Abstract
The current study aimed to evaluate and compare the effect of different concentrations (0.0–200.0 µM) of jasmonic acid (JA), methyl jasmonate (MeJA), and salicylic acid (SA) on suspension culture mass production and untargeted metabolic profiling of the medicinal plant Cymbopogon schoenanthus subsp. proximus. The addition of 50 µM MeJA improved the fresh weight of embryogenic tissue significantly. MeJA and SA did not affect tissue dry weight, whereas JA significantly decreased it. Based on 1H and 1H–13C NMR data and NMR databases, 50 compounds were identified. The addition of stress hormones resulted in the biosynthesis of novel metabolites like theophylline and syringate that were absent in control samples. In addition, significant variations in the concentrations of numerous compounds, including sugars, amino acids, organic acids, phenols, and alkaloids, were observed. The upregulation of trigonelline concentration was observed upon the addition of a higher concentration of MeJA (200 µM), whereas all tested concentrations of SA resulted in its upregulation. Addition of JA and SA causes significant changes in aminoacyl-tRNA biosynthesis pathway and amino acid metabolism pathways, such as alanine-aspartate and glutamate metabolism and arginine and proline metabolism. MeJA had significant impacts on glycolysis and starch-glucose metabolism pathways in addition to amino acids metabolism pathways. The present findings were successful in demonstrating a correlation and distinction between the effects of JA, MeJA, and SA, on the metabolome of Cymbopogon schoenanthus, a valuable medicinal plant. The identified metabolites and their associated pathways would be valuable in future biotechnology applications of the genus Cymbopogon.
Key Message
Exogenous application of stress hormones to Cymbopogon schoenanthus suspension culture impacted metabolic pathways involved in amino acids and protein metabolism, induced novel medicinal compounds (theophylline and syringate) and upregulated osmoprotectants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Cymbopogon, family Poaceae, comprises a large number of aromatic species which possess commercial importance in the fields of drug, food and cosmetics industries (Avoseh et al. 2015). Cymbopogon schoenanthus subsp. proximus is a densely tufted aromatic grass that grows at Gabel Elba and Egypt’s southern borders—an adequate habitat for growing the wild population (Sayed 1980). The wild plant has been utilized for centuries in folk medicine in treatment of renal calculi (Sayed 1980; Heiba and Rizk 1986). The wild plant shoot is a rich source of bioactive metabolites including terpenoid, phenols and alkaloids, which are used in drug production and the cosmetics industries (Abdelsalam et al. 2017; Malin et al. 2018). Proximol®, for example, is a wild plant-based medication used to expel urinary calculi and as an antispasmodic in the treatment of renal colic (Abdullah et al. 2021). The aerial part of the wild plant was found to have varied biological activities including antioxidant (Selim 2011) and anticancer properties against human breast cancer and human colon adenocarcinoma cell lines (Yagi et al. 2020), antimicrobial activity (Moglad et al. 2020) and insecticidal activities (Hasaballah 2018).
Somatic embryogenesis and organogenesis are generally applied for regeneration and micropropagation in various Cymbopogon species, including Cymbopogon pendulus and Cymbopogon winterianus (Bhattacharya et al. 2010; Dey et al. 2015). In previous work, we reported the effects of various factors such as media composition, growth regulators, and sugar concentrations on in vitro regeneration of Cymbopogon schoenanthus subsp. proximus using various tissue culture techniques such as suspension culture (El-Bakry and Abdelsalam 2012), somatic embryogenesis (Abdelsalam et al. 2015), indirect organogenesis (Abdelsalam et al. 2018), and direct tillering (Abdelsalam et al. 2017). In addition, the genetic fidelity of in vitro regenerated plants grown in different tissue culture techniques has been described (Abdelsalam et al. 2019).
Despite the economic significance of the genus Cymbopogon, phytochemical research has concentrated mostly on the essential oil content (Kaur et al. 2021; Kumar et al. 2021), whereas the study of untargeted metabolic profiling is limited to a few studies. Untargeted metabolic profiling of Cymbopogon citratus (Madi et al. 2020) and Cymbopogon schoenanthus (Abdelsalam et al. 2017, 2021, 2022) have been reported. Also, the use of untargeted metabolomic profiling in the distinguishing of Cymbopogon species has been demonstrated (Singh et al. 2017; Otify et al. 2022).
Stress hormone signals, such as salicylic acid (SA), abscisic acid (ABA), and jasmonic acid (JA) enable plants to adapt to biotic and abiotic conditions and enhance the production of bioactive metabolites (Nguyen et al. 2016). These stress hormones regulate a cascade of physiological and biochemical processes that increase the plant’s stress tolerance. For example, ABA closes stomata to reduce water loss and prevent microbe invasion, and it enhances the production of osmoprotectant metabolites like proline (Bharath et al. 2021). JA stimulates the expression of antioxidant genes, and enhances the accumulation of osmo-protective metabolites (Tang et al. 2020; Wang et al. 2021). External application of SA leads to accumulation of a variety of secondary metabolites like phenols and alkaloids compounds (Ali 2021). As evidenced by transcriptome and metabolome data, these effects are associated with alterations and complex interactions within the metabolic pathways. MeJA and SA enhanced the wheat plant’s resistance to Puccinia triticina caused fungal infection by modulating multiple metabolic pathways, such as phenyl alanine biosynthesis, which contributes to flavonoid biosynthesis and consequently improved plant resistance to fungal infection (Kim et al. 2020). Chen et al. 2020 reported that the application of JA to Chrysanthemum enhanced the plant's resistance to western flower thrips via proteomic and metabolic modifications. SA and MeJA both triggered metabolic alterations in Jacobaea vulgaris and J. Aquatica with MeJA having a greater effect than SA (Wei et al. 2021). MeJA stimulated the production of osmoprotectant metabolites and secondary compounds in in vitro-regenerated Cymbopogon schoenanthus subsp. proximus shoots (Abdelsalam et al. 2021).
Cell suspension culture is an appropriate tool for the study and exploration of different biological processes in the plant cell as well as in the production of secondary metabolites as it produces large amounts of uniform material in a relatively short time frame (Casimiro et al. 2023). Suspension culture is frequently used in the production of bioactive metabolites from economically valuable medicinal plants like Dracocephalum polychaetum (Taghizadeh et al. 2019), Dracocephalum kotschyi and D. polychaetum (Taghizadeh et al. 2020), and from Tanacetum parthenium (Pourianezhad et al. 2019). The effects of several biotic elicitors, particularly JA and SA, on the physiological and molecular characteristics of suspension culture of various plant species have been examined in many studies. They are considered to be efficient elicitors for the production of, for example, alkaloids from Papaver armeniacum (Naeini et al. 2021) and improved the phenols and flavonoids content in Prunella vulgaris plants (Tang et al. 2022).
The metabolic reactions of cells in suspension cultures of genus Cymbopogon have not been thoroughly studied. Since the efficiency of biotechnological systems is tied to familiarity with the biosynthetic machinery of a given culture, investigating metabolomic changes is of the utmost importance.
The goal of the present study was to investigate and compare metabolomic changes, identify key metabolites, and determine the biological pathways and their impact, as associated with, the addition of different concentrations of jasmonic acid (JA), methyl jasmonate (MeJA), and salicylic acid (SA) to suspension culture of the wild grass Cymbopogon schoenanthus sub. proximus.
Materials and methods
Plant material
Mature inflorescences of C. Schoenanthus sub. proximus (Hochst. ex A.Rich.) Maire & Weiler were obtained from the botanical garden at Aswan University (coordinate location 24.0° N, 32.5° E) and stored in paper bags at 25 °C in the dark until use. Professor Hasnaa Hosni, professor of plant taxonomy, the Botany department, Cairo University, Egypt, identified the plant material. Three plant specimens were deposited in the Herbarium of the Faculty of Science at Helwan University in Egypt.
Establishment of embryogenic callus culture
As previously described (El-Bakry and Abdel-Salam 2012), the induction and maintenance of embryogenic calli have been performed. Briefly, healthy seeds were rinsed for 15 min in tap water and then washed for 5 min in distilled water. The seed was surface sterilized by dipping it in 70% ethanol for 1 min before agitating for 20 min in 1.05% (v/v) sodium hypochlorite. Seeds were then rinsed three times each for 15 min in sterile double distilled water.
Embryogenic calli were induced by aseptically culturing sterile seeds for four weeks on Murashige and Skoog medium (Murashige and Skoog 1962) with B5 vitamins (Gamborg et al. 1968) (MSB5) medium, supplemented with 1 mg/l 2,4-Dichlorophenoxy acetic acid (2,4-D) and 0.5 mg/l 6-Benzyl amino purine (BAP), 3% sucrose and solidified using 2 g/l phytagel. After subculture on the same medium composition and growth regulator concentrations for another 6 weeks, friable, embryogenic calli were obtained. Embryogenic calli were incubated at 25 °C for 8 h under cool white, fluorescent light (3000 lx).
Cell suspension culture establishment
A preliminary investigation was conducted to determine the appropriate media composition, inoculum weight, growth curve, and liquid media addition for establishing efficient suspension cultures system and are described as follows:
-
1.
1. Effect of different inoculums weight on suspension culture
Various weights of embryogenic callus (0.03, 0.06, 0.125, 0.25, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 10 g) were inoculated into 25 ml MSB5 media supplemented (in 100 ml Erlenmeyer flask) with 1 mg/l 2,4-D and 0.5 mg/l BAP (determined based on previous experiment results). After 4 weeks of culture initiation, fresh and dry weights were recorded. The growth rate was measured in terms of the growth index (GI) for both fresh and dry weights, which was computed using the following equation:
$$GI = \left( {final \, weight {-} initial \, weight} \right) / initial \,weight$$ -
2.
2. Cell growth curve
To determine the cell suspension culture growth curve 0.25 g of fresh embryogenic callus was added to 25 ml MSB5 media supplemented with 1 mg/l 2,4-D in a 100 ml Erlenmeyer flask. The fresh and dry weights of suspension-culture cells were observed at seven-day intervals from the first day to the end of fourth week.
Effect of stress hormones on suspension culture growth and metabolic profiling
Suspension cultures were initiated by inoculating 0.25 g of friable embryogenic calli into a 125 ml Erlenmeyer flask containing 25 ml liquid MSB5 medium supplemented with the same type and concentration of growth regulators as used with solid media according to (El-Bakry and Abdel-Salam 2012). After two weeks, cultures were treated with 5 ml of the same MSB5 medium composition and growth concentrations plus (0.0, 50, 100, 200 µM) jasmonic acid (JA) or methyl jasmonate (MeJA) or salicylic acids (SA). JA, MeJA and SA were sterilized using 0.45 µm filter (Minisart®, Sartorius, Germany). Twelve replicates were carried out for each type and concentration of the stress hormone. Cultures were harvested after 7 days from the stress hormones addition. Suspension cultures were filtered under vacuum using Whatman® filter paper No. 1 and immersed directly in liquid nitrogen, then lyophilized for 24 h using (Labcono®, Kansas City, MO, USA) lyophlizer.
Fresh weight and dry weight data (twelve replicates from each treatment) were statistically analyzed using Minitab® 17 software by one-way ANOVA. Where between treatment differences are significant, treatment means were compared by Fisher least significant difference (LSD) with 95% confidence level.
NMR sample collection, metabolite extraction, data collection and analysis
The dry embryogenic tissue was completely homogenized, and 20 mg of each sample was used for metabolite extraction. Methanol, chloroform, and water were used in a constant ratio of 2:2:1.8 to extract the metabolites, as described by Kim et al. 2010, through the utilization of dry mass and the water loss ratio. The hydrophilic upper layer was separated from the extract and vacuum-dried with a centrivap (Labcono®, Kansas City, MO, USA) for 24 h. The hydrophilic extracts were resuspended in 620 µl of TMSP NMR buffer, which consisted of 1 mM deuterated Trimethylsilylpropanoic acid (TMSP-d4), 100 mM sodium phosphate buffer, and 0.1% sodium azide dissolved in 99.9% D2O. The 700 MHz Bruker Avance™ III spectrometer was used to collect NMR data, with a spectral width of 16.0 ppm and 64 K points, resulting in an acquisition time of 2.9 s. On-resonance pre-saturation was utilized for solvent suppression during a 3 s recycle delay. With 120 scans, 4 dummy scans, a 3 s relaxation delay, and pre-saturation at the residual water frequency, the first increment of the presat-noesy spectra was collected. Each sample’s 90° pulse width was determined using Topspin 2.1.1’s automatic pulse calculation experiment (pulsecal) (Bruker BioSpin, Billerica, MA). Two dimensional 1H–13C HSQC data were collected using a Bruker hsqcedetgpsisp 2.2 pulse sequence. The 1H was observed in the F2 channel with a spectral width of 11 ppm while the 13C was observed in the F1 channel with a spectral width of 180 ppm.
Metabolite identification and NMR-data analysis
The metabolites were identified by comparing the 1H data of the suspension culture with the data present in Chenomx NMR Suite (Chenomx Inc., Edmonton, Alberta, Canada). The 1H–13C HSQC data were compared with 1H–13C HSQC data that was published in the literature as well as data that was available online in HMDB (The Human Metabolome Database, https://hmdb.ca) in order to confirm the Chenomx assignments, particularly in the case of overlapping peaks. MetaboAnalyst 5.0 software (MetaboAnalyst 5.0—a comprehensive server for metabolomic data analysis, https://www.metaboanalyst.ca/home.xhtml) was used to perform statistical analyses on the normalized NMR data. Principal Component Analysis (PCA) was adjusted to 95% confidence intervals. Clustering analyses (heat map and hierarchical cluster) were constructed using Euclidean distance measurement and Ward clustering algorithms. One way analysis of variance (ANOVA) was adjusted to p > 0.05 and Fisher’s LSD post-hoc analysis. Boxplots were generated for the 30 most significant metabolites selected based on p-values calculated using ANOVA. The metabolic pathways that were significantly changed during stress hormone treatment were visualized using enrichment analysis and pathway analysis. Enrichment analysis was performed using metabolite sets based on KEGG human metabolic pathways. Pathway analysis based on significant metabolites was carried out using Oryza sativa (Japanese rice) (KEGG) as a reference.
Results
The present study investigated the effects of various concentrations of jasmonic acid (JA), methyl jasmonate (MeJA), and salicylic acid (SA) (represented as J, M and S respectively, followed by 1, 2 or 3 depending on the concentrations, in the figures) on suspension culture mass production and metabolic profiling of the medicinal aromatic herb Cymbopogon schoenanthus subsp. proximus. To establish an efficient suspension culture system, different factors have been studied. Based on these findings, 0.25 g inoculum weight had the highest values in both fresh and dry weights of suspension cultures (Fig. 1 in the supplementary material). Based on fresh and dry weight data, a growth curve was generated, with the greatest increase in fresh weight occurring in the first two weeks of culture initiation. After four weeks, the growth rate in fresh weight was negligible, while it remained constant for dry weight (Fig. 2 in the supplementary data).
Figure 1 shows the stages of embryogenic callus induction and maturation from natural seeds, as well as the suspension culture of the plant.
Cell suspension culture establishment from Cymbopogon schoenanthus; A The wild plant showing the mature inflorescences; B The wild seeds; C 4 week-old callus culture showing somatic embryos; D 6 week-old embryogenic friable callus showing scutellar stage; E 6 week -old embryogenic friable callus showing mature somatic embryos; F 7 day old suspension culture and G suspension culture filtrate
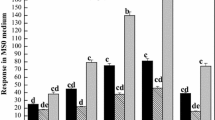
MeJA at 50 µM concentration improved the fresh weight of embryogenic tissue. All JA concentrations lowered both the fresh and dry weights of embryogenic tissue (Fig. 2). The lower concentrations of SA (50.0 and 100.0 µM) significantly lowered the fresh weight of calli, while the higher concentration of SA (200 µM) was statistically similar to the control.
Effect of jasmonic acid (J), methyl jasmonate (M) and salicylic acid (S) on the fresh and dry weight of three weeks old suspension culture from the C. schoenanthus. The letters (a, b, c) assigned to each column indicates the significance between mean of the group being compared at p < 0.05 level according to Fisher test
To determine the effect of stress hormones on the metabolic profiling of the plant suspension culture, 1H and 1H–13C (HSQC) NMR data were generated. Based on the metabolites in the Chenomx database, fifty metabolites were identified among the various NMR signals that characterized the polar extract of suspension culture. The metabolites were identified in the spectral region δ 0.5–10.0 ppm. The identified metabolites include 45% amino acids, 12.5% carbohydrates, 10.4% carboxylic acids, and 8% alkaloids (Fig. 3). Signals of eighteen aliphatic amino acids e.g. alanine, asparagine, and arginine, as well as amines and organic acids, have been identified in the spectrum region δ 0.5–3.0 ppm (Fig. 4A). Glucose, fructose, sucrose, L-fucose, trehalose and xylose signals have been detected in the sugar enhanced signals region (δ 3.5–5.5 ppm) (Fig. 4B). The signals of aromatic amino acid (e.g., tryptophan, phenylalanine) and alkaloids (trigonelline, xanthine, oxypurinol, theophylline) acids were identified in the aromatic area (δ 5.5–9.5 ppm) (Fig. 4C). The chemical shifts of all identified metabolites are listed in Table 1 supplementary data.
Ethanolamine was present in JA and MeJA treatments, as well as, in the control, but not detected in all SA treatments. 2,3-Butanediol and acetate were present in SA treatments and control samples. All concentrations of SA were characterized by the presence of theophylline. Sarcosine was detected only in the 50 µM SA concentration (Table 1).
Glycine and syringate were present in different concentrations of MeJA, but absent in all other treatments (JA, SA) and in the control.
Irrespective of the type and concentration of stress hormone treatment, the following seven compounds were induced, but were absent in the control, namely glycine, malate, sarcosine, syringate, theophylline and uridine.
Table 1 in the supplementary data lists the identified metabolites, along with their chemical formulas, molecular weights, coupling constants, and 1H and 13C chemical shifts.
Principal component analysis (PCA) 2D and 3D scores plots, as well as ANOVA, were used to show the metabolic association between the different stress hormone treatments and the control (Fig. 5). The effect of different JA concentrations is shown in Fig. 4A. The lower concentration (50 µM) overlapped with control in 2D and 3D scores plots, and clearly separated from the other concentrations. PC1 and PC2 explained a total of 74% of the variance and with PC3, 84.4% of total variance (Fig. 5A1 and A2). ANOVA showed 59 metabolites changed significantly in concentration (Fig. 5A3).
Principal component analysis (PCA) 2D (A1, B1, C1), 3D (A2, B2, C2) and One-way analysis of variance (ANOVA) (A3, B3, C3), showing significant metabolite changes between control and A JA, B MeJA and C SA treated samples. ANOVA was calculated based on p-value threshold ˂ 0.05 and using post-hoc tests—Fisher’s least significant difference (LSD). J1 = 50 µM JA; J2 = 100 µM JA; J3 = 200 µM JA; M1 = 50 µM MeJA; M2 = 100 µM MeJA; M3 = 200 µM Me JA; S1 = 50 µM SA; S2 = 100 µM SA; S3 = 200 µM SA and C = control
The effect of MeJA concentrations is shown in Fig. 5B. In a 2D scores plot, the control sample is separated from all various MeJA treatments. The 100.0 and 200.0 µM concentrations overlapped (Fig. 5B1). On the other hand, in a 3D scores plot, all treatments are clearly separated (Fig. 5B2). PC1 and PC2 explained a total of variance 59% while PC1, PC2 and PC3 explained 75.6% of the total variance. ANOVA revealed significant concentration changes in 85 metabolites (Fig. 5B3).
Figure 5C shows the effect of different SA concentrations. All treatments are well separated in 2D and 3D scores plots (Fig. 5C2). ANOVA revealed significant concentration changes in 77 metabolites. PC1 and PC2 explained 55.8% of the total variance while PC1, PC2, and PC3 accounted for 75.7% (Fig. 5B3).
The metabolic relationship between all studied stress hormones and the control are presented in Fig. 6. The PCA 2D scores plot revealed that control, J1 and J3 samples overlap and are present in the same quartile. Also, the samples of the J2, M2, and S2 treatments are overlapping. Additionally, M1, M3, S1 and S3 samples are present in the same quartile (Fig. 6A). Results from hierarchical cluster analysis (HCA) were consistent with those from principal components analysis (PCA). In HCA, there were two main clusters, the first contained the control, J1, and J3 samples, while the second contained all remaining treatments (Fig. 6B).
A Principal component analysis (PCA) and B Hierarchical cluster analysis (HCA) dendrogram showing metabolite relationship between different C. schoenanthus cultures treated with JA, MeJA, and SA. J1 = 50 µM JA; J2 = 100 µM JA; J3 = 200 µM JA; M1 = 50 µM MeJA; M2 = 100 µM MeJA; M3 = 200 µM Me JA; S1 = 50 µM SA; S2 = 100 µM SA; S3 = 200 µM SA and C = control
Figure 7 shows one way Analysis of Variance (ANOVA) showing 98 significant metabolites among control, JA, MeJA and SA treatments.
One-way analysis of variance (ANOVA) showing the significant and non-significant metabolites between C. schoenanthus suspension culture treated with different concentrations of JA, MeJA and SA. ANOVA was calculated based on p-value threshold ˂ 0.05 and using post-hoc tests—Fisher’s least significant difference (LSD)
A heat map shows the relative concentrations of metabolites between the control group and the various types and concentrations of growth hormones in Fig. 8. Based on heat map data, J1 treatment raised the concentration of ornithine, while J2 treatment increased the concentrations of glutamine, β-alanine, glutamate, and serine. Metabolites putrescine and threonine were both increased by J3 addition. M2 treatment showed higher levels of proline, asparagine, and pyruvate, while M3 samples have a larger quantity of betaine. The amounts of trans-aconitate and trehalose increased in samples S2 and S3, respectively. The levels of proline and asparagine increased in M2 and S2 samples.
Heat map analysis created by MetaboAnalyst 5.0 software, using Euclidean distance measurement and Ward clustering algorithms. Each row represents a metabolite, and each column represents a sample. J1 = 50 µM JA; J2 = 100 µM JA; J3 = 200 µM JA; M1 = 50 µM MeJA; M2 = 100 µM MeJA; M3 = 200 µM Me JA; S1 = 50 µM SA; S2 = 100 µM SA; S3 = 200 µM SA and C = control
The relative concentrations of the significant metabolites (selected based on ANOVA test) are shown in Fig. 8. Figure 9A presents the relative concentrations of metabolites associated with the addition of exogenous JA. The accumulation of histamine was greatest when 50 µM JA (J1) was added to suspension cultures compared to other JA concentrations and the control samples. Metabolites glutamine, leucine, isoleucine, ethanolamine, and sucrose were present in control and J1 samples at comparable concentrations. In comparison to other treatments, the addition of 100 µM JA (J2) resulted in higher accumulation of serine, phenylalanine, ornithine, pyroglutamate, β-alanine, glutamate, and oxypurinol. On the other hand, J2 samples showed a downregulation in the amounts of alanine, glutamine, isoleucine, ethanolamine, and glucose-6-P when compared to the control samples. Many metabolites, including proline, threonine, and betaine, were upregulated in samples treated with 200 µM JA (J3).
Boxplots of relative concentrations of the most significant metabolites (based on ANOVA analysis) that significantly changed between control and A Jasmonic acid = J (J1 = 50 µM JA; J2 = 100 µM JA; J3 = 200 µM JA), B Methyl jasmonate = M (M1 = 50 µM MeJA; M2 = 100 µM MeJA; M3 = 200 µM Me JA), and C Salicylic acid = S (S1 = 50 µM SA; S2 = 100 µM SA; S3 = 200 µM SA). The black dots represent the concentration of the selected metabolite in each replicate. The notch indicates the 95% confidence interval around the median of each treatment, defined as ± 1.58* IQR/sqrt (n). The relative concentration of each treatment is indicated by the yellow diamond. The Y axis defines the relative abundances of the specific metabolite and X axis defines the treatment group
With the addition of MeJA, the metabolites isoleucine, valine, leucine, alanine and fructose were found to be downregulated (Fig. 9B). The addition of 50 µM MeJA (M1) enhanced the accumulation of glutamate. Trigonelline, syringate, trehalose, glucose, glucose-6-P, proline, asparagine, and glutamate were all upregulated with 100 µM MeJA (M2). The highest concentration of MeJA (M3) increased the levels of sucrose, betaine, arginine and putrescine.
SA in various concentrations boosted trigonelline, arginine and glutamine accumulation while downregulated alanine, acetate, glycerol and phenylalanine relative concentrations. Concentrations of SA had varying effects on the accumulation of metabolites; for example, 50 µM SA (S1) increased the accumulation of serine, choline, betaine, malate, and malonate, while 100 µM SA (S2) increased the accumulation of asparagine, proline, 4-aminobutyrate, putrescine, and trans-aconitate. The addition of 200 µM SA resulted in a significant increase in the levels of metabolites like trigonelline, arginine, trehalose, glutamate, and ornithine compared to both the control and other SA treatments (Fig. 9C).
The influence of different concentrations of JA, MeJA, and SA on the C. schoenanthus suspension culture's physiological pathways is shown in Fig. 10. The significance of the pathways is characterized in Fig. 10A by the enrichment analysis (analysis based on the metabolites set) and the pathways analysis Fig. 10B (analysis describing the metabolites set and their interaction).
Metabolic pathways in C. schoenanthus polar extract that are significantly altered after stress hormone treatments. A Interactive bar-chart of the enrichment analysis (based on KEGG database) for the effect of A1 = JA, A2 = MeJA, A3 = SA and A4 = combined data of JA, MeJA and SA on suspension culture; The most significant p-values are represented by dark red columns, while the color decreases gradually with decreasing p-values, with pale yellow representing the least significant; the length of the column represent the enrichment ratio; B Pathway analysis (based on KEGG database) showing significantly changed metabolic pathways in response to B1 = JA, B2 = MeJA, B3 = SA and B4 = combined data of JA, MeJA and SA on Cymbopogon schoenanthus suspension culture. The dark red color circles show that the pathways which were strongly affected by stress hormones; As the p-value increases, the color progressively fades, whereas the larger the circle size, the greater the pathway’s influence
Pathway analysis revealed that the addition of JA significantly altered 29 metabolic pathways (Table 2 supplementary data). The effect of different concentrations of JA was observed in aminoacyl-tRNA biosynthesis, arginine and proline metabolism and glutathione metabolism pathways based on both enrichment analysis and pathway analysis (Fig. 10A1 and B1). Amino sugar-nucleotide sugar metabolism and galactose metabolism were the least significantly affected based on enrichment analysis and pathway analysis respectively. Alanine-aspartate-glutamate metabolism, arginine-proline metabolism, and glycine-serine-threonine metabolism pathways represented the main impact during exogenous JA addition (Fig. 10B1). Seven significant metabolites were involved in the aminoacyl-tRNA biosynthesis pathway, including proline, arginine, isoleucine, and glutamine (Fig. 11A). In arginine and proline metabolism pathway, five significant metabolites were involved (arginine, proline, putrescine, glutamate, and ornithine).
The addition of MeJA significantly altered 30 metabolic pathways, according to pathway analysis (Table 3 supplementary data). Aminoacyl-tRNA biosynthesis, starch-sucrose metabolism, and valine-leucine-isoleucine biosynthesis were the pathways most significantly affected by the addition of exogenous MeJA. Valine-leucine-isoleucine biosynthesis produced a higher enrichment ratio (Fig. 10A2). On the other hand, pathway analysis (Fig. 10B2) showed the most significantly altered pathways were aminoacyl-tRNA biosynthesis, alanine-aspartate-glutamate metabolism, arginine-proline metabolism, and pyruvate metabolism, while the highly impacted pathways were phenylalanine metabolism, and alanine-aspartate-glutamate metabolism. The relationship between the significant metabolic pathways and the significant metabolites that were changed because of MeJA addition is shown in Fig. 11B. The high significance of the aminoacyl-tRNA biosynthesis pathway stems from its association with ten significant amino acids, including arginine, proline, phenylalanine, and asparagine. Other metabolic pathways have been altered by the addition of MeJA, which stimulates alkaloid production including tropan, bipyridine, and pyridine alkaloids.
Adding SA to a suspension culture significantly alters 34 metabolic pathways (Table 4 supplementary data). The three most significant pathways were aminoacyl-tRNA biosynthesis, arginine-proline metabolism, and alanine-aspartate-glutamate metabolism, with inositol phosphate metabolism being the least significant (Fig. 10A3 and B3). Alanine-aspartate-glutamate metabolism is the most impacted pathway due to SA addition: this pathway incorporated many key metabolites (alanine, glutamine, asparagine, aspartate) that are involved in many other significantly altered pathways like aminoacyl-tRNA biosynthesis, glycoxalate and dicarboxylate metabolism and C-fixation processes (Fig. 11C).
The combined data for the effect of JA, MeJA, and SA on the metabolic pathways of the C. schoenanthus suspension culture showed that, aminoacyl-tRNA biosynthesis, valine-leucine-isoleucine biosynthesis are the most significantly altered pathways based on enrichment analysis (Fig. 10A4). Pathway analysis (Fig. 10B4) illustrated that the most significantly influenced pathways were aminoacyl-tRNA biosynthesis, glutathione metabolism, and glyoxylate and dicarboxylate metabolism. The phenylalanine metabolism pathway showed the lowest significance according to both enrichment and pathway analysis.
Discussion
In the last two decades, scientists have learned a lot about stress hormones like jasmonate and salicylic acid, as well as their biosynthesis pathways, cell signaling, and roles in plant growth, development, and protection from biotic and abiotic stress (Zhang and Li 2019; Yang et al. 2019; Ghorbel et al. 2021). Jasmonate and salicylic acid are known to play a role in the elicitation of bioactive metabolites (Liu et al. 2023). Most of these studies were carried out on dicotyledonous plants, with monocotyledonous plants receiving far less research. Their impact on the metabolic profile of the genus Cymbopogon has yet to be studied. In the present study, we describe the effect of various concentrations of jasmonic acid, methyl jasmonate, and salicylic acid on the growth as manifested by fresh weight and dry weights of suspension culture, as well as, on the metabolic profile of the polar extract of the medicinal aromatic plant C. schoenanthus subsp. proximus.
The data showed that 50 µM MeJA improves the fresh weight of embryogenic suspension culture, while different JA concentrations decrease both the fresh and dry weights. MeJA has been reported to enhance embryogenic callus biomass in Cymbopogon schoenanthus, whereas it dramatically decreased the biomass of shoots and roots generated from direct organogenesis (Abdelsalam et al. 2021). There have been reports of various effects of MeJA on various plant species, such as the external addition of MeJA increasing the biomass of Melastoma malabathricum suspension culture (See et al. 2011). MeJA at higher concentrations has been reported to inhibit the root and branch growth of the Pharbitis nil plant. (Maciejewska and Kopcewicz 2002). There is evidence that JA controls the balance between plant growth and defensive mechanisms against pathogenic invasions (Hewedy et al. 2023) and induced microspore embryogenesis in Brassica napus (Ahmadi et al. 2014).
According to the present investigation, the fresh weight of suspension cultures treated with SA at varying concentrations did not increase when compared to the control. Dong et al. 2010 have found comparable outcomes. After 48 h of treatment, the addition of SA to a Salvia miltiorrhiza suspension culture had no effect on the growth of cells.
A total of fifty polar metabolites were identified from the polar extract of suspension culture cells when treated with different stress hormone types and concentrations. Among the identified metabolites was trehalose, which was detected in all suspension culture treatments. Previous studies on the metabolic profiling of C. schoenanthus shoots and calli revealed the presence of trehalose only in the polar extract of wild plant shoots, while it was absent in shoots regenerated from various tissue culture systems and greenhouse shoots, as well as embryogenic and organogenic calli (Abdelsalam et al. 2017, 2022). Extreme environmental stress is associated with the presence of trehalose in the shoots of wild plants (Abdelsalam et al. 2017). Therefore, the suspension culture induced comparable metabolic changes to those induced by environmental stress in the wild plant.
Sugars of various types were detected in polar extracts from suspension, with some of those sugars, such as L-fucose and xylose, not previously identified in metabolic profiling of wild, greenhouse, direct and indirect regenerated shoots (Abdelsalam et al. 2017) and embryogenic and organogenic calli (Abdelsalam et al. 2022). This indicates that, compared to other in vitro culture techniques, the suspension culture approach successfully promoted the production of novel and valuable metabolites. L-fucose is a pentose sugar and considered a rare sugar; it has a variety of cosmetic, agricultural, and medical applications (Kim et al. 2019; Iqbal et al. 2021). L-fucose has many potential applications, including pharmaceuticals as anticancer and antitumor (Tomsik et al. 2011; Adhikari et al. 2022) and boosting the immune system; cosmetics for treating skin discoloration and dryness; and for preventing and reversing the effects of aging (Péterszegi et al. 2003a, b; Robert et al. 2005). L-fucose biosynthesis in plants plays a multifunctional role in plant immunity and defense against stress conditions (Zhang et al. 2019a, b).
In the present work, alkaloids (trigonelline, xanthine, oxypurinol and theophylline) constituted 8% of the identified metabolites. Various species of the genus Cymbopogon, such as C. citratus and C. nardus, have been reported to contain alkaloid and phenol compounds (Ang and Manuales 2022; Solekha et al. 2022). We previously documented the presence of trigonelline and xanthine in wild and in vitro regenerated shoots from the same plant (Abdelsalam et al. 2017, 2022).
According to our results, PCA and HCA clearly show that cells' metabolic responses to MeJA and SA are fundamentally distinct in C. schoenanthus suspension culture. MeJA samples are characterized by the presence of the phenolic compound syringate, whereas SA treatments are characterized by the presence of the alkaloid theophylline. Neither metabolite has been previously reported in wild or in vitro-regenerated Cymbopogon schoenanthus plants. Syringate and theophylline have been reported to have therapeutic properties. Theophylline is a purine alkaloid which used to treat lung diseases like chronic obstructive pulmonary disease and asthma (Barnes 2013; Jilani et al. 2022). It acts as an anti-inflammatory agent, bronchodilator, and immunomodulatory agent (Vassallo and Lipsky 1998; Kanehara et al. 2008). It has been reported that light can stimulate theophylline biosynthesis in the leaves of Coffea arabica (Pompelli et al. 2013). In response to drought and salinity stress, theophylline accumulates in Coffea canephora plant (Kumar et al. 2015). Syringate is a naturally occurring phenolic compound which is biosynthesized by some plants like pumpkin, olives and grapes (Srinivasulu et al. 2018). It exhibits useful pharmaceutical properties such as antioxidant, anti-microbial, anti-inflammation, anti-cancer, and anti-diabetic (Srinivasulu et al. 2018). Syringate triggered Arabidopsis plants to accumulate more lignin in their interfascicular fibers (Adams et al. 2020).
PCA scores plots revealed that the lower concentration of JA (50 µM) is more like control on a metabolic level than the same concentration of MeJA and SA, implying that the metabolic changes generated by MeJA and SA were greater than those caused by JA. In addition, analysis of variance revealed that MeJA and SA significantly altered more metabolites than JA. Creelman and Mullet (1997) and Stitz et al. (2011) reported that MeJA is a highly volatile compound that enters the plant cytoplasm and crosses cell membranes more efficiently than JA. It has been proven that MeJA is more effective than other forms of exogenous jasmonates (Kamińska 2021). Also, many studies have shown that MeJA is more effective than JA in terms of plant development and protection from various stress conditions; the inductive impact of exogenous MeJA is more effective than that of JA in enhancing Larix olgensis seedlings resistance to insects (Wang et al. 2015). MeJA enhanced the accumulation of rosmarinic acid in peppermint plants more effectively than JA (Krzyzanowska et al. 2012). In comparison to MeJA, the negative effects of JA on the growth and yield of Oryza sativa were less pronounced (Bhavanam and Stout 2021).
The relative concentrations of metabolites associated with the addition of exogenous jasmonic acid showed that 100 µM JA upregulated metabolites like phenylalanine, ornithine, pyroglutamate, β-alanine, glutamate and oxypurinol. The role of these metabolites in plant protection during stress conditions have been previously reported. Under drought and salt stress, Arabidopsis plants upregulated ornithine production, which helps cope with stress (Kalamaki et al. 2009). Ornithine and glutamate are aliphatic amino acids that serve as precursors to a variety of chemicals, including arginine, proline, putrescine, and polyamines, which help plants withstand stress (Correa-Aragunde et al. 2016). In tomato seedlings cultivated under Pb stress, Bali et al. (2019) found that glutamate, ornithine, and β-alanine levels increased. Glutamate has been reported to accumulate in Setaria italica in response to high soil temperature (Aidoo et al. 2016). Glutamine and pyroglutamate accumulated in kiwi plant in response to pathogen infection (Li et al. 2020).
The addition of higher concentrations of JA (200 µM) improved the accumulation of proline, threonine, betaine, arginine, putrescine, and sarcosine in the polar extract of suspension cultures. According to Sharma et al. (2019), phytohormones such as jasmonates regulate the accumulation of osmolytes such as proline, betaine, and polyamines. Moreover, in watermelon plants, JA signal transduction is responsible for glycine betaine biosynthesis and contributes to plant hardening under osmotic stress (Xu et al. 2018). Putrescine has been found to accumulate during stress conditions and proved to serve several roles in plant defense and protection, such as cation balancing, antioxidant, reactive oxygen species mediated signaling, osmolyte or pH regulation when there is a deficiency of K+ (Alcázar et al. 2010; Cui et al. 2020).
The alkaloid trigonelline and the syringate phenol compound accumulated more when MeJA was added in concentrations of 100 µM. This outcome matched with the results of the pathway studies that revealed that a number of biochemical pathways that were involved in the synthesis of phenolic and alkaloid metabolites such tropan, bipyridine, and pyridine alkaloids as well as phenylalanine metabolism were altered by the addition of MeJA. Trigonelline is a pyridine alkaloid with a variety of therapeutic benefits (Liang et al. 2023). According to Abdelsalam et al. (2021), trigonelline accumulation was increased when exogenous MeJA was added to tissue culture medium of C. schoenanthus organogenic shoots, although the effect on the bioactive sesquiterpene proximadiol differed depending on the MeJA concentration. Exogenous MeJA was used in the elicitation of alkaloid and phenol compound production from the suspension culture of several plants (Mendoza et al. 2018; Zhang et al. 2022). MeJA has been proven to promote the activity of the enzyme-like phenyl-ammonialyase and catalase, which are involved in the production of a range of secondary metabolites (Nabi 2021). MeJA induced the accumulation of alkaloids concentrations in Dendrobium officinale and Nicotiana tabacum plants (Kajikawa et al. 2009; Chen et al. 2019).
In the present study, the carbohydrates trehalose, glucose, and glucose-6-P have been upregulated by the addition of 100 µM MeJA. Carbohydrates are the plant’s principal metabolites and can be produced through the pathways of glycolysis, starch and sucrose metabolism, and galactose metabolism. They play a crucial part in the plant's defense against stress. Trehalose accumulation in watermelon plants was linked positively with the addition of MeJA, and the inhibition of their hydrolysis aids in cell protection during stress conditions (Zhu et al. 2022). MeJA causes a reduction in the expression of the gene’s trehalose-6-phosphate phosphatase and trehalose-6-phosphate synthase in the Populus plant (Gao et al. 2021).
Our data revealed that the addition of 200 µM methyl jasmonate boosted the formation of a range of amino acids such betaine, pyroglutamate, and 4-aminobutyrate, all of which are known to protect plant cells from biotic and abiotic stress (Annunziata et al. 2019; Jiménez-Arias et al. 2019; Shelp et al. 2021).
This study's findings demonstrated that SA, at a variety of concentrations, induced trigonelline accumulation. According to Beygi et al. (2021), the suspension culture of Trigonella foenum-graecum L. accumulated trigonelline in response to the addition of SA. Salicylic acid has been implicated in the accumulation of secondary metabolites in numerous plant species, including Salvia miltiorrhiza and Ginkgo biloba cell culture (Xu et al. 2009; Dong et al. 2010).
Trehalose and arginine were upregulated with the addition of 100 and 200 µM SA. The accumulation of trehalose, which plays an important role as an osmo-protectant metabolite during stress conditions, is reported to be stimulated by SA in spinach leaves when exposed to freezing stress (Min et al. 2018). SA enhanced the accumulation of sugars in shoot and root of cucumber seedling and Arabidopsis leaves (Dong et al. 2011; Gebauer et al. 2017).
Metabolites asparagine and 4-aminobutyrate have been accumulated at a higher rate with 100 µM SA. Similar accumulation of asparagine and 4-aminobutyrate have been observed in response to numerous abiotic and biotic stressors across a wide range of species, including pathogen infection, environmental deprivation (such as high salt concentrations or water deficiency), and nutritional deficiencies (Brikis et al. 2018; Oddy et al. 2020). Many studies have documented the influence of SA on osmoprotectant component accumulation in plants, including Ctenanthe setosa and Saponaria officinalis (Demiralay et al. 2013; Xu et al. 2022).
According to enrichment and pathway analysis in the present study, the addition of stress hormones influences various biological pathways, with the aminoacyl-tRNA biosynthesis pathway, as well as, the amino acid metabolic pathways as arginine and proline metabolism and arginine biosynthesis being the most significant. All these pathways have been reported to be modulated in plants under stress conditions.
The aminoacyl-tRNA biosynthesis pathway is a critical pathway in plant stress responses. This pathway is responsible for the attachment of particular amino acids to their respective transfer RNAs (tRNAs), resulting in the formation of aminoacyl-tRNAs, which are a necessary component in protein synthesis (Yao and Fox 2020). The aminoacyl-tRNA biosynthesis pathway has been reported in numerous studies as the most significantly altered pathway during plant stress. It was significantly altered in ryegrass seedlings exposed to tetracycline (Han et al. 2019), in Elodea nuttallii plants exposed to heavy metals (Cosio and Renault 2020) and was recognized as the most significantly altered pathway in response to heat stress in the Sargassum fusiforme leaf (Liu and Lin 2020). The process of acylating amino acids has the potential to regulate plant immunity against pathogens and pests through the synthesis of defensive plant metabolites or the modulation of plant hormone signalling pathways (Eskandari et al. 2018). The following explains the mechanism underlying the modification of the aminoacyl-tRNA biosynthesis pathway under conditions of stress: The aminoacyl-tRNA biosynthesis pathway is required for the synthesis of stress-related proteins (Fu et al. 2012). Also, because stress can cause translation mistakes, resulting in the biosynthesis of non-functional, misfolded, or toxic proteins, the precise aminoacylation of tRNAs by aminoacyl-tRNA synthetases (enzymes involved in this process) contributes to translation fidelity by ensuring that the proper amino acids are integrated into the developing polypeptide chain (Ranjan and Rodnina 2016). In addition to their role in protein synthesis, under stress, tRNAs alter the level of aminoacylation to become uncharged, and these uncharged tRNAs act as effector molecules that control gene expression, which helps the plant adapt to its stressful environment (Li and Zhou 2009).
Amino acid metabolic pathways constitute an integral part of the plant immune system (Moffett and Namboodiri 2003; Bender 2012). The amino acid metabolism under stress conditions is crucial for cellular viability, as it facilitates energy production, enhances osmotic regulation, and sustains the stability of cell membranes (Wang et al. 2022). Arginine-proline metabolism pathway is one of the main pathways involved in the biosynthesis of arginine and proline. Changes in arginine and proline metabolism have been linked to stress in several plants (Adamipour et al. 2020). The modulation of arginine and proline metabolism during stressful conditions helps plants cope with stressors and maintain cellular homeostasis (Shafi et al. 2019; Du et al. 2021). Proline functions as an osmo-protectant; it serves to uphold cellular water potential, stabilise proteins, eliminate reactive oxygen species, and protect cell membranes against stress-induced damage (Alhasnawi 2019). Arginine participates in the synthesis of polyamines (Grillo and Colombatto 2004), which contribute to stress tolerance by modulating ion transport, scavenging reactive oxygen species, and regulating gene expression (Pottosin et al. 2014; Shi and Chan 2014). According to Huang et al. (2021), the regulation of arginine and proline metabolism in papaya fruit is crucial for overcoming chilling stress. Additionally, the arginine-proline metabolic pathway has been identified as a key pathway in the response to heat stress in Clematis crassifolia (Qian et al. 2022).
The present study demonstrated that the metabolic pathway of alanine-aspartate-glutamate (AAG) displayed a higher impact value in response to all examined stress hormones compared with the control samples. The AAG pathway is known to have a crucial role in the regulation of compatible solutes such as proline, glutathione, and glycine betaine, which act as osmo-protectants. This is because glutamate serves as a precursor for glutathione and proline synthesis (Tapiero et al. 2002; Verslues and Sharma 2010), while aspartate is involved in the biosynthesis of glycine betaine (Han et al. 2021). Moreover, aspartate plays a crucial role in the tricarboxylic acid (TCA) cycle and is also involved in the biosynthesis of malate. Consequently, this process enables the generation of energy that is imperative for coping with stress (Sweetlove et al. 2010). The alteration of alanine-aspartate-glutamate in plants has been documented in relation to various abiotic stresses, including salt stress in two distinct sesame genotypes (Zhang et al. 2019a, b) and heat stress in Sargassum fusiforme (Liu and Lin 2020).
The pathway and enrichment analysis in the present work demonstrated that the addition of varying concentrations of MeJA significantly affected glycolysis and the starch-glucose metabolism pathways. Both pathways function as pivotal centres for energy generation in the plant under adverse conditions. The process of glycolysis serves as the primary stage in cellular respiration, whereby glucose is transformed into pyruvate, resulting in the production of ATP and NADH. These energy-rich molecules play a critical role in energy production and signal transduction during plant stress (Li et al. 2017). Moreover, the process of glycolysis exerts an impact on the manifestation of genes that respond to stress via diverse mechanisms. The multifunctional properties of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have been well documented, with its involvement in transcriptional regulation being one of its known moonlighting functions. The protein GAPDH has the ability to interact with transcription factors that are associated with stress and subsequently regulate their activity, thereby impacting the expression of genes that respond to stress (Plaxton 1996; Zhang et al. 2020). Additionally, the process of glycolysis plays a crucial role in maintaining the redox balance of the cell by generating NADH, which facilitates the reduction of reactive oxygen species (ROS) and thereby mitigates the risk of oxidative harm to cellular constituents (Dumont and Rivoal 2019). Furthermore, glycolysis intermediates are utilised in the process of synthesising secondary metabolites that serve as protective agents, such as flavonoids and phenolics (Bhatla et al. 2018).
The metabolism of starch and sucrose functions as the predominant fuel for a multitude of physiological mechanisms, including the ability to adapt to environmental challenges (Dong and Beckles 2019). Several research studies have documented starch metabolism in response to diverse abiotic stress conditions, as reported by Halmann and Santelia in 2017. The change in starch metabolism can be attributed to either a reduction in the biosynthesis of starch due to a malfunction in the photosynthesis process or an increase in the breakdown of starch into sugars for the purpose of energy production and the synthesis of stress-related sugars such as trehalose, which serves as an osmo-protectant (Dong and Beckles 2019). Sugars have been identified as potential signalling molecules that engage in cross-talk with the ABA-dependent signalling pathway, thereby eliciting the activation of downstream components in the stress response cascade, as reported by Rook et al. (2006). Numerous studies have reported a significant change in starch-sucrose metabolism in various plant species under biotic stress conditions. For instance, Kaur et al. (2019) reported changes in this metabolic pathway in maize plants subjected to heat stress, while Zeng et al. (2021) reported similar findings in peanut plants exposed to waterlogging stress.
Conclusion
The present research elucidated the metabolic response of C. schoenanthus suspension culture treated with different concentrations of 3 different stress hormones (JA, MeJA, SA). Major changes in the metablome included amino acids, carbohydrates, organic acids, phenols and alkaloids. Also, compounds of high medicinal value, namely theophylline and syringate were present in the treated cultures and were not previously recorded. Also, higher concentration of trigonelline has been recorded with SA treated samples. The analysis of compound enrichment and pathway impact demonstrated a significant effect in the aminoacyl-tRNA biosynthesis pathway, arginine and proline metabolism and arginine biosynthesis pathways leading to amino acid increase in JA and SA treatments, and a different response leading to the increase the sugars trehalose and sucrose in MeJA treated samples.
The research results are of clear significance in the future application in biotechnology either through the production of biologically active compounds and the control of their concentration and stable productivity from cell cultures, as well as, their use in metabolic engineering. The understanding of the physiological and molecular changes in gene expression in response to stress hormones treatment are important for both basic science and for their different applications.
Data availability
All data generated or analyzed during this study are included in this manuscript and are available from the corresponding author on reasonable request.
References
Abdelsalam M, Chowdhury K, El Bakry AA (2015) Effect of sugar types, culture age, concentrations of 2, 4-D and sucrose on somatic embryogenesis of Cymbopogon schoenanthus subsp. proximus. Plant Tissue Culture Biotechnol 25:51–62
Abdelsalam A, Mahran E, Chowdhury K, Boroujerdi A, El-Bakry A (2017) NMR-based metabolomic analysis of wild, greenhouse, and in vitro regenerated shoots of Cymbopogon schoenanthus subsp. proximus with GC–MS assessment of proximadiol. Physiol Mol Biol Plants 23:369–383. https://doi.org/10.1007/s12298-017-0432-0
Abdelsalam A, Chowdhury K, El Bakry A (2018) Efficient adventitious morphogenesis from in vitro cultures of the medicinal plant Cymbopogon schoenanthus. Plant Tissue Culture Biotechnol 5:147–160
Abdelsalam A, Chowdhury K, El-Bakry A (2019) Genetic polymorphism of the wild and in vitro regenerated plants of the medicinal grass Cymbopogon schoenanthus subsp. proximus. Notulae Sci Biol 28:222–232
Abdelsalam A, Mahran E, Chowdhury K, Boroujerdi A, El-Bakry A (2021) Effect of exogenous methyl jasmonate on in vitro propagation, metabolic profiling and proximadiol production from Cymbopogon schoenanthus subsp. proximus. Plant Physiol Rep 26:548–560. https://doi.org/10.1007/s40502-021-00608-x
Abdelsalam A, Chowdhury K, Boroujerdi A, El-Bakry A (2022) Nuclear magnetic resonance characterizes metabolic differences in Cymbopogon schoenanthus subsp. proximus embryogenic and organogenic calli and their regenerated shoots. Plant Cell Tissue Organ Cult 149:225–241. https://doi.org/10.1007/s11240-021-02202-3
Abdullah A, Mahdi Younis S, Khudair AN, Selman Abdullah A (2021) Effect of Ammi visnaga water extract compared to proximol (Halfa Bar Extract) in treatment of induced renal lithiasis in a mature female rabbit. Indian J Forensic Med Toxicol 15:2512–2520. https://doi.org/10.37506/ijfmt.v15i4.17083
Adamipour N, Khosh-Khui M, Salehi H, Razi H, Karami A, Moghadam A (2020) Metabolic and genes expression analyses involved in proline metabolism of two rose species under drought stress. Plant Physiol Biochem 155:105–113. https://doi.org/10.1016/J.PLAPHY.2020.07.028
Adams E, Miyazaki T, Moon JY, Sawada Y, Sato M, Toyooka K, Hirai MY, Shin R (2020) Syringic acid alleviates cesium-induced growth defect in Arabidopsis. Int J Mol Sci 21:1–17. https://doi.org/10.3390/ijms21239116
Adhikari E, Burton C, Mockabee-Macias A, Lester DK, Lau E (2022) L-fucose, a sugary regulator of antitumor immunity and immunotherapies. Mol Carcinog 61:439–453. https://doi.org/10.1002/mc.23394
Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA (2014) Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tiss Organ Cult (PCTOC) 116:343–351. https://doi.org/10.1007/s11240-013-0408-x
Aidoo MK, Bdolach E, Fait A, Lazarovitch N, Rachmilevitch S (2016) Tolerance to high soil temperature in foxtail millet (Setaria italica L.) is related to shoot and root growth and metabolism. Plant Physiol Biochem 106:73–81. https://doi.org/10.1016/j.plaphy.2016.04.038
Alcázar R, Planas J, Saxena T, Zarza X, Bortolotti C, Cuevas J, Bitrián M, Tiburcio AF, Altabella T (2010) Putrescine accumulation confers drought tolerance in transgenic Arabidopsis plants over-expressing the homologous Arginine decarboxylase 2 gene. Plant Physiol Biochem 48:547–552. https://doi.org/10.1016/J.PLAPHY.2010.02.002
Alhasnawi AN (2019) Role of proline in plant stress tolerance: a mini review. Res Crops 20:223–229
Ali B (2021) Salicylic acid: an efficient elicitor of secondary metabolite production in plants. Biocatal Agric Biotechnol 31:101884. https://doi.org/10.1016/J.BCAB.2020.101884
Ang AMG, Manuales ADF (2022) Total Alkaloid and Saponin content of the Ethanolic leaf extracts of Cassia alata, Chrysophyllum cainito, Cymbopogon citratus, Lantana camara and Terminalia catappa. Asian J Biol Life Sci 11:157–160. https://doi.org/10.5530/ajbls.2022.11.21
Annunziata MG, Ciarmiello LF, Woodrow P, Dell’aversana E, Carillo P (2019) Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front Plant Sci 10:230. https://doi.org/10.3389/fpls.2019.00230
Avoseh ON, Oyedeji OO, Aremu K, Nkeh-Chungag BN, Songca SP, Oluwafemi SO, Oyedeji AO (2015) Chemical composition and anti-inflammatory activities of the essential oils from Acacia mearnsii de Wild. Nat Prod Res 18:1184–1188
Bali S, Jamwal VL, Kohli SK, Kaur P, Tejpal R, Bhalla V, Ohri P, Gandhi SG, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ali HM, Ahmad P (2019) Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 235:734–748. https://doi.org/10.1016/J.CHEMOSPHERE.2019.06.188
Barnes PJ (2013) Theophylline. Am J Respir Crit Care Med 188:901–906. https://doi.org/10.1164/rccm.201302-0388PP
Bender DA (2012) The metabolism of “surplus” amino acids. Br J Nutr 108:113–121
Beygi Z, Nezamzadeh Z, Rabiei M, Mirakhorli N (2021) Enhanced accumulation of trigonelline by elicitation and osmotic stresses in fenugreek callus culture. Plant Cell Tiss Organ Cult (PCTOC) 147:169–174. https://doi.org/10.1007/s11240-021-02055-w
Bharath P, Gahir S, Raghavendra AS (2021) Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front Plant Sci 12:615114. https://doi.org/10.3389/fpls.2021.615114
Bhatla SC, Lal AM, Bhatla SC (2018) Secondary metabolites. Plant physiology, development and metabolism. Springer, Berlin
Bhattacharya S, Bandopadhyay TK, Ghosh PD (2010) Somatic embryogenesis in Cymbopogon pendulus and evaluation of clonal fidelity of regenerants using ISSR marker. Sci Hortic 123:505–513. https://doi.org/10.1016/j.scienta.2009.10.011
Bhavanam S, Stout M (2021) Seed treatment with Jasmonic acid and methyl Jasmonate induces resistance to insects but reduces plant growth and yield in rice Oryza sativa. Front Plant Sci. https://doi.org/10.3389/fpls.2021.691768
Brikis CJ, Zarei A, Chiu GZ, Deyman KL, Liu J, Trobacher CP, Hoover GJ, Subedi S, DeEll JR, Bozzo GG, Shelp BJ (2018) Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic Res 5:61. https://doi.org/10.1038/s41438-018-0069-3
Casimiro B, Mota I, Veríssimo P, Canhoto J, Correia S (2023) Enhancing the production of hydrolytic enzymes in elicited tamarillo (Solanum betaceum Cav.) cell suspension cultures. Plants 12:190. https://doi.org/10.3390/plants12010190
Chen Y, Wang Y, Lyu P, Chen L, Shen C, Sun C (2019) Comparative transcriptomic analysis reveal the regulation mechanism underlying MeJA-induced accumulation of alkaloids in Dendrobium officinale. J Plant Res 132:419–429. https://doi.org/10.1007/s10265-019-01099-6
Chen G, Kim HK, Klinkhamer PG, Escobar-Bravo R (2020) Site-dependent induction of jasmonic acid-associated chemical defenses against western flower thrips in Chrysanthemum. Planta 251:1–4
Correa-Aragunde N, Negri P, del Castello F, Foresi N, Polacco JC, Lamattina L (2016) The antioxidant power of arginine/nitric oxide attenuates damage induced by methyl viologen herbicides in plant cells. Redox state as a central regulator of plant-cell stress responses. Springer International Publishing, Cham, pp 349–363
Cosio C, Renault D (2020) Effects of cadmium, inorganic mercury and methyl-mercury on the physiology and metabolomic profiles of shoots of the macrophyte Elodea nuttallii. Environ Pollut 257:113557
Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Biol 48:355–381
Cui J, Pottosin I, Lamade E, Guillaume T (2020) What is the role of putrescine accumulated under potassium deficiency? Plant Cell Environ 43:1331–1347. https://doi.org/10.1111/pce.13740
Demiralay M, Saǧlam A, Kadioǧlu A (2013) Salicylic acid delays leaf rolling by inducing antioxidant enzymes and modulating osmoprotectant content in Ctenanthe setosa under osmotic stress. Turk J Biol 37:49–59. https://doi.org/10.3906/biy-1205-16
Dey T, Saha S, Ghosh PD (2015) Somaclonal variation among somatic embryo derived plants—Evaluation of agronomically important somaclones and detection of genetic changes by RAPD in Cymbopogon winterianus. S Afr J Bot 96:112–121. https://doi.org/10.1016/j.sajb.2014.10.010
Dong S, Beckles DM (2019) Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J Plant Physiol 234:80–93. https://doi.org/10.1016/j.jplph.2019.01.007
Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol 148:99–104. https://doi.org/10.1016/j.jbiotec.2010.05.009
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129:629–636. https://doi.org/10.1016/J.SCIENTA.2011.05.005
Du C, Li H, Liu C, Fan H (2021) Understanding of the postgerminative development response to salinity and drought stresses in cucumber seeds by integrated proteomics and transcriptomics analysis. J Proteomics 232:104062
Dumont S, Rivoal J (2019) Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front Plant Sci 10:166
El-Bakry AA, Abdel-Salam AM (2012) Regeneration from embryogenic callus and suspension cultures of the wild medicinal plant Cymbopogon schoenanthus. Afr J Biotech 43:10098–10107. https://doi.org/10.5897/AJB12.331
Eskandari S, Khoshgoftarmanesh AH, Sharifnabi B (2018) The effect of foliar-applied manganese in mineral and complex forms with amino acids on certain defense mechanisms of cucumber (Cucumis sativus L.) against powdery mildew. J Plant Growth Regul 37:481–490
Fu J, Momčilović I, Prasad PV (2012) Roles of protein synthesis elongation factor EF-Tu in heat tolerance in plants. J Bot 1:8. https://doi.org/10.1155/2012/835836
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gao Y, Yang X, Yang X, Zhao T, An X, Chen Z (2021) Characterization and expression pattern of the trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase gene families in Populus. Int J Biol Macromol 187:141–8130. https://doi.org/10.1016/j.ijbiomac.2021.07.096
Gebauer P, Korn M, Engelsdorf T, Sonnewald U, Koch C, Voll LM (2017) Sugar accumulation in leaves of arabidopsis sweet11/sweet12 double mutants enhances priming of the salicylic acid-mediated defense response. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01378
Ghorbel M, Brini F, Sharma A, Landi M (2021) Role of jasmonic acid in plants. Plant Cell Rep 40:1471–1494
Grillo MA, Colombatto S (2004) Metabolism and function in animal tissues of agmatine, a biogenic amine formed from arginine. Amino Acids 26:3–8
Han T, Liang Y, Wu Z, Zhang L, Liu Z, Li Q, Chen X, Guo W, Jiang L, Pan F, Ge S (2019) Effects of tetracycline on growth, oxidative stress response, and metabolite pattern of ryegrass. J Hazard Mater 15:120885
Han M, Zhang C, Suglo P, Sun S, Wang M, Su T (2021) L-Aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 26:1887
Hasaballah AI (2018) The Biological role of Cymbopogon proximus leaf extracts against the malaria vector, anopheles pharoensis. Egyptian Acad J Biol Sci Entomol 11:63–76
Heiba HI, Rizk AM (1986) Constituents of Cymbopogon species. Qatar Univ Sci Bull 6:53–75
Hewedy OA, Elsheery NI, Karkour AM, Elhamouly N, Arafa RA, Mahmoud GAE, Dawood MFA, Hussein WE, Mansour A, Amin DH, Allakhverdiev SI, Zivcak M, Brestic M (2023) Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ Exp Bot 208:105260. https://doi.org/10.1016/J.ENVEXPBOT.2023.105260
Huang Q, Song H, Pan Y, Zhang Z (2021) Exogenous arginine enhances the chilling tolerance in postharvest papaya fruit by regulating arginine and proline metabolism. J Food Process Preserv 4:e15821. https://doi.org/10.1111/jfpp.15821
Iqbal MW, Riaz T, Mahmood S, Ali K, Khan IM, Rehman A, Zhang W, Mu W (2021) A review on selective l-fucose/d-arabinose isomerases for biocatalytic production of l-fuculose/d-ribulose. Int J Biol Macromol 168:558–571. https://doi.org/10.1016/J.IJBIOMAC
Jilani TN, Preuss CV, Sharma S (2022) Theophylline. StatPearls Publishing, Tampa
Jiménez-Arias D, García-Machado FJ, Morales-Sierra S, Luis JC, Suarez E, Hernández M, Valdés F, Borges AA (2019) Lettuce plants treated with L-pyroglutamic acid increase yield under water deficit stress. Environ Exp Bot 158:215–222. https://doi.org/10.1016/J.ENVEXPBOT.2018.10.034
Kajikawa M, Hirai N, Hashimoto T (2009) A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Mol Biol 69:287–298. https://doi.org/10.1007/s11103-008-9424-3
Kalamaki MS, Merkouropoulos G, Kanellis AK (2009) Can ornithine accumulation modulate abiotic stress tolerance in Arabidopsis? Plant Signal Behav 4:1099–1101. https://doi.org/10.4161/psb.4.11.9873
Kamińska M (2021) Role and activity of jasmonates in plants under in vitro conditions. Plant Cell Tissue Organ Culture (PCTOC) 146:425–447
Kanehara M, Yokoyama A, Tomoda Y, Shiota N, Iwamoto H, Ishikawa N, Taooka Y, Haruta Y, Hattori N, Kohno N (2008) Anti-inflammatory effects and clinical efficacy of theophylline and tulobuterol in mild-to-moderate chronic obstructive pulmonary disease. Pulm Pharmacol Ther 21:874–878. https://doi.org/10.1016/J.PUPT.2008.09.003
Kaur H, Kaur K, Gill GK (2019) Modulation of sucrose and starch metabolism by salicylic acid induces thermotolerance in spring maize. Russ J Plant Physiol 66:771–777
Kaur H, Bhardwaj U, Kaur R (2021) Cymbopogon nardus essential oil: a comprehensive review on its chemistry and bioactivity. J Essent Oil Res 33:205–220. https://doi.org/10.1080/10412905.2021.1871976
Kim HK, Choi YH, Verpoorte R (2010) NMR-based metabolomic analysis of plants. Nat Protoc 5:536–549. https://doi.org/10.1038/nprot.2009.237
Kim IJ, Kim DH, Nam KH, Kim KH (2019) Enzymatic synthesis of l-fucose from l-fuculose using a fucose isomerase from Raoultella sp. And the biochemical and structural analyses of the enzyme. Biotechnol Biofuels. https://doi.org/10.1186/s13068-019-1619-0
Kim M, Lee A, Roh YJ, Lee HM, Jo Y, Cho H, Choi DW, Choi M, Eyun SI, Choi C, Chung N (2020) Gene expression and metabolomics profiling of the common wheat obtaining leaf rust resistance by salicylic or jasmonic acid through a novel detached leaf rust assay. Agronomy 10:1668. https://doi.org/10.3390/agronomy10111668
Krzyzanowska J, Czubacka A, Pecio L, Przybys M, Doroszewska T, Stochmal A, Oleszek W (2012) The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tiss Organ Cult (PCTOC) 108:73–81. https://doi.org/10.1007/s11240-011-0014-8
Kumar A, Naik GK, Simmi PS, Giridhar P (2015) Salinity and drought response alleviate caffeine content of young leaves of Coffea canephora var Robusta cv S274. J Appl Biol Biotechnol 3:50–60
Kumar A, Jnanesha AC, Lal RK (2021) Coppicing impact on the essential oil yield and its chemical composition of lemongrass cultivars of the genus Cymbopogon under the semi-arid region of South India. Acta Ecol Sin. https://doi.org/10.1016/J.CHNAES.2021.05.005
Li Y, Zhou H (2009) tRNAs as regulators in gene expression. Sci China Ser C 52–245:252. https://doi.org/10.1007/s11427-009-0039-y
Li M, Zhang K, Long R, Sun Y, Kang J, Zhang T, Cao S (2017) iTRAQ-based comparative proteomic analysis reveals tissue-specific and novel early-stage molecular mechanisms of salt stress response in Carex rigescens. Environ Exp Bot 143:99–114. https://doi.org/10.1016/j.envexpbot.2017.08.010
Li Y, Wang X, Zeng Y, Liu P (2020) Metabolic profiling reveals local and systemic responses of kiwifruit to Pseudomonas syringae pvactinidiae. Plant Direct. https://doi.org/10.1002/pld3.297
Liang Y, Dai X, Cao Y, Wang X, Lu J, Xie L, Liu K, Li X (2023) The neuroprotective and antidiabetic effects of trigonelline: a review of signaling pathways and molecular mechanisms. Biochimie 206:93–104. https://doi.org/10.1016/j.biochi.2022.10.009
Liu L, Lin L (2020) Effect of heat stress on Sargassum fusiforme leaf metabolome. J Plant Biol 63:229–241. https://doi.org/10.1007/s12374-020-09247-5
Liu Y, Tang Y, Zhang W, Liang R, Luo K, Jiang X, Yang P, Xu L, Ming J (2023) Postharvest methyl jasmonate treatment enhanced biological activity by promoting phenylpropanoid metabolic pathways in Lilium brownii var. viridulum. Sci Hortic 308:111551. https://doi.org/10.1016/J.SCIENTA.2022.111551
Maciejewska B, Kopcewicz J (2002) Inhibitory effect of methyl jasmonate on flowering and elongation growth in Pharbitis nil. J Plant Growth Regul 21:216–223. https://doi.org/10.1007/s003440010061
Madi YF, Choucry MA, El-Marasy SA, Meselhy MR, El-Kashoury ESA (2020) UPLC-Orbitrap HRMS metabolic profiling of Cymbopogon citratus cultivated in Egypt; neuroprotective effect against AlCl3-induced neurotoxicity in rats. J Ethnopharmacol 259:112930. https://doi.org/10.1016/J.JEP.2020.112930
Malin MA, Ali MM, Ramadhani AM (2018) GC-MS analysis and antimicrobial activities of Cymbopogon proximus essential oil and phytochemical screening of its crude extracts. J Med Plants 6:117–122
Mendoza D, Cuaspud O, Arias JP, Ruiz O, Arias M (2018) Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol Rep 19:e00273. https://doi.org/10.1016/J.BTRE.2018.E00273
Min K, Showman L, Perera A, Arora R (2018) Salicylic acid-induced freezing tolerance in spinach (Spinacia oleracea L.) leaves explored through metabolite profiling. Environ Exp Bot 156:214–227. https://doi.org/10.1016/J.ENVEXPBOT.2018.09.011
Moffett JR, Namboodiri MA (2003) Tryptophan and the immune response. Immunol Cell Biol 81:247–265
Moglad EH, Hamad AM, Fatima F, Seshadri VD, Naz M (2020) Antimicrobial and wound healing activities of certain Sudanese medicinal plants. Saudi J Biol Sci 27:1766–1772
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Plant Physiol 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nabi N, Singh S (2021) Responses of in vitro cell cultures to elicitation: regulatory role of jasmonic acid and methyl jasmonate: a review. Cell Develop Biol. https://doi.org/10.1007/s11627-020-10140-6/Published
Naeini SM, Naghavi MR, Bihamta MR, Sabokdast M, Salehi M (2021) Production of some benzylisoquinoline alkaloids in Papaver armeniacum L. hairy root cultures elicited with salicylic acid and methyl jasmonate. Vitro Cell Develop Biol Plant 57:261–271
Nguyen D, Rieu I, Mariani C, van Dam NM (2016) How plants handle multiple stresses: hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol Biol 91:727–740. https://doi.org/10.1007/s11103-016-0481-8
Oddy J, Raffan S, Wilkinson MD, Elmore JS, Halford NG (2020) Stress, nutrients and genotype: understanding and managing asparagine accumulation in wheat grain. CABI Agricult Biosci. https://doi.org/10.1186/s43170-020-00010-x
Otify AM, Serag A, Porzel A, Wessjohann LA, Farag MA (2022) NMR metabolome-based classification of Cymbopogon species: a prospect for phyto-equivalency of its different accessions using chemometric tools. Food Anal Methods 15:2095–2106. https://doi.org/10.1007/s12161-022-02257-8
Péterszegi G, Fodil-Bourahla I, Robert AM, Robert L (2003a) Pharmacological properties of fucose. Applications in age-related modifications of connective tissues. Biomed Pharmacother 57:240–245. https://doi.org/10.1016/S0753-3322(03)00028-3
Péterszegi G, Isnard N, Robert AM, Robert L (2003b) Studies on skin aging. Preparation and properties of fucose-rich oligo- and polysaccharides. Effect on fibroblast proliferation and survival. Biomed Pharmacother 57:187–194. https://doi.org/10.1016/S0753-3322(03)00031-3
Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. https://doi.org/10.1146/annurev.arplant.47.1.185
Pompelli MF, Pompelli GM, de Oliveira FM, Antunes WC (2013) The effect of light and nitrogen availability on the caffeine, theophylline and allantoin contents in the leaves of Coffea arabica L. AIMS Environ Sci 1:1–11. https://doi.org/10.3934/environsci.2013.1.1
Pottosin I, Velarde-Buendía AM, Bose J, Zepeda-Jazo I, Shabala S, Dobrovinskaya O (2014) Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: implications for plant adaptive responses. J Exp Bot 65:1271–1283
Pourianezhad F, Rahnama H, Mousavi A, Khosrowshahli M, Mafakheri S (2019) Effects of combined elicitors on parthenolide production and expression of parthenolide synthase (TpPTS) in Tanacetum parthenium hairy root culture. Plant Biotechnol Reports 12:211–218
Qian R, Hu Q, Ma X, Zhang X, Ye Y, Liu H, Gao H, Zheng J (2022) Comparative transcriptome analysis of heat stress responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biol 22(1):1–6. https://doi.org/10.1186/s12870-022-03497-w
Ranjan N, Rodnina MV (2016) tRNA wobble modifications and protein homeostasis. Translation (austin) 28:e1143076. https://doi.org/10.1080/21690731.2016.1143076
Robert C, Robert AM, Robert L (2005) Effect of a preparation containing a fucose-rich polysaccharide on periorbital wrinkles of human voluntaries. Skin Res Technol. https://doi.org/10.1111/j.1600-0846.2005.00100.x
Rook F, Hadingham SA, Li Y, Bevab MW (2006) Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ 29:426–434
Sayed MD (1980) Traditional medicine in health care. J ethnopharmacol 1:19–22
See SK, Bhatt A, Lai Keng C (2011) Effect of sucrose and methyl jasmonate on biomass and anthocyanin production in cell suspension culture of Melastoma malabathricum (Melastomaceae). Rev de Biol Trop 59:597–606
Selim SA (2011) Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas Aceites 62:55–61. https://doi.org/10.1515/znc-2020-0126
Shafi A, Zahoor I, Mushtaq U (2019) Proline accumulation and oxidative stress: diverse roles and mechanism of tolerance and adaptation under salinity stress. Salt Stress Microbes Plant Interact Mech Mol Approaches 2–269:300
Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 17:285. https://doi.org/10.3390/biom9070285
Shelp BJ, Aghdam MS, Flaherty EJ (2021) γ-aminobutyrate (GABA) regulated plant defense: mechanisms and opportunities. Plants 17:1939. https://doi.org/10.3390/plants10091939
Shi H, Chan Z (2014) Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J Integr Plant Biol 56:114–121
Singh P, Bajpai V, Kumar S, Srivastava M, Kumar B (2017) Metabolic profiling and discrimination of Cymbopogon species using direct analysis real time mass spectrometry and principal component analysis. J Med Plants Stud 5:384–391
Solekha R, Ayu P, Setiyowati I, Sahara B, Kusumanegara M, Tri C, Sari U (2022) Phytochemical screening of ethanol extract on stems, leaves, and roots of citronella grass (Cymbopogon nardus L.). BEST J 5:141–147. https://doi.org/10.30743/best.v5i1.5320
Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Suresh Kumar C (2018) Syringic acid (SA)—a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother 108:547–557. https://doi.org/10.1016/J.BIOPHA.2018.09.069
Stitz M, Gase K, Baldwin IT, Gaquerel E (2011) Ectopic expression of AtJMT in Nicotiana attenuata: creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression. Plant Physiol 157:341–154
Sweetlove LJ, Beard KF, Nunes-Nesi A, Fernie AR, Ratcliffe RG (2010) Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci 1:462–470. https://doi.org/10.1016/j.tplants.2010.05.006
Taghizadeh M, Nasibi F, Kalantari KM, Ghanati F (2019) Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation. Plant Cell Tiss Organ Cult (PCTOC) 15:489–498. https://doi.org/10.1007/s11240-018-01530-1
Taghizadeh M, Nasibi F, Kalantari KM, Benakashani F (2020) Callogenesis optimization and cell suspension culture establishment of Dracocephalum polychaetum Bornm and Dracocephalum kotschyi Boiss: An in vitro approach for secondary metabolite production. S Afr J Bot 1:79–86. https://doi.org/10.1016/j.sajb.2020.04.015
Tang G, Ma J, Hause B, Nick P, Riemann M (2020) Jasmonate is required for the response to osmotic stress in rice. Environ Exp Bot 175:104047. https://doi.org/10.1016/J.ENVEXPBOT.2020.104047
Tang H, Hu J, Zhao M, Cao L, Chen Y (2022) Comparative study of the physiological responses, secondary metabolites, and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiol Plant 45:20. https://doi.org/10.1007/s11738-022-03498-0
Tapiero H, Mathe G, Couvreur P, Tew KD (2002) Glutamine and glutamate. Biomed Pharm 56:446–457
Tomsik P, Soukup T, Cermakova E, Micuda S, Niang M, Sucha L, Rezacova M (2011) L-rhamnose and L-fucose suppress cancer growth in mice. Cent Eur J Biol 6:1–9. https://doi.org/10.2478/s11535-010-0087-0
Vassallo R, Lipsky JJ (1998) Theophylline: recent advances in the understanding of its mode of action and uses in clinical practice. Mayo Clin Proc 73:346–354. https://doi.org/10.1016/S0025-6196(11)63701-4
Verslues PE, Sharma S (2010) Proline metabolism and its implications for plant-environment interaction. Am Soc Plant Biol 8:e0140
Wang Y, Jia Q, Zhang L, Zhang Z, Zhang H (2015) Allelic variation in cinnamyl alcohol dehydrogenase (LoCAD) associated with wood properties of Larix olgensis. Forests 12:1649–1665
Wang Y, Mostafa S, Zeng W, Jin B (2021) Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int J Mol Sci 22:8568. https://doi.org/10.3390/ijms22168568
Wang L, Wang G, Cui J, Wang X, Li M, Qi X, Li X, Li Y, Ma L (2022) Transcriptomics, metabolomics, antioxidant enzymes activities and respiration rate analysis reveal the molecular responses of rice to Cd stress and/or elevated CO2 concentration. Plant Soil 8:1–22
Wei X, Vrieling K, Kim HK, Mulder PP, Klinkhamer PG (2021) Application of methyl jasmonate and salicylic acid lead to contrasting effects on the plant’s metabolome and herbivory. Plant Sci 303:110784. https://doi.org/10.1016/j.plantsci.2020.110784
Xu M, Dong J, Wang H, Huang L (2009) Complementary action of jasmonic acid on salicylic acid in mediating fungal elicitor-induced flavonol glycoside accumulation of Ginkgo biloba cells. Plant Cell Environ 32:960–967. https://doi.org/10.1111/j.1365-3040.2009.01976.x
Xu Z, Sun M, Jiang X, Sun H, Dang X, Cong H, Qiao F (2018) Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells. Front Plant Sci 12:1469. https://doi.org/10.3389/fpls.2018.01469
Xu L, Chen H, Zhang T, Deng Y, Yan J, Wang L (2022) Salicylic acid improves the salt tolerance capacity of Saponaria officinalis by modulating its photosynthetic rate, osmoprotectants, antioxidant levels, and ion homeostasis. Agronomy. https://doi.org/10.3390/agronomy12061443
Yagi S, Mohammed AB, Tzanova T, Schohn H, Abdelgadir H, Stefanucci A, Mollica A, Zengin G (2020) Chemical profile, antiproliferative, antioxidant, and enzyme inhibition activities and docking studies of Cymbopogon schoenanthus (L.) Spreng. and Cymbopogon nervatus (Hochst.) Chiov. from Sudan. J Food Biochem 44:13107
Yang J, Duan G, Li C, Liu L, Han G, Zhang Y, Wang C (2019) The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front Plant Sci 18:1349. https://doi.org/10.3389/fpls.2019.01349.eCollection
Yao P, Fox PL (2020) Aminoacyl-tRNA synthetases in cell signaling. Biology of aminoacyl-tRNA synthetases, vol 48. Academic Press, Cambridge, p 243275
Zeng R, Chen T, Wang X, Cao J, Li X, Xu X, Chen L, Xia Q, Dong Y, Huang L, Wang L (2021) Physiological and expressional regulation on photosynthesis, starch and sucrose metabolism response to waterlogging stress in peanut. Front Plant Sci 12:601771
Zhang Y, Li X (2019) Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol 50:29–36. https://doi.org/10.1016/J.PBI.2019.02.004
Zhang L, Paasch BC, Chen J, Day B, He SY (2019a) An important role of l-fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. New Phytol 222:981–994. https://doi.org/10.1111/nph.15639
Zhang Y, Li D, Zhou R, Wang X, Dossa K, Wang L, Zhang Y, Yu J, Gong H, Zhang X, You J (2019b) Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol 19:1–4. https://doi.org/10.1186/s12870-019-1665-6
Zhang Y, Sampathkumar A, Kerber SM, Swart C, Hille C, Seerangan K, Graf A, Sweetlove L, Fernie AR (2020) A moonlighting role for enzymes of glycolysis in the co-localization of mitochondria and chloroplasts. Nat Commun 11:4509. https://doi.org/10.1038/s41467-020-18234-w
Zhang B, Niu Z, Li C, Hou Z, Xue Q, Liu W, Ding X (2022) Improving large-scale biomass and total alkaloid production of Dendrobium nobile Lindl using a temporary immersion bioreactor system and MeJA elicitation. Plant Methods 18:10. https://doi.org/10.1186/s13007-022-00843-9
Zhu F, Li M, Sun M, Jiang X, Qiao F (2022) Plant hormone signals regulate trehalose accumulation against osmotic stress in watermelon cells. Protoplasma 5:1351–1369. https://doi.org/10.1007/s00709-021-01715-0
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors participated in the idea and design of the research. AA was responsible for the preparation of materials, collecting of data, and analysis. AB was responsible for gathering NMR data and identifying metabolites. AA wrote the first draft of the manuscript. AE made considerable changes and adjustments to the initial text, focusing on essential themes and the manuscript's primary objective. All authors revised the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. AA, KC, AB, and AE declare that they have no conflict of interest.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Additional information
Communicated by Agnieszka Szopa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelsalam, A., Chowdhury, K., Boroujerdi, A. et al. Effect of stress hormones on the metabolome of a suspension culture of the aromatic medicinal plant Cymbopogon schoenanthus subsp. proximus. Plant Cell Tiss Organ Cult 155, 137–163 (2023). https://doi.org/10.1007/s11240-023-02560-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02560-0