Abstract
With the discovery in 2015 of the ability to induce somatic embryos in Cyathea delgadii, learning more about the relationship between the structure of apoplast and cell differentiation has become possible among ferns. In this study, the distribution of arabinogalactan proteins, pectins, extensins, and callose with specific epitopes recognized by monoclonal antibodies was investigated during direct somatic embryogenesis (SE) of C. delgadii. Eight antigens against the arabinogalactan proteins (JIM8, JIM13, LM2), pectins (JIM5, JIM7), extensins (JIM11, JIM12), and callose (anti-1 → 3-β-glucan) were selected. Two types of explants were analyzed, i.e. stipe fragments and internodes, which give rise to embryos of unicellular and multicellular origin, respectively. The study showed that embryogenic transition in C. delgadii is preceded by cell wall remodeling of initial explants. Dynamic changes in JIM13, JIM12, and anti-1 → 3-β-glucan localization were observed. The differences in the distribution of studied epitopes were observed between the cell walls of the epidermis and those located in the other layers of the explant. Moreover, within the somatic embryos, a stronger fluorescence of the examined antibodies was observed, mainly those reacting with arabinogalactan proteins, extensins, and callose. These results also implicated that, with the exception of the earlier appearance of callose in the stipe explants, the uni- and multicellular pathways of somatic embryo differentiation do not differ in the quality of cell wall components. The presented studies document the first time that SE in ferns can be regulated by changes in apoplast structure and they provide a basis for more detailed research.
Key Message
The cell wall plays a significant role in somatic embryogenesis of Cyathea delgadii and changes in its composition may be a marker of embryogenicity for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant cells are supported by relatively thin but mechanically strong cell walls, which together with extracellular spaces, xylem, phloem, and tracheids form the apoplastic pathway involved in the movement of water and dissolved minerals along cell walls and extracellular spaces (Zhang et al. 2021). Plant cell walls are crossed by plasmodesmata which are the cytoplasmic channels passing sugars, ions, and even proteins and RNA from one cell to another. Symplastic (consists of the entire cytosol of all living plant cells) and apoplastic systems together are the way of cell-to-cell communication and play a crucial role in plant development (Otero et al. 2016).
Plant cell walls primarily consist of cellulose, polysaccharides (hemicelluloses and pectins), lignin, and several groups of structural glycoproteins: hydroxyproline-rich glycoproteins (HRGPs), glycine-rich proteins (GRPs), and lightly glycosylated proline-rich proteins (PRPs) (for review see Zhang et al. 2021). Pectins are major chemical components of primary cell walls with homogalacturonan as the most abundant component. This complex and heterogenic group of acidic polysaccharides is synthesized in the Golgi apparatus and secreted into the cell wall in a highly methyl-esterified form. They modify the mechanical properties of cell walls (i.e. porosity, elasticity, adhesion, binding of ions) by changes in the methyl-esterification status. Such a modification is important for cell adhesion, division, and expansion and consequently for proper organ and plant development (Xu et al. 2011; Pérez-Pérez et al. 2019). Arabinogalactan proteins (AGPs) are a large, complex, and diverse class of heavily glycosylated proteins. These plant-specific proteoglycans belong to the family of HRGPs and have been identified in distinct cells and tissues and are expressed at particular stages of plant development. Beyond the cell wall, AGPs were found in the plasma membrane, apoplastic space, and in secretions e.g. extracellular matrix and wound exudates (Su and Higashiyama 2018; Silva et al. 2020). Extensins are cell wall proteins characterized by the repeated occurrence of serine followed by several prolines. They are a diverse family of HRGPs implicated in nearly all aspects of plant growth and development including cell division, cell differentiation, response to wounding, and pathogen infection (Liu et al. 2016; Wu et al. 2017; Mishler-Elmore et al. 2021).
The composition of cell walls in ferns is certainly not as widely studied as in seed plants. Despite that, some biochemical analyses indicate they are similar in both groups (Leroux et al. 2013, 2015). In ferns, mannose-containing polysaccharides (e.g., mannan and glucomannan) appear to be abundant, whereas pectins are found in lower concentrations than in other plants (Silva et al. 2011). Detailed characterization of AGPs in various ferns revealed that the amount of protein in these glycoproteins is comparable to most spermatophyte AGPs and was within the 6–12% range. Further, the carbohydrate molecules showed typical features known for type II arabinogalactans known from seed plants and cross-reacted with antibodies against AGPs (Bartels and Classen 2017).
Through their location, cell walls are essential for maintaining a specific shape, and architecture and defining the plane of division and differentiation into different cell types (Malinowski and Filipecki 2002). In addition, signaling factors exported from the cytoplasm to the cell wall can diffuse through the apoplast to surrounding cells and stimulate developmental processes. During differentiation, both the structure and composition of the cell wall can change (Šamaj et al. 2005). Remodeling of the apoplast in developmental processes is usually associated with the synthesis, deposition, or degradation of macromolecules, such as hemicelluloses, AGPs, and pectins (Pilarska et al. 2013; Betekhtin et al. 2016). Thus, it can include a change in their content and localization in the cell walls, which can be visualized using well-characterized antibodies specific to them (Filipović et al. 2021). The use of this approach provides evidence suggesting that AGPs, pectins, and extensins play an important role during somatic to embryogenic cell transition as well as in the somatic embryo development (Šamaj et al. 2005; Xu et al. 2011; Pilarska et al. 2013; Betekhtin et al. 2016; Potocka et al. 2018; Pérez-Pérez et al. 2019). The modifications of the cell walls during somatic embryogenesis (SE) may also include the appearance of an extracellular matrix, which, as observed in Bactris gasipaes, surrounds the somatic embryo at an early stage of development (Steinmacher et al. 2012), accumulation of cutin surrounding the developing somatic embryo, as seen in Humulus lupulus (Fortes et al. 2002) or thickening of the embryogenic cell wall in comparison to non-embryogenic one as was shown for Syagrus oleracea (de Araújo Silva-Cardoso et al. 2020). Callose (1,3-β-glucan) is a polysaccharide produced by plant cells and deposited on the inside of the cell wall in response to various stresses. It was found to accumulate in high levels in response to both biotic and abiotic stresses (Zavaliev et al. 2011). Studies show that it may also be involved in morphogenetic processes (Worrall et al. 1992; Grimault et al. 2007) as one of the elements regulating symplastic communication in plants.
Despite most of the previous research on the cell wall of non-flowering plants contributing to a deeper understanding of plant evolution, the involvement of the cell wall in the SE of ferns or any cryptogamic plants has never been explored so far. Detailed studies related to the dynamics of cell walls in embryogenic systems are still very limited, especially when considering non-seed-producing species. Therefore, using a very well-developed system of direct SE in the tree fern C. delgadii, in which the somatic embryos originate from single cells of the epidermis of stipe explants or from a group of epidermal and cortical cells of internodes (Mikuła et al. 2022) we analyzed the composition of cell walls during SE-inducing in vitro culture. To that end, analyses of the distribution of monoclonal antibodies within the explants and early somatic embryos were undertaken to answer whether there is a correlation between changes in cell wall composition and cell differentiation during SE of C. delgadii.
Materials and methods
Plant material and culture conditions
The research was carried out using the axenic cultures of the tree fern C. delgadii obtained as described by Mikuła and co-workers (2015). The plant material was collected from the youngest fronds of 5-month-old in vitro-grown sporophytes maintained in darkness. Two types of explants derived from etiolated plants that had developed 2 or 3 leaves were analyzed. These were stipe and internode fragments, which under the same culture conditions produce somatic embryos by unicellular and multicellular pathways, respectively (Grzyb and Mikuła 2019). Stipe (about 2.5 mm in length) and internode (about 1.5 mm in length) explants were freshly cut off from the first fronds of donor sporophytes. These explants were cultured on a hormone-free agar medium containing half-strength macro- and micronutrients of basal Murashige and Skoog medium (1/2 MS) supplemented with the full set of vitamins and 1% sucrose (Murashige and Skoog 1962).
To carry out microscopic analyses the plant material was successively collected.
Immunocytochemical analyses
Cell wall epitopes and callose were localized in initial stipe and internode explants (control; freshly excised from sporophyte) and during induction and early expression of SE (between 2nd and 14th and 2nd and 12th day of culture, for stipe and internode explants respectively). Differences in the timing of explant collection were due to their different responses and the associated dynamics of somatic embryo formation (Grzyb and Mikuła 2019). Attention was focused on the epitopes that have been confirmed in SE for other plants. Thus, seven primary antibodies for the detection of three groups of epitopes: pectins (i.e., JIM5, JIM7), AGPs (i.e. JIM8, JIM13, LM2), and extensins (i.e., JIM11, JIM12) and one against callose (anti-1 → 3-β-glucan) were used (Table 1).). Anti-1 → 3-β-glucan was ordered from Biosupplies (Australia), the other antibodies used in this study were purchased from Plant Probes (Leeds, UK).
The samples were fixed in 4% paraformaldehyde prepared on MSB buffer (microtubule-stabilizing buffer) for 2 h at room temperature. Then the materials were dehydrated in a graded ethanol series, embedded in BMM (butyl-methyl methacrylate) resin, and sectioned at 2 µm on the microtome (Sujkowska-Rybkowska et al. 2022). After resin removal with acetone, sections were incubated in a blocking solution containing 3% bovine serum albumin (BSA) in PBS (phosphate-buffered saline) for 1 h before the application of the primary monoclonal antibody. Sections were washed in PBS and incubated with the solution (1:3) of primary monoclonal antibodies with 0.5% BSA for the night at 4 °C before washing in PBS and applying a secondary (1:2000) goat anti-rat antibody conjugated to Alexa Fluor 488 or Fluorescein isothiocyanate (FITC) (Molecular Probes) for 1.5 h in darkness.
Sections were observed under an epifluorescence microscope with a triple DFTR filter (Olympus-Provis, Japan). Immunocytochemical analyses were performed on at least 5 explants for each time point and the 5 sections for each were analyzed. Negative controls were performed by incubation in PBS instead of the primary antibodies and by incubating the sections with the blocking buffer solution. They did not show any labeling (data not shown).
Light and scanning electron microscopy
To visualize the course of SE induced on both explants, microscopic observations using an environmental scanning electron microscope (FEI QUANTA 200; 0.75 Tr, at a relative humidity of up to 100%, and reduced pressure of less than 10−4 Pa) were carried out. For this purpose, the material did not require any fixation, so it was taken directly from the induction medium at various stages of culture. For general structure analysis, explants were also fixed in the mix of 2.5% paraformaldehyde and 2.5% glutaraldehyde, post-fixed in 2% osmium tetroxide, dehydrated in a graded ethanol series, and infiltrated in Epon epoxy resin as described in Grzyb and Mikuła (2019). Semithin sections were obtained with a microtome, and they were examined using a Vanox light microscope (Olympus, Japan) with a computer image analysis system (cellSens Standard ver. 1.7).
Results
Antibody distribution within initial explants
Immunocytochemical analyses show that the epitope distribution in the initial stipe and internode explants was similar (Table 2; 0 day of culture).
It was observed that JIM5 (recognizing the low-methyl esterified pectins epitope) in the stipes gives fluorescence in the epidermis and slightly weaker signal in cortex cells (directly under the epidermis) and in the vascular bundle (Fig. 1a). Similar distribution but the weaker signal of JIM5 was observed in the internodes (Fig. 1a’). The second pectin antibody binding with their high-methyl esterified forms—JIM7 gave uniform fluorescence in all cell walls of both explants however, stronger in the stipes (Fig. 1b, b’).
The distribution of AGP epitopes recognized by JIM8, JIM13, and LM2 was not uniform throughout the layers of the initial explants. The first antibody JIM8 gave strong fluorescence in the vascular bundle and much weaker in some epidermal cells. Such a distribution was observed for both initial stipes and internodes (Fig. 1c, c’). Subsequent JIM13 and LM2, on the other hand, reacted evenly with epitopes located in the epidermis, cortex, and vascular bundle of stipes as well as internodes (Fig. 1d, d’, e, e’). However, the signal for JIM13 was weaker than for LM2.
For extensin epitopes, the JIM11 antibody showed response only in the vascular tissue of both analyzed explants (Fig. 1f, f’) while JIM12 was evenly distributed throughout their layers (Fig. 1g, g’). The callose-detecting antibody demonstrated fluorescence in the epidermis, cortex, and vascular bundle and the signal was evenly distributed throughout the stipes and internodes (Fig. 1 h, 1 h’).
Antibody distribution within explant during SE induction and early somatic embryo formation
During the induction of the SE, which for the stipe explants was analyzed until day 8 of culture and for the internodes until day 4 of the culture, the distribution of the studied cell wall epitopes remained mostly constant until embryogenic divisions began on both stipe and internode explants (Table 2). Although there were differences between the different layers of explants, they remained constant during in vitro culture in both explants. Moreover, in the case of pectin, the fluorescence of both analyzed antibodies did not change from that seen in initial explants during the entire period of SE-inducing culture of stipes and internodes.
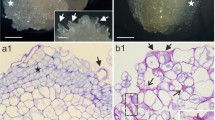
Only change in the distribution of callose in some cells of the stipe epidermis was observed (Fig. 2). Closer look at these cells showed that 1 → 3-β-glucan was localized mainly in the anticlinal cell walls and those located between the epidermis and the cortex (Fig. 2b) and also sometimes in the cortical cells (Fig. 2c). There was no increase in the signal for anti-1 → 3-β-glucan detected in internodes by the 4th day of culture (Table 2).
Distribution of the antibody recognizing 1 → 3-β-glucan within stipe explants of Cyathea delgadii after 8 days of culture. a Strong fluorescence of the antibody detected in some epidermal cells (marked with a rectangle). b A closer look at the area marked in Fig “a” indicates strong fluorescence of antibodies in the anticlinal walls (dashed line) and those located between the epidermis and the cortex (arrow). c Strong fluorescence of the antibody detected in the cortical cell. Bars = 50 µm (a), 10 µm (b, c). Ep epidermal cells, Cx cortical cells, Vb vascular bundle
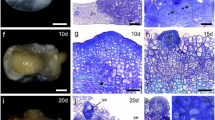
In the stipe explants culture, the individual epidermal cells dividing give rise to the linear somatic embryos on the 14th day of culture (Fig. 3a–c). In turn, on the internode explants, simultaneous divisions of neighboring epidermal and cortical cells starting on the 4th day of culture lead to the formation of somatic embryos much faster, and as early as the 12th day of culture embryos significantly protruding above the surface of the explants can be seen (Fig. 3d–f). More details on the formation of somatic embryos in C. delgadii on both the stipe and internode explants have previously been described (Grzyb and Mikuła 2019).
Direct somatic embryogenesis obtained in Cyathea delgadii during 14- and 12-day cultures of the stipe (a–c) and internode (d–f) explants, respectively. a Top surface view of the middle part of the initial stipe explant. b Longitudinal section of the stipe explant showing linear embryos originating from a single cell of epidermis. c View on the stipe explant surface after 14 days of culture with multiple somatic embryos of unicellular origin (marked by ^ in yellow). d Top surface view of initial internode explant. e Longitudinal section of the internode explant showing embryo differentiated from the multiple-divided epidermal and cortical cells. f View on the internode explant surface after 12 days of culture with three somatic embryos of multicellular origin. Light (b, e) and an environmental scanning electron (a, c, d, f) microscope visualization. Bars = 500 µm (a, d), 20 µm (b, e), 50 µm (c, f). Cx cortex, Se somatic embryo
In linear somatic embryos initiated on stipe explants, we detect signals for AGPs bearing the JIM8, JIM13, and LM2 epitopes (Table 2). However, the signal for JIM13 was the strongest, and the signals for the other two antibodies in somatic embryos were comparable to those observed in undivided epidermal cells and did not change during embryogenic culture. As we have shown here, JIM13 was already present in the few-cell somatic embryo of unicellular origin and its signal intensified on day 14 of the culture. Strong fluorescence of the antibodies reacting with extensins—JIM12 was also observed (Table 2). JIM13 and JIM12 fluorescence was localized in all somatic embryo body cell walls (Fig. 4a, b). Similarly, to the two previous ones, the callose antibody strongly labeled the anticlinal cell walls of the linear embryos and those separating it from the explant (Fig. 4c, d).
Distribution of the antibody recognizing extensins (a), AGPs (b), and callose (c, d) within the stipe explant and somatic embryo of Cyathea delgadii after 14 days of culture. Longitudinal (a, b, d) and transverse (c) section. Bars = 20 µm (a–d). Ep epidermal cells, Cx cortical cells, Vb vascular bundle, Se somatic embryo
As for the stipes, the signal for JIM13 increased most strongly of all AGPs antibodies also in the divided cells of the internodal epidermis starting from day 4th of the culture (Table 2, Fig. 5b). The signals for JIM8 and LM2 in embryos of multicellular origin were comparable to those observed in undivided epidermal cells and did not change during embryogenic culture. Also, an increase in fluorescence of the antibodies reacting with extensins—JIM12 was observed in divided epidermal and cortical cells for these explants (Table 2, Fig. 5a). Starting from day 4 of culture, strong callose labeling has been shown in dividing epidermal cells of internodes and weaker in cortical cells which enter the path of divisions almost at the same time (Table 2). It was shown that the anticlinal cell walls of the divided epidermis and those adjacent to the explant were labeled by anti-1 → 3-β-glucan. In addition, much stronger saturation with antibodies was observed in the walls separating the two divided cells of the epidermis (Fig. 5c).
Discussion
Both SE itself and the role of the cell wall in regulating this process are being undertaken by researchers. However, this research is limited to the group of seed plants. With the discovery of the ability to induce somatic embryos in C. delgadii, the first species of fern for which SE has been described, learning about the relationship between the structure of apoplast and cell differentiation has also become possible among this group of plants. Because somatic embryos of C. delgadii are formed directly from explant cells (without callus phase), the species provides great opportunities for the exploration of cell-to-cell communication during the acquisition of embryogenic competence (Grzyb et al. 2020).
Embryogenic transition
The primary plant cell wall is a highly dynamic structure that constantly undergoes remodeling during cell growth, division, and differentiation. It is also thought that similar modifications of cell wall composition give rise to competent cells during the very early stages of embryogenic cell differentiation (Pedroso and Pais 1995; Verdeil et al. 2001).
Pectins, as major plant cell wall polysaccharides, have been studied also in the context of SE. Previous data suggest that both low- and highly-methyl-esterified HG epitopes are developmentally regulated in diverse stages of the process however, most focus on later stages with already differentiated embryos or proembryos. Studies from Arabidopsis thaliana show some characteristics of embryogenic cells that were “positively” marked by the JIM7 and LM5 pectic epitopes (Potocka et al. 2018). On the contrary, studies on SE of bananas (Musa spp. AAA) mapping of numerous pectic epitopes on immunodots and tissue sections, suggest that embryogenic cells were rich with fully de-esterified and low methyl-esterified ones (among them JIM5) while highly methyl-esterified ones (such as JIM7 and LM20) antibodies were hardly detected in these cells (Xu et al. 2011). Using a homogeneous Cocos nucifera L. callus strain (L82) obtained from immature floral meristems it was shown that the acquisition of embryogenic competence during SE is linked to the appearance of an outer layer of fibrillar material containing mainly un-methyl-esterified pectin epitope which fully coating the embryogenic cells (Verdeil et al. 2001). This data suggests that both low- and highly-methyl-esterified pectin epitopes are developmentally regulated in the initial stage of SE. However, they are inconsistent with our studies as we did not notice any changes in the pectin distribution during the embryogenic transition. Although we observed differences in the distribution of pectins recognized by JIM5 between the different layers of the initial explants, they remain constant during in vitro culture in both stipe and internode explants. The second pectin-recognizing antibody JIM7 gave a stable signal throughout the explant layers and also did not change during the SE-inducing culture of both stipes and internodes. In general, both antibodies against pectin showed rather weak fluorescence during in vitro culture of stipe and internode explants and their distribution did not change over the course of the experiment. This may indicate a lack of involvement of these polysaccharides in somatic to embryogenic cell transition in C. delgadii or may be related to their lower content as shown in ferns (Silva et al. 2011).
Many researchers over the years have confirmed the role of AGPs in inducing SE by showing that released or added to the culture medium they were able to induce somatic embryo formation in diverse plant species (reviewed in Šamaj et al. 2005). Studies from A. thaliana show that embryogenic cells were “negatively” marked by the LM2 AGPs epitope, while the JIM4 AGPs epitope appeared to be highly specific for differentiated cells with a distinct morphology (Potocka et al. 2018). Contrary, in Brachypodium distachyon AGP epitopes recognized by the JIM16 and LM2 antibodies were characteristic cell wall components of embryogenic calli (Betekhtin et al. 2016). In the studies presented here, we observed differences in the distribution of AGPs recognized by JIM8 between the different layers of the initial explants. However, it had not changed by the time embryogenic divisions began on both stipe and internode explants. The other two antibodies for AGPs JIM13 and LM2 marked evenly whole explants and gave stable fluorescence until embryogenic divisions started. This could mean that in C. delgadii AGPs are not associated with embryogenic transition but rather with embryogenic divisions at a later stage of the SE in this fern. All of the above together indicate that the distribution of this diverse epitope group may be species or SE-inducing system dependent.
The involvement of extensin in embryogenic transition during SE was not as intensively studied as other epitopes and their localization using the immunocytochemical approach has been shown only in a few works (Zagorchev and Odjakova 2011; Lee et al. 2013; Betekhtin et al. 2016). Despite limited data, some characteristics of the cell wall of embryogenic cells were suggested for these proteins. In B. distachyon, the surface of embryogenic cells was “positively” marked by the JIM11 and “negatively” marked by JIM12 antibodies for extensin epitopes (Betekhtin et al. 2016). JIM11 and other extensin-labeled epitopes JIM20 were also observed on the surface of embryogenic cells in the protocorm-like bodies of the Phalaenopsis tissue culture (Lee et al. 2013). Moreover, in Dactylis glomerata localization of JIM12-labeled proteins in a medium of a salt-adapted embryogenic suspension culture was shown (Zagorchev and Odjakova 2011). The above research proves that antibodies for different extensin epitopes could be used as a marker to survey the embryogenic competence of some embryogenic culture systems. However, as we have not observed changes in their distribution in C. delgadii seems that for our model, as in the case of AGPs, they are not relevant at this stage of the SE.
Callose deposition in plasmodesmata which, as shown in A. thaliana precedes the expression of the WUSCHEL-related homeobox 2 gene, was observed at future sites of somatic embryo development. As well, it was also required for symplastic isolation and embryogenic transition of somatic cells in this model plant (Godel-Jedrychowska et al. 2020). The same as for A. thaliana, embryogenic cells of coconut (Verdeil et al. 2001), Trifolium repens (Maheswaran and Williams 1985) and Cichorium hybrid clone "474" (Dubois et al. 1991; Grimault et al. 2007) were indicated by the existence of callose in the modified cell wall surrounding them which confirms at least their temporary isolation. It is clear from our research that in C. delgadii the earliest change that stipe explant undergoes during SE-inducing in vitro culture is the accumulation of callose in some epidermal cells. This agrees with previous studies in which, using low-molecular-weight symplast transport fluorochromes, we showed the isolation of epidermal cells of stipes (Grzyb et al. 2020). These observations together indicate that symplastic isolation of epidermal cells which underlies the initiation of embryogenic divisions in these cells and further development of the somatic embryo may be regulated by callose. However, we did not observe an amplification of the signal for the antibody recognizing callose at such an early stage of SE induced on internode explants of C. delgadii. This may indicate that regulation of plasmodesmata permeability by callose does not take place or is little involved in the initiation of somatic embryos through the multicellular pathway.
An early stage of somatic embryo development
According to the previous results, the distribution of cell wall epitopes within the explants changes during SE in terms of their presence in the cells that realize different developmental programs (Potocka et al. 2018). Remodeling of the composition of cell wall polysaccharides, resulting in changes in their mechanical properties, allows cell division and expansion to proceed normally (Barnes and Anderson 2018). With the progression of embryogenic division in C. delgadii explants strong fluorescence of the antibodies reacting with AGPs, extensin, and callose in the cells involved in embryo formation was observed for both stipes and internodes. This clearly points out their important roles in embryogenic division coordination and the development of the tree fern somatic embryo.
During the very early development of C. delgadii somatic embryos, we detected signals for AGPs bearing the JIM8, JIM13, and LM2 epitopes. However, the signal for JIM13 was the strongest, and the signals for the other two antibodies in somatic embryos were comparable to those observed in undivided epidermal cells and did not change during embryogenic culture. As we have shown here, JIM13 was already present in the few-cell somatic embryo of unicellular origin and in divided epidermal cells of internodes at an early stage of embryos of multicellular origin. Similar to C. delgadii results, an intense signal for JIM13 was shown in the embryogenic sector of peach palm (Bactris gasipaes Kunth) and polarized localization during the initial development of somatic embryos in this species was also observed (Steinmacher et al. 2012). However, in Trifolium nigrescens the only AGP epitope detected in somatic embryos was the LM2 epitope which was present solely at an early stage of embryogenic development (Pilarska et al. 2013). Previous studies in bananas have shown a diversity of immunolabeled AGPs that decreased with somatic embryo maturation (Pan et al. 2011). Contradictory data reported in Quercus suber have shown that AGP-recognizing antibodies LM6 and LM2 were characterized by the highest labeling intensity in the cotyledonary embryo stage compared to the proembryogenic mass stage. In the same study, increasing expression of three AGP-encoding genes and a gradual increase in the level of AGP accumulation along with the progression of SE were shown (Pérez-Pérez et al. 2019). Despite the varying data in terms of immunodetection at later stages of somatic embryo development and the type of detected AGPs, what is consistent is the fact of their key role in the initiation of embryogenic development.
In C. delgadii, we report an increased signal for the JIM12 antibody which recognizes a protein component in the glycoprotein region of extensin epitope in embryogenically dividing epidermal cells of stipes and internodes. The location and function of extensins in somatic embryo differentiation are unknown. The few studies to date have focused on analyzing these epitopes mainly in callus tissue. From these studies some antibodies directed against extensins, among them also JIM12, appear as a marker of embryogenic cells. Due to limited data, it is difficult to interpret their role in embryo development. However, considering they regulate the growth and properties of the cell walls (Lamport et al. 2011) their involvement in the induction of embryogenic divisions and embryonic development can be postulated.
Callose observed in some epidermal cells of stipes as early as day 4 of culture was also strongly labeled at further SE stages in these explants. This sugar, as indicated by strong antibody saturation, was present in high amounts in the epidermal and cortical cells undertaking embryogenic divisions in the internodes. In plant cells, callose is distributed in specific cell walls, such as cell plates, the outer wall of the pollen wall, and also in plasmodesmata (Wang et al. 2022). Thus, its role during somatic embryo differentiation can be of a dual nature. Firstly related to the intense divisions associated with the formation of the somatic embryo body. Secondly, by regulation of communication between cells carrying out different developmental programs through plasmodesmata. Callose's presence associated with dividing cells of somatic embryos is known from previous reports. In several-celled embryos of chicory, callose deposits were found in the newly formed cell walls and slight callose deposition remained in the external cell wall (Dubois et al. 1991; Grimault et al. 2007). We showed earlier that the formation of the somatic embryo by the single-cell pathway is associated with its isolation from the initial explant (Grzyb et al. 2020). Moreover, within the embryo, communication between its cells was also reduced. Thus, the accumulation of callose in stipes subjected to embryogenic culture may be related to the isolation shown earlier. Additional studies are needed to show whether the same mechanism is involved in the differentiation of embryos of multicellular origin induced on internodes.
Conclusion
The multiplicity of the SE systems (that are species-specific) and different ways of somatic embryo differentiation (direct or indirect SE; uni- or multicellular origin) make it difficult to compare immunocytochemical results with each other. Thus, analyzing the earliest stages of SE, including embryogenic transition, faces many obstacles. Still, not enough plants and SE-inducing systems have been studied to conclude on shared mechanisms for different species or groups of plants. In the research presented here, we showed that changes in the fate of explant cells during early SE in the tree fern C. delgadii are related to the modifications in the chemical composition of the cell walls. Callose deposition in epidermal cells of stipe explants seems to be one of the first signs indicating that these cells have undertaken embryogenic development, and thus it can be considered as an early marker of SE in C. delgadii. The uni- and multicellular pathways of somatic embryo differentiation do not differ in the quality of cell wall components, however, they may differ in the intensity of their synthesis. Within the somatic embryos, strong fluorescence of the tested antibodies was observed, mainly those reacting with extensins, AGPs, and callose, which may indicate their increased synthesis during induction of SE. Based on these findings, it can be assumed that the cell wall plays a key role in the acquisition of embryogenic capacity in C. delgadii and that changes in its composition may be a good marker of embryogenic cells for this species and should be further explored.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Barnes WJ, Anderson CT (2018) Release, recycle, rebuild: cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol Plant 11:31–46. https://doi.org/10.1016/j.molp.2017.08.011
Bartels G, Classen B (2017) Structural investigations on arabinogalactan-proteins from a lycophyte and different monilophytes (ferns) in the evolutionary context. Carbohydr Polym 172:342–351. https://doi.org/10.1016/j.carbpol.2017.05.031
Betekhtin A, Rojek M, Milewska-Hendel A, Gawecki R, Karcz J, Kurczynska E, Hasterok R (2016) Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS ONE 11:e0167426. https://doi.org/10.1371/journal.pone.0167426
Clausen MH, Willats WGT, Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338:1797–1800. https://doi.org/10.1016/S0008-6215(03)00272-6
de Araújo Silva-Cardoso IM, Meira FS, Gomes ACMM, Scherwinski-Pereira JE (2020) Histology, histochemistry and ultrastructure of pre-embryogenic cells determined for direct somatic embryogenesis in the palm tree Syagrus oleracea. Physiol Plant 168:845–875. https://doi.org/10.1111/ppl.13026
Dubois T, Guedira M, Dubois J, Vasseur J (1991) Direct somatic embryogenesis in leaves of Cichorium. Protoplasma 162:120–127. https://doi.org/10.1007/BF02562555
Filipović BK, Trifunović-Momčilov MM, Simonović AD, Jevremović SB, Milošević SM, Subotić AR (2021) Immunolocalization of some arabinogalactan protein epitopes during indirect somatic embryogenesis and shoot organogenesis in leaf culture of centaury (Centaurium erythraea Rafn). In Vitro Cell Dev Biol Plant 57:470–480. https://doi.org/10.1007/s11627-020-10143-3
Fortes AM, Testillano PS, Risueno MD, Pais MS (2002) Studies on callose and cutin during the expression of competence and determination for organogenic nodule formation from internodes of Humulus lupulus var. Nugget Physiol Plant 116:113–120. https://doi.org/10.1034/j.1399-3054.2002.1160114.x
Godel-Jedrychowska K, Kulinska-Lukaszek K, Horstman A, Soriano M, Li M, Malota K, Boutilier K, Kurczynska EU (2020) Symplasmic isolation marks cell fate changes during somatic embryogenesis. J Exp Bot 71:2612–2628. https://doi.org/10.1093/jxb/eraa041
Grimault V, Helleboid S, Vasseur J, Hilbert JL (2007) Co-localization of ß-1,3-glucanases and callose during somatic embryogenesis in Cichorium. Plant Signal Behav 2:455–461. https://doi.org/10.4161/psb.2.6.4715
Grzyb M, Mikuła A (2019) Explant type and stress treatment determine the uni- and multicellular origin of somatic embryos in the tree fern Cyathea delgadii Sternb. PCTOC 136:221–230. https://doi.org/10.1007/s11240-018-1507-5
Grzyb M, Wróbel-Marek J, Kurczyńska E, Sobczak M, Mikuła A (2020) Symplasmic isolation contributes to somatic embryo induction and development in the tree fern Cyathea delgadii Sternb. PCP 61:1273–1284. https://doi.org/10.1093/pcp/pcaa058
Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J 1:317–326. https://doi.org/10.1046/j.1365-313X.1991.t01-9-00999.x
Lamport DTA, Li T, Held M, Kieliszewski MJ (2018) The role of the primary cell wall in plant morphogenesis. Int J Mol Sci 19:2674. https://doi.org/10.3390/ijms19092674
Lee YI, Hsu ST, Yeung EC (2013) Orchid protocorm-like bodies are somatic embryos. Am J Bot 100:2121–2131. https://doi.org/10.3732/ajb.1300193
Leroux O, Eeckhout S, Viane RLL, Popper ZA (2013) Ceratopteris richardii (C-Fern): a model for investigating adaptive modification of vascular plant cell walls. Front Plant Sci 4:367. https://doi.org/10.3389/fpls.2013.00367
Leroux O, Sørensen I, Marcus SE, Viane RLL, Willats WGT, Knox JP (2015) Antibody-based screening of cell wall matrix glycans in ferns reveals taxon, tissue and cell-type specific distribution patterns. BMC Plant Biol 15:56. https://doi.org/10.1186/s12870-014-0362-8
Liu X, Wolfe R, Welch LR, Domozych DS, Popper ZA, Showalter AM (2016) Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS ONE 11(2):e0150177. https://doi.org/10.1371/journal.pone.0150177
Maheswaran G, Williams EG (1985) Origin and development of somatic embryoids formed directly on immature embryos of Trifolium repens in vitro. Ann Bot 56:619–630. https://doi.org/10.1093/oxfordjournals.aob.a087052
Malinowski R, Filipecki M (2002) The role of cell wall in plant embryogenesis. Cell Mol Biol Lett 7:1137–1151
McCabe PF, Valentine TA, Forsberg LS, Pennell RI (1997) Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell 9:2225–2241. https://doi.org/10.1105/tpc.9.12.2225
Meikle PJ, Bonig I, Hoogenraad MJ, Clarke AE, Stone BA (1991) The location of (1–3)-beta-glucans in the walls of pollen tubes of Nicotiana alata using a (1–3)-beta-glucan-specific monoclonal antibody. Planta 185:1–8. https://doi.org/10.1007/BF00194507
Mikuła A, Gaj M, Grzyb M, Hazubska-Przybył T, Kępczyńska E, Kępczyński J, Rybczyński J, Tomiczak K, Wójcik AM (2022) Polish contribution to global research on somatic embryogenesis. Acta Soc Bot Pol. https://doi.org/10.5586/asbp.9115
Mikuła A, Pożoga M, Tomiczak K, Rybczyński JJ (2015) Somatic embryogenesis in ferns: a new experimental system. Plant Cell Rep 34:783–794. https://doi.org/10.1007/s00299-015-1741-9
Mishler-Elmore JW, Zhou Y, Sukul A, Oblak M, Tan L, Faik A, Held MA (2021) Extensins: self-assembly, crosslinking, and the role of peroxidases. Front Plant Sci 12:664738. https://doi.org/10.3389/fpls.2021.664738
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Otero S, Helariutta Y, Benitez-Alfonso Y (2016) Symplastic communication in organ formation and tissue patterning. Curr Opin Plant Biol 29:21–28. https://doi.org/10.1016/j.pbi.2015.10.007
Pan X, Yang X, Lin G, Zou R, Chen H, Šamaj J, Xu C (2011) Ultrastructural changes and the distribution of arabinogalactan proteins during somatic embryogenesis of banana (Musa spp. AAA cv ‘Yueyoukang 1’). Physiol Plant 142:372–389. https://doi.org/10.1111/j.1399-3054.2011.01478.x
Pedroso MC, Pais MS (1995) Factors controlling somatic embryogenesis. Cell wall changes as an in vivo marker of embryogenic competence. PCTOC 43:147–154. https://doi.org/10.1007/BF00052170
Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart J, Roberts K (1991) Developmental regulation of a plasma-membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3:1317–1326. https://doi.org/10.1105/tpc.3.12.1317
Pérez-Pérez Y, Carneros E, Berenguer E, Solís MT, Bárány I, Pintos B, Gómez-Garay A, Risueño MC, Testillano PS (2019) Pectin de-methylesterification and AGP increase promote cell wall remodeling and are required during somatic embryogenesis of Quercus suber. Front Plant Sci 9:1915. https://doi.org/10.3389/fpls.2018.01915
Pilarska M, Knox JP, Konieczny R (2013) Arabinogalactan-protein and pectin epitopes in relation to an extracellular matrix surface network and somatic embryogenesis and callogenesis in Trifolium nigrescens Viv. PCTOC 115:35–44. https://doi.org/10.1007/s11240-013-0337-8
Potocka I, Godel K, Dobrowolska I, Kurczyńska EU (2018) Spatio-temporal localization of selected pectic and arabinogalactan protein epitopes and the ultrastructural characteristics of explant cells that accompany the changes in the cell fate during somatic embryogenesis in Arabidopsis thaliana. Plant Physiol Biochem 127:573–589. https://doi.org/10.1016/j.plaphy.2018.04.032
Šamaj J, Bobák M, Blehová A, Pret'ová A (2005) Importance of cytoskeleton and cell wall in somatic embryogenesis. In: Mujib A, Šamaj J (eds) Somatic embryogenesis. Plant cell monographs, vol 2, pp 35–50. Springer, Berlin. https://doi.org/10.1007/7089_024
Silva GB, Ionashiro M, Carrara TB, Crivellari AC, Tiné MA, Prado J, Carpita NC, Buckeridge MS (2011) Cell wall polysaccharides from fern leaves: evidence for a mannan-rich Type III cell wall in Adiantum raddianum. Phytochemistry 72:2352–2360. https://doi.org/10.1016/j.jplph.2021.153526
Silva J, Ferraz R, Dupree P, Showalter AM, Coimbra S (2020) Three decades of advances in arabinogalactan-protein biosynthesis. Front Plant Sci 11:610377. https://doi.org/10.3389/fpls.2020.610377
Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198:452–459. https://doi.org/10.1007/BF00620063
Steinmacher DA, Saare-Surminski K, Lieberei R (2012) Arabinogalactan proteins and the extracellular matrix surface network during peach palm somatic embryogenesis. Physiol Plant 146:336–349. https://doi.org/10.1111/j.1399-3054.2012.01642.x
Sujkowska-Rybkowska M, Rusaczonek A, Kochańska A (2022) Exploring apoplast reorganization in the nodules of Lotus corniculatus L. growing on old Zn–Pb calamine wastes. J Plant Physiol 268:153561. https://doi.org/10.1016/j.jplph.2021.153561
Su S, Higashiyama T (2018) Arabinogalactan proteins and their sugar chains: functions in plant reproduction, research methods, and biosynthesis. Plant Reprod 31:67–75. https://doi.org/10.1007/s00497-018-0329-2
Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferriere N, Nicole M (2001) Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann Bot 88:9–8. https://doi.org/10.1006/anbo.2001.1408
Wang B, Andargie M, Fang R (2022) The function and biosynthesis of callose in high plants. Heliyon 8:e09248. https://doi.org/10.1016/j.heliyon.2022.e09248
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771. https://doi.org/10.1105/tpc.4.7.759
Wu Y, Fan W, Li X, Chen H, Takáč T, Šamajová O, Fabrice MR, Xie L, Ma J, Šamaj J, Xu C (2017) Expression and distribution of extensins and AGPs in susceptible and resistant banana cultivars in response to wounding and Fusarium oxysporum. Sci Rep 20:42400. https://doi.org/10.1038/srep42400
Xu C, Zhao L, Pan X, Šamaj J (2011) Developmental localization and methylesterification of pectin epitopes during somatic embryogenesis of banana (Musa spp. AAA). PLoS ONE 6:e22992. https://doi.org/10.1371/journal.pone.0022992
Yates EA, Valdor J-F, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6:131–139. https://doi.org/10.1093/glycob/6.2.131
Zagorchev L, Odjakova M (2011) Hydroxiproline rich proteins in salt adapted embryogenic suspension cultures of Dactylis glomerata L. Biotechnol Biotechnol Equip 25:2321–2328. https://doi.org/10.5504/BBEQ.2011.0050
Zavaliev R, Ueki S, Epel BL, Citovsky V (2011) Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 248:117–130. https://doi.org/10.1007/s00709-010-0247-0
Zhang B, Gao Y, Zhang L, Zhou Y (2021) The plant cell wall: biosynthesis, construction, and functions. J of Integr Plant Biol 63:251–272. https://doi.org/10.1111/jipb.13055
Funding
This work was supported by the Polish National Science Centre (NCN) Grant No. 2017/27/N/NZ3/00434.
Author information
Authors and Affiliations
Contributions
MG designed and performed the experiments, analyzed the data, and wrote the paper; MS-R contributed to the data collection and analysis for immunostaining; AM supervised the research. All authors were involved in editing the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Victor M. Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzyb, M., Sujkowska-Rybkowska, M. & Mikuła, A. Cell wall remodeling and callose deposition during the embryogenic transition in the tree fern Cyathea delgadii Sternb.. Plant Cell Tiss Organ Cult 156, 30 (2024). https://doi.org/10.1007/s11240-023-02654-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02654-9