of Plantago sinaica were transferred to half-strength MS medium containing 1.23, 2.46, 4.96, and 9.80 M of IBA or 1.35, 2.46, 5.37, and 10.74 µM of NAA in order to promote the formation of microshoot roots.
Depending on the concentration of IBA and NAA, the rooting percentage, the mean number of roots per each shoot, and the length of roots varied significantly (Table 6 and Fig. 4a and b). By comparing IBA and NAA, IBA was more promising than NAA in inducing roots. Table 6 illustrates that MS media enriched with 1.23 or 2.46 µM IBA was more suitable for inducing roots. After six weeks of culture, both exhibited the highest rooting percentage (100%). They also produced the most significant number of roots per shoot. MS medium enriched with 2.46 µM IBA gave a 24.0 mean number of roots per shoot, while MS medium enriched with 1.23 µM IBA demonstrated a 22.75 mean number of shoots. Differences between the two concentrations of IBA were insignificant in terms of the mean number of roots. Although the medium contains 1.23 µM, IBA demonstrated the most significant mean length of root formed per explant. It was also observed that high concentrations of IBA (4.96 and 9.80 µM) lead to decreasing the rooting percentage (60%) and also decreasing in length and the mean number of roots formed per shoot.
In vitro rooting and acclimatization of Plantago sinaica plantlets; (a) In vitro rooting on half-strength MS basal medium with IBA at 2.46 µM. (b) Preparation of plantlets for acclimatization. (c) Acclimatization of the in vitro rooted plantlets under plastic bags. (d) The acclimatized plants after 30 days in the greenhouse. (e) Acclimatized plants in open light after five months of growth
Rooted microshoots were successfully transplanted into plastic pots accommodating sand, vermiculite, and peat moss (1: 1:1) covered by transparent plastic bags (Fig. 4c) under the greenhouse conditions. After 21 days, the plastic bags were gradually removed (Fig. 4d), and the fully hardened plants were finally transferred to open light (Fig. 4e) with 90% success.
Molecular identification of Plantago sinaica by DNA barcode analysis
The expected product molecular sizes of the primer pairs for the three barcodes rbcL, psbA–trnH, and ITS were approximately 600 bp, 340 bp, and 800 bp, respectively. Sequences were subjected to the GenBank database and were given the following accession numbers successively ON854978, OP295408, and ON844118.1.
The obtained sequences were identified using the BLASTn tool available at the National Centre of Biotechnology Information (NCBI). DNA barcode analysis was carried out to identify and classify the rare near endemic Plantago sinaica plant for the purpose of conservation. The BLASTn results and the obtained phylogenetic trees analysis of Plantago sinaica are depicted in Table 7, 8, and 9 & Figs. 5, 6, and 7. Only the plant species with identity percentages of 98% or higher were considered. The query coverage for the alignments of of rbcL, psbA–trnH, and ITS sequences against GenBank accessions ranged from 95 to 100%, 90 to 98%, and 100%, respectively (Table 7, 8, and 9).
Sequence alignment analysis of the barcode rbcL has revealed 100% identification of Plantago sinaica to the genus and species levels. In contrast, psbA–trnH and ITS barcode sequences have only revealed 100% identification at the level of the Plantago genus (Figs. 5, 6, and 7).
Discussion
In vitro propagation of Plantago sinaica
Four types of explants (shoot tip, stem node section, cotyledonary node, and root) were successfully cultured in vitro on MS medium enriched with different concentrations of Kin, BA, and 2iP to obtain a high rate of shoot bud induction and proliferation in in-vitro propagation of Plantago sinaica. Numerous species of the Plantago genus, including Plantago asiatica (Makowczynska and Golec 2003), Plantago lanceolata (Budzianowska et al. 2004), Plantago camtschatica (Golec and Makowczynska 2008), Plantago maritima (Makowczynska and Golec 2009), and Plantago lanceolata (Rahamooz-Haghighi et al. 2020) are propagated in vitro using seedling explants.
The PGRs are regarded as one of the most significant factors influencing the success of micropropagation of plants. PGRs inhibit the physiological cell responses in vitro, thereby inducing the development of various plant parts (Amoo and Staden 2013; Youssef et al. 2021). Cytokinin is a kind of plant growth regulator essential for plant growth and maturation and is used to enhance the differentiation of cells and division. In the present study, the effect of three cytokinins, Kin at 1.16, 2.32, and 3.48 µM, BA at 1.11, 2.22 and 3.33 µM and 2iP at 1.23, 2.46, and 3.69 µM on the proliferation and multiplication of Plantago sinaica was clarified individually. Of the three tested cytokinins, BA at the three concentrations showed the most optimal response and significant differences in growth induction percentage, the mean number of shoots, and the mean length of shoots observed with all tested explants except the cotyledonary node explants. Furthermore, the optimal concentration of BA 3.33 µM was the optimum for the Plantago sinaica proliferation from stem nodes, shoot tips, and root explants. The efficacy of aromatic BA may be attributable to its greater in vivo stability than other cytokinins, and it is well known that each plant species prefers a particular type and concentration of cytokinin based on the endogenous plant hormone (Kadota and Niimi 2003; Amoo et al. 2014). The superiority of BA over other cytokinins was also observed among other medicinal species such as Capsicum frutescens (Kumar et al. 2007), Withania somnifera (Fatima and Anis 2012), Bacopa monniera (Haque et al. 2017), and Breynia disticha (Abd El-Kafie et al. 2018). It was observed that the cotyledonary node explants exhibited insignificant variations across all tested cytokinins, including BA. These explants thrived best in a PGRs-free MS medium, yielding the highest number of shoots at 3.55. This outcome might be attributed to the inherent auxin levels present in the cotyledonary node explants. Such endogenous auxin content potentially increases shoot formation with the control medium and inhibits shoot organogenesis in other tested media, likely due to the interactions between the natural auxins and varying levels of externally applied PGRs present in other media formulations (Hu et al. 2017; Mwaniki et al. 2019).
Merging the auxin and cytokinin in MS medium inducing a cross-talk controls the formation of shoot meristems responsible for whole plant establishment (Ying-Hua et al. 2011). BA is essential for bud breaking, but an augmentation of the low amount of auxin with BA increased the shoot multiplication rate (Choudhary et al. 2020). In the present study, augmenting the BA-enriched full MS medium with two concentrations of NAA, a considerable enhancement was observed in both the number and length of shoots formed per explant for shoot tip, stem nodes and root explants. The optimum concentration for shoot proliferation from shoot tip and stem nodes was MS medium enriched with 0.54 µM NAA along with 3.33 µM BA while MS medium plus 0.54 µM NAA along with 2.22 µM BA was more suitable for shoot proliferation from root explants. It is clear from the results that the interaction between NAA and BA was important in controlling the developmental processes responsible for increasing the mean number and length of shoots. The synergistic effect of auxin and cytokinin in shoot proliferation was also reported on Plantago asiatica (Makowczynska and Golec 2003), Plantago camtschatica (Golec and Makowczynska 2008), and Plantago maritima (Makowczynska and Golec 2009).
The capability of the in vitro multiplied shoots to form roots is based on the synergy between endogenous and exogenous factors. Auxins plays an essential role in root development (Sharma et al. 2010).
In this study, the regenerated microshoots of Plantago Sinaica were forced to form roots in vitro in half MS medium enriched with various concentrations of NAA or IBA. Out of different auxins, IBA is a stable auxin with a low rate of oxidative and is widely used in clonal propagation due to its efficiency in regulating in vitro root formation by converting it into IAA (Bai et al. 2020). Half-strength MS medium enriched with 1.23 or 2.46 µM IBA was proved to be optimal for root formation in micropropagated shoots of Plantago sinaica. Each of the two concentrations resulted in a 100% rooting percentage with the highest mean number of roots per shoot. The highest mean length of roots was found in both mediums containing 1.23 µM IBA and the control medium. IBA was also optimal for rooting in Plantago lanceolata since the in vitro multiplied shoots were rooted efficiently on MS medium + 2.46 µM IBA (Rahamooz-Haghighi et al. 2020). Regarding other Plantago species, different responses were observed on rooting. For example, MS medium enriched with 0.5 µM of NAA and MS without auxin was the best media for rooting Plantago camtschatica (Golec and Makowczynska 2008). MS medium + 1.0 µM NAA gave the highest rooting percentage and the highest number and length of roots in Plantago major (Mederos et al. 1997). The optimum medium for rooting Plantago maritima was MS medium enriched with 0.5 µM NAA, which resulted in a 90% rooting percentage (Makowczynska and Golec 2009). In contrast, Plantago lanceolata favors a 100% root induction percentage in MS medium containing 5.7µM IAA (Budzianowska et al. 2004). The fully hardened plantlets in the greenhouse were finally placed in open light with 90% success.
Identification of Plantago sinaica by DNA barcode analysis
To our knowledge, this is the first study of the psbA–trnH and ITS barcodes for the rare near endemic Plantago sinaica plant. The universal tool of DNA barcoding has been integrated with traditional taxonomy tools for identifying and classifying plants, particularly plants that have been poorly studied and native to poorly considered regions (Carneiro de Melo Moura et al. 2019; Pires and Marinoni 2010). DNA barcoding has proven effective in identifying rare and endemic plant species, thereby facilitating evolutionary and ecological research and validating conservation priorities (Hashim et al. 2021; Hosein et al. 2017; Techen et al. 2014).
DNA barcoding has not only been utilized for the identification and/or confirmation of species but also for determining their genetic relationships (Jiang et al. 2022; Hashim et al. 2021). DNA barcoding is a reliable method for identifying different plant species since it relies on a short genetic sequence belonging to a specific genome region. BLASTn and phylogenetic tree analyses of Plantago sinaica using the three barcodes, rbcL, psbA–trnH, and ITS, successfully identified it to the genus level Plantago. In contrast, rbcL only was successful in identifying it to the species level of Plantago sinaica. This is because the psbA–trnH and ITS sequences for Plantago sinaica have not been previously submitted to the GenBank database, confirming the novelty of our submission of these sequences for the first time to the GenBank database. The highest similarity percentages between Plantago sinaica and the other recorded Plantago species ranged between 100% and 98.4. The rbcL barcode sequence showed 100% similarity with the same species, Plantago sinaica (unpublished data). The phylogenetic trees created by the BLASTn for Plantago sinaica using the three barcode markers: rbcL, psbA–trnH, and ITS supported the inclusion of Plantago sinaica in the genus Plantago. It was also found that the most closely related species are Plantago sinaica.
Due to the advocacy of the Consortium for the Barcode of Life (CBOL) (CBOL Plant Working Group 2009) plant working group, the rbcL barcode was selected as it is considered a core barcode for plant species ITS and psbA–trnH was chosen as a supplement barcode. In addition, it was determined that the combination of psbA–trnH and ITS performs better or the same comparisons with other combinations in most investigated taxonomic groups (Pang et al. 2012). Phylogenetic relationship studies are most effective when combining nuclear DNA (ncDNA) and chloroplast DNA (cpDNA) to evaluate the evolutionary relationships between and within species (Amandita et al. 2019). The current study results successfully identify Plantago sinaica on both the species and genus levels. Two new significant barcode sequences were introduced for the first time to the GenBank databases, which contributes to the potential of DNA barcoding in providing nucleotide data of the different taxa DNA sequences database (Hosein et al. 2017). This study confirms the potential use of DNA barcode analysis to document endemic endangered species to their adequate taxonomic position.
Conclusion
Protection and prevention of the threatened species' extinction is a top priority of the 2030 Agenda and the Sustainable Development Goals (SDGs). Therefore, in order to save rare and endangered species, especially endemic ones, it is necessary to take prompt and significant action. In vitro propagation and DNA barcoding are considered to be essential tools for protecting endangered species within and beyond their nature reserves.
In conclusion, to our knowledge, this is the first report describing the in vitro propagation protocol and publishing two new DNA barcodes for Plantago sinaica, a near-endemic and rare plant. The in vitro propagation of the plant was conducted using four types of explants excised from in vitro germinated seedlings. Furthermore, the DNA barcode analysis was performed using three DNA barcodes for identifying the plant on the molecular level. As Plantago sinaica is a rare plant, in vitro micropropagation and DNA barcoding are required to protect our intellectual property rights and achieve one of the most significant Sustainable Development Goals related to the conservation and management of endemic and endangered species.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ANOVA:
-
Analysis of variance
- BA:
-
6-Benzyl adenine
- BLAST:
-
Basic local alignment search tool
- CBOL:
-
Consortium for the barcode of life
- cpDNA:
-
Chloroplast DNA
- IBA:
-
Iindole-3-butyric acid
- 2iP:
-
N6-(2-isopentenyl) adenine
- ITS :
-
Internal transcribed spacer
- IUCN:
-
International Union for Conservation of Nature
- Kin:
-
Kinetin
- MS:
-
Murashige and Skoog
- NAA:
-
α– Naphthaleneacetic acid
- NaOCl:
-
Sodium hypochlorite
- NCBI:
-
National Center of Biotechnology Information
- ncDNA:
-
Nuclear DNA
- PGRs:
-
Plant growth regulators
- psbA – trnH :
-
Plastid photosystem II protein D1 intergenic spacer region
- rbcL :
-
Ribulose-1,5- bisphosphate carboxylase/oxygenase large subunit
- SKP:
-
Saint Katherine protectorate
References
Abdelaal M, Fois M, Fenu G, Bacchetta G (2018) Critical checklist of the endemic vascular. Flora of Egypt. Phytotaxa 360:19–34
Abd El-Kafie OM, El-Banna HY, Elsharqawi AA (2018) Effects of Plant Growth Regulators on Frequency Shoot Multiplication of an Important Ornamental and Medicinal Plant, Snowbush (Breynia disticha). J Plant Production 9(6):515–520. https://doi.org/10.21608/jpp.2018.36340
Alsaraf KM, Mohammad MH, Al-Shammari AM, Abbas IS (2019) Selective cytotoxic effect of Plantago lanceolata L. against breast cancer cells. J Egypt Natl Canc Inst 31(1):10. https://doi.org/10.1186/s43046-019-0010-3
Amandita FY, Rembold K, Vornam B, Rahayu S, Siregar IZ, Kreft H, Finkeldey R (2019) DNA barcoding of flowering plants in Sumatra Indonesia. Ecol Evol 9(4):1858–1868
Amoo SO, Staden JV (2013) Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micro propagated Huernia hystrix. Plant Cell Tissue Organ Culture 112:249–256
Amoo SO, Aremu AO, Moyo M, Szucova L, Dolezal K, Staden JV (2014) Physiological effects of a novel aromatic cytokinin analogue in micropropagated Aloe arborescens and Harpagophytum procumbens. Plant Cell Tiss Organ Cult 116:17–26. https://doi.org/10.1007/s11240-013-0377-0
Bai T, Dong Z, Zheng X, Song S, Jiao J, Wang M, Song C (2020) Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front Plant Sci 11:574881. https://doi.org/10.3389/fpls.2020.574881
Baka ZAM, Mousa MMA (2020) In vitro and in vivo, biocontrol activity of extracts prepared from Egyptian indigenous medicinal plants for the management of anthracnose of mango fruits. Archives of Phytopathology and Plant Protectionhttps://doi.org/10.1080/03235408.2020.1794308
Boulos L (2002) Flora of Egypt, vol 3. Al-Hadara Publishing, Cairo
Boulos L (2009) Flora of Egypt checklist. Al-Hadara Publishing, Cairo, Revised annotated edition, p 410
Budzianowska A, Skrzypczak L, Budzianowski J (2004) Phenylethanoid glucosides from in vitro propagated plants and callus cultures of Plantago lanceolata. Planta Med 70:834–840. https://doi.org/10.1055/s2004-827232
Carneiro de Melo Moura C, Brambach F, Jair Hernandez Bado K, Krutovsky KV, Kreft H, Tjitrosoedirdjo SS, Siregar IZ, Gailing O (2019) Integrating DNA barcoding and traditional taxonomy for the identification of dipterocarps in remnant lowland forests of Sumatra. Plants (Basel). 8(11):461. https://doi.org/10.3390/plants8110461
CBOL Plant Working Group (2009) A DNA barcode for land plants. Proc Natl Acad Sci. USA, 106:12794–12797. https://doi.org/10.1073/pnas.0905845106
Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K, Li Y, Li X, Jia X, Lin Y, Leon C (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS one 5(1):e8613
Choudhary D, Rai MK, Shekhawat NS, Kataria V (2020) In vitro propagation of Farsetia macrantha Blatt. & Hallb.: an endemic and threatened plant of Indian Thar Desert. Plant Cell, Tissue Organ Cult 142:519–526
Duncan DB (1955) Multiple range and multiple “F” test. Biometrics 11:1–42
EL-Khalafy MM, Shaltout KH, Ahmed DA (2021) Updating and assessing plant endemism in Egypt. Phytotaxa 502:(3):237–258. 10.11646
Farcaș CAD, Moț AC, Pârvu AE, Toma VA, Popa MA, Mihai MC, Sevastre B, Roman I, Vlase L, Pârvu M (2019) In vivo pharmacological and anti-inflammatory evaluation of xerophyte Plantago sempervirens. Oxid Med Cell Longev, pp 13. https://doi.org/10.1155/2019/5049643
Fatima DN, Anis M (2012) Role of growth regulators on in vitro regeneration and histological analysis in Indian ginseng (Withania somnifera L.). Physiol Mol Biol Plants 18(1):59–67. doi: https://doi.org/10.1007/s12298-011-0099-x
Fattorini S (2017) Endemism in historical biogeography and conservation biology: concepts and implications. Biogeographia – The Journal of Integrative Biogeography 32:47–75
Ghaly O (2015) Taxonomical and molecular characterization of endemic species at Saint Katherine Protectorate Sinai- Egypt. PhD Mansoura University (unpublished)
Golec EA, Makowczynska J (2008) Micropropagation of Plantago camtschatica Link. Acta Soc Bot Pol 77(4):269–273
Goncalves S, Romano A (2015) The medicinal potential of plants from the genus Plantago (Plantaginaceae). Ind Crops Prod 83:213–226. https://doi.org/10.1016/j.indcrop.2015.12.038
Grainger J (2003) The Management and development plan; Saint Katherine Protectorate, a World Heritage Site, IUCN Category IV Protected Landscape
Haddadian K, Haddadian K, Zahmatkash M (2014) A review of Plantago plant. Indian J Trad Knowl 13(4):681–685
Haque SM, Chakraborty A, Dey D, Mukherjee S, Nayak S, Ghosh B (2017) Improved micropropagation of Bacopa monnieri (L.) Wettst. (Plantaginaceae) and antimicrobial activity of in vitro and ex vitro raised plants against multidrug-resistant clinical isolates of urinary tract infecting (UTI) and respiratory tract infecting (RTI) bacteria. Clinical Phytoscience 3:17. https://doi.org/10.1186/s40816-017-0055-6
Hashim AM, Alatawi A, Altaf FM, Qari SH, Elhady ME, Osman GH, Abouseadaa HH (2021) Phylogenetic relationships and DNA barcoding of nine endangered medicinal plant species endemic to Saint Katherine Protectorate. Saudi J Biol Sci 28(3):1919–1930
Hebert PD, Cywinska A, Ball SL, de Waard JR (2003) Biological identifications through DNA barcodes. Proc Biol Sci 270:313–321
Heywood VH (2014) An overview of in situ conservation of plant species in the Mediterranean. Fl Medit 24:5–24. https://doi.org/10.7320/FlMedit24.005
Hosein FN, Austin N, Maharaj S, Johnson W, Rostant L, Ramdass AC, Rampersad SN (2017) Utility of DNA barcoding to identify rare endemic vascular plant species in Trinidad. Ecol Evol 7(18):7311–7333
Hosni H, Hosny A, Shamso E, Hamdy R (2013) Endemic and near-endemic taxa in the flora of Egypt. Egypt J Bot 53:357–383
Hu W, Fagundez S, Grazzini LK, Li Y, Li W, Chen Y, Wang X, Deng Z, Xie S, R Avoy JM, Li Y (2017) Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Horticulture Res 4(1):17071. https://doi.org/10.1038/hortres.2017.71
Jiang S, Chen F, Qin P, Xie H, Peng G, Li Y, Guo X (2022) The specific DNA barcodes based on chloroplast genes for species identification of Theaceae plants. Physiol Mol Biol Plants 28(4):837–848
Kadota M, Niimi Y (2003) Effects of cytokinin types and their concentrations on shoot proliferation and hyperhydricity in in vitro pear cultivar shoots. Plant Cell, Tissue Organ Cult 72:261–265. https://doi.org/10.21608/ejchem.2020.45909.2934
Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) PNAS 102(23):8369–8374
Kumar V, Sharma A, Prasad BCN, Gururaj HB, Giridhar P, Ravishankar GA (2007) Direct shoot bud induction and plant regeneration in Capsicum frutescens Mill.: influence of polyamines and polarity. Acta Physiol Plant 29:11–18
Li DZ, Gao LM, Li HT, Wang H, Ge XJ, Liu JQ, Chen ZD, Zhou SL, Chen SL, Yang JB (2011) Comparative analysis of a large dataset indicates that internal transcribed spacer (ITS) should be incorporated into the core barcode for seed plants. Proc Natl Acad Sci 108(49):19641–19646
Loera-Sánchez M, Studer B, Kölliker R (2020) DNA barcode psbA–trnH is a promising candidate for efficient identification of forage legumes and grasses. BMC Res Notes 13(1):1–6
Makowczynska J, Golec EA (2003) Micropropagation of Plantago asiatical L. through culture of shoot tips. Acta Societatis Botanicorum Poloniae 72(3): 191–194
Makowczynska J, Golec EA (2009) Micropropagation of Plantago maritima L. a vanishing species in Poland. Acta Societatis Botanicorum Poloniae 78(1):13–18
Mederos S, Martin C, Navarro F, Ayuso MJ (1997) Micropropagation of a medicinal plant Plantago Major. Biol Ogia Plantarum 40(3):465–468
Moustafa AA, Zaghloul MS, Al-Sharkawy DH (2015) Seed bank approach for conservation of two threatened endemic medicinal plant species; Hypericum sinaicum Hocsht. & Steud ex Boiss. and Plantago sinaica (Barneoud) Decne. American-Eurasian J Agric & Environ. Sci 15 (12): 2512–2520. doi: https://doi.org/10.5829/idosi.aejaes.2015.15.12.12691
Mohsenzadeh S, Sheidai M, Koohdar F (2020) Populations genetic study of the medicinal species Plantago afra L. (Plantaginaceae). Caryologia 73(2):73–80. doi: https://doi.org/10.13128/caryologia-135
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052x
Mwaniki WI, Lubabali AH, Asava KK, Agwanda CO, Anami SE (2019) Effects of genotype and plant growth regulators on callus induction in leaf cultures of Coffea arabica L. F1 hybrid. African Journal of Biotechnology 18(31):1004–1015. https://doi.org/10.5897/AJB2019.16913
Nazarizadeh A, Mikaili P, Moloudizargari M, Aghajanshakeri S, Javaherypour S (2013) therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. Journal of Basic and Applied Scientific Research 3(9):212–221
Pang X, Liu C, Shi L, Liu R, Liang D, Li H, Cherny SS, Chen S (2012) Utility of the trnH–psbA intergenic spacer region and its combinations as plant DNA barcodes: a meta-analysis. PLoS One 7(11):e48833
Pires AC, Marinoni L (2010) DNA barcoding and traditional taxonomy unified through integrative taxonomy: a view that challenges the debate questioning both methodologies. Biota Neotrop 10:339–346
Piyaviriyakul KS, Thongpraditchote S, Siripong P, Vallisuta O (2017) Effects of Plantago major extracts and its chemical compounds on proliferation of cancer cells and cytokines production of lipopolysaccharide-activated THP-1 macrophages. Pharmacogn Mag 13(51):393–399
POWO (2023). "Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:685660-1#synonyms
Rabei S, Khedr AH, Elgamal I (2021) Floristic study of Saint Katherine Protectorate, Sinai: with one new record to flora of Egypt. Taeckholmia 41:32–55
Rahamooz-Haghighi S; Bagheri K; Bagheri K; Danafar H, Sharafi A (2020) Tissue culture, in vitro organogenesis and regeneration of Plantago lanceolata.J Appl Biotechnol Rep 7(4):258–265. https://doi.org/10.30491/JABR.2021.122254
Rohwer JG, Trofimov D, Mayland-Quellhorst E, Albach D (2019) Incongruence of morphological determinations and DNA barcode sequences: a case study in Cinnamomum (Lauraceae). Willdenowia 49(3):383–400
Sang T, Crawford DJ, Stuessy TF (1997) Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am J Bot 84:1120–1136
Shaltout KH, Eid EM, Al-Sodany YM, Heneidy SZ, Shaltout SK, El-Masry SA (2021) Effect of protection of mountainous vegetation against over-grazing and over-cutting in South Sinai Egypt. Diversity 13:113. https://doi.org/10.3390/d13030113
Sharma S, Kamal B, Rathi N, Chauhan S, Jadon V, Vats N, Gehlot A, Arya S (2010) In vitro rapid and mass multiplication of highly valuable medicinal plant Bacopa monnieri (L.) Wettst. African Journal of Biotechnology 9(49):8318–8322. ISSN 1684–5315.
Sharma M, Kumari A, Mahant E (2017) Micropropagation and phytochemical profile analysis of tissue culture grown Plantago ovata Forsk 10(4) doi: https://doi.org/10.22159/ajpcr.2017.v10i4.16532
Snedecor GW, Cochran WG (1990) Statistical methods, 8th edn. Iowa State University Press, Ames, Iowa, USA
Soliman MA, Galal TM, Naim MA, Khalafallah AA (2022) Seasonal variation in the secondary metabolites and antimicrobial activity of Plantago major L. from Egyptian heterogenic habitats. Egypt J Bot 62(1):255–273
Sun Z, Chen S (2013) Identification of cortex herbs using the DNA barcode nrITS2. J Nat Med 67:296–302. https://doi.org/10.1007/s11418-012-0681-8
Tate JA, Simpson BB (2003) Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst Bot 28:723–737
Techen N, Parveen I, Pan Z, Khan IA (2014) DNA barcoding of medicinal plant material for identification. Curr Opin Biotechnol 25:103–110
Tripathi AM, Tyagi A, Kumar A, Singh A, Singh S, Chaudhary LB, Roy S (2013) The internal transcribed spacer (ITS) region and trnH-psbA are suitable candidate loci for DNA barcoding of tropical tree species of India. PLoS One 8(2):e57934
Ying-Hua S, Yu-Bo L, Xian-Sheng Z (2011) Auxin–cytokinin interaction regulates meristem development. Mol Plant 4(4):616–625. https://doi.org/10.1093/mp/ssr007
Youssef NM, Tahaa LS, Abd El-Khalekb SN (2021) Secondary metabolites characterization of in vitro propagated Antigononleptopus cultures Egypt. J Chem 64(2):923–932. https://doi.org/10.21608/ejchem.2020.45909.2934
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
HG put the plan of the in vitro propagation section, did the in vitro propagation experiments of the plant, did the analysis of data, written the in vitro propagation section and corresponding the publication. HA. did the DNA barcoding section, analysis, written the barcoding section, and All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by Melekşen Akın
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghareb, H.ES., Abouseadaa, H.H. In vitro propagation and DNA barcoding of the rare near endemic Plantago sinaica (Barnéoud) plant in Saint Katherine, Sinai. Plant Cell Tiss Organ Cult 156, 69 (2024). https://doi.org/10.1007/s11240-024-02689-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-024-02689-6