Abstract
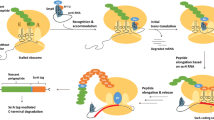
Transfer RNAs (tRNAs) hold a central place in protein synthesis by interpreting the genetic information stored in DNA into the amino acid sequence of protein, thus functioning as “adaptor” molecules. In recent years, however, various studies have shown that tRNAs have additional functions beyond participating in protein synthesis. When suffering from certain nutritional stresses, tRNAs change the level of aminoacylation to became uncharged, and these uncharged tRNAs act as effector molecules to regulate global gene expression, so that the stressed organism copes with the adverse environmental stresses. In budding yeast and certain mammalian cells, the retrograde movement of mature tRNAs from cytoplasm to nucleus serves as a mechanism for the surveillance system within the nucleus to continue monitoring the integrity of tRNAs. On the other hand, this retrograde action effectively reduces the global protein synthesis level under conditions of nutritional starvation. Quite recently, various publications have shown that tRNAs are not stable molecules in an absolute sense. Under certain physiological or environmental stresses, they are specifically cleaved into fragments of different lengths in the anticodon loop or anticodon left arm. These cleavages are not a meaningless random degradation phenomenon. Instead, a novel class of signal molecules such as tRNA halves or sitRNAs may be produced, which are closely correlated with the modulation of global gene expression. Investigation of the regulatory functions of tRNAs is a frontier, which seeks to reveal the structural and functional diversity of tRNAs as well as their vital functions during the expression of genetic information.
Similar content being viewed by others
References
Hopper A K, Phizicky E M. tRNA transfers to the limelight. Genes Dev, 2003, 17(2): 162–180 12533506, 10.1101/gad.1049103, 1:CAS:528:DC%2BD3sXmtFyitw%3D%3D
Wang D B, Liu W Y. Transfer RNA-structure, function and sythesis. Hangzhou: Zhejiang Science and Technology Press, 1995. 144–152
McFarland R, Elson J L, Taylor R W, et al. Assigning pathogenicity to mitochondrial tRNA mutations: when “definitely maybe” is not good enough. Trends Genet, 2004, 20(12): 591–596 15522452, 10.1016/j.tig.2004.09.014, 1:CAS:528:DC%2BD2cXpt1Whtb8%3D
Cashel M, Gallant J. Two compounds implicated in the function of the RC gene of E. coli. Nature, 1969, 221: 838–841 4885263, 10.1038/221838a0, 1:CAS:528:DyaF1MXpsVWgsw%3D%3D
Cashel M, Gentry D R, Hernandez V J, et al. The stringent response. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington DC: ASM Press, 1996. 1458–1496
Magnusson L U, Farewell A, Nystrom T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol, 2005, 13(5): 236–242 15866041, 10.1016/j.tim.2005.03.008, 1:CAS:528:DC%2BD2MXjslKhtro%3D
Chatterji D, Fujita N, Ishihama A. The mediator for stringent control, ppGpp, binds to the β-subunit of Escherichia coli RNA polymerase. Genes Cells, 1998, 3(5): 279–287 9685179, 10.1046/j.1365-2443.1998.00190.x, 1:CAS:528:DyaK1cXltFOqtbg%3D
Toulokhonov I I, Shulgina I, Hernandez V J. Binding of the transcription effector ppGpp to Escherichia coli RNA polymerase is allosteric, modular, and occurs near the N terminus of the beta′-subunit. J Biol Chem, 2001, 276(2): 1220–1225 11035017, 10.1074/jbc.M007184200, 1:CAS:528:DC%2BD3MXmtVyruw%3D%3D
Kuroda A, Murphy H, Cashel M, et al. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J Biol Chem, 1997, 272(134): 21240–21243 9261133, 10.1074/jbc.272.34.21240, 1:CAS:528:DyaK2sXls1OjtLs%3D
Kuroda A, Tanaka S, Ikeda T, et al. Inorganic polyphosphate kinase is required to stimulate protein degradation and for adaptation to amino acid starvation in Escherichia coli. Proc Natl Acad Sci USA, 1999, 96(25): 14264–14269 10588694, 10.1073/pnas.96.25.14264, 1:CAS:528:DyaK1MXnvFKltb8%3D
Chatterji D, Ojha A K. Revisiting the stringent response, ppGpp and starvation signaling. Curr Opin Microbiol, 2001, 4(2): 160–165 11282471, 10.1016/S1369-5274(00)00182-X, 1:CAS:528:DC%2BD3MXjvVart7g%3D
Grundy F J, Winkler W C, Henkin T M. tRNA-mediated transcription anti-termination in vitro: codon-anticodon pairing independent of the ribosome. Proc Natl Acad Sci USA, 2002, 99(17): 11121–11126 12165569, 10.1073/pnas.162366799, 1:CAS:528:DC%2BD38XmslSmtrY%3D
Nelson A R, Henkin T M, Agris P F. tRNA regulation of gene expression: interactions of an mRNA 5′-UTR with a regulatory tRNA. RNA, 2006, 12(7): 1254–1261 16741230, 10.1261/rna.29906, 1:CAS:528:DC%2BD28Xms1Cqurc%3D
Dong J, Qiu H, Garcia-Barrio M, et al. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell, 2000, 6(2): 269–279 10983975, 10.1016/S1097-2765(00)00028-9, 1:CAS:528:DC%2BD3cXmsFWmsb4%3D
Hao S, Sharp J W, Ross-Inta C M, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science, 2005, 307(5716): 1776–1778 15774759, 10.1126/science.1104882, 1:CAS:528:DC%2BD2MXit1yrs78%3D
Wilson W A, Roach P J. Nutrient-regulated protein kinases in budding yeast. Cell, 2002, 111(2): 155–158 12408859, 10.1016/S0092-8674(02)01043-7, 1:CAS:528:DC%2BD38Xotlaiu7k%3D
Hinnebusch A G, Natarajan K. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot Cell, 2002, 1(1): 22–32 12455968, 10.1128/EC.01.1.22-32.2002, 1:CAS:528:DC%2BD38XitVSls78%3D
Yang R, Wek S A, Wek, R C. Glucose limitation induces GCN4 translation by activation of Gcn2 protein kinase. Mol Cell Biol, 2000, 20(8): 2706–2717 10733573, 10.1128/MCB.20.8.2706-2717.2000, 1:CAS:528:DC%2BD3cXit1ymu78%3D
Wolin S L, Matera A G. The trials and travels of tRNA. Genes Dev, 1999, 13(1): 1–10 9887094, 10.1101/gad.13.1.1, 1:CAS:528:DyaK1MXnvF2jsw%3D%3D
Nakanishi K, Nureki O. Recent progress of structural biology of tRNA processing and modification. Mol Cells, 2005, 19(2): 157–166 15879697, 1:CAS:528:DC%2BD2MXksFamu7c%3D
Ibba M, Söll D. Aminoacyl-tRNAs: setting the limits of the genetic code. Genes Dev, 2004, 18: 731–738 15082526, 10.1101/gad.1187404, 1:CAS:528:DC%2BD2cXjsVGksb4%3D
Lund E, Dahlberg J E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science, 1998, 282(5396): 2082–2085 9851929, 10.1126/science.282.5396.2082, 1:CAS:528:DyaK1cXotVyjs7c%3D
Takano A, Endo T, Yoshihisa T. tRNA actively shuttles between the nucleus and cytosol in yeast. Science, 2005, 309(5731): 140–142 15905365, 10.1126/science.1113346, 1:CAS:528:DC%2BD2MXlsF2ht78%3D
Shaheen H H, Hopper A K. Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc Natl Acad Sci USA, 2005, 102(32): 11290–11295 16040803, 10.1073/pnas.0503836102, 1:CAS:528:DC%2BD2MXoslKit7w%3D
Whitney M L, Hurto R L, Shaheen H H, et al. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol Biol Cell, 2007, 18(7): 2678–2686 17475781, 10.1091/mbc.E07-01-0006, 1:CAS:528:DC%2BD2sXnsVWqtL0%3D
Shaheen H H, Horetsky R L, Kimball S R, et al. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci USA, 2007, 104(21): 8845–8850 17502605, 10.1073/pnas.0700765104, 1:CAS:528:DC%2BD2sXmt1aku74%3D
Deutscher M P. Degradation of stable RNA in bacteria. J Biol Chem, 2003, 278(46): 45041–45044 12941949, 10.1074/jbc.R300031200, 1:CAS:528:DC%2BD3sXoslyqt7o%3D
Kaufmann G. Anticodon nucleases. Trends Biochem Sci, 2000, 25(2): 70–74 10664586, 10.1016/S0968-0004(99)01525-X, 1:CAS:528:DC%2BD3cXht1Krsrg%3D
Li Z W, Reimers S, Pandit S, et al. RNA quality control: degradation of defective transfer RNA. EMBO J, 2002, 21(5): 1132–1138 11867541, 10.1093/emboj/21.5.1132, 1:CAS:528:DC%2BD38XitFyltrY%3D
Lee S R, Collins K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J Biol Chem, 2005, 280(52): 42744–42749 16272149, 10.1074/jbc.M510356200, 1:CAS:528:DC%2BD2MXhtlCmsLbN
Ogawa T, Tomita K, Ueda T, et al. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science, 1999, 283(5410): 2097–2100 10092236, 10.1126/science.283.5410.2097, 1:CAS:528:DyaK1MXit12gu7s%3D
Tomita K, Ogawa T, Uozumi T, et al. A cytotoxic ribonuclease which specifically cleaves four isoaccepting arginine tRNAs at their anticodon loops. Proc Natl Acad Sci USA, 2000, 97(15): 8278–8283 10880568, 10.1073/pnas.140213797, 1:CAS:528:DC%2BD3cXlt1Ggt7Y%3D
Claessen D, de Jong W, Dijkhuizen L, et al. Regulation of Streptomyces development: reach for the sky! Trends Microbiol, 2006, 14(7): 313–319 16759865, 10.1016/j.tim.2006.05.008, 1:CAS:528:DC%2BD28XmsFSlt70%3D
Haiser H J, Karginov F V, Hannon G J, et al. Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic Acids Res, 2008, 36(3): 732–741 18084030, 10.1093/nar/gkm1096, 1:CAS:528:DC%2BD1cXitVKktr4%3D
Jöchl C, Rederstorff M, Hertel J, et al. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res, 2008, 36(8): 2677–2689 18346967, 10.1093/nar/gkn123
Fu H J, Feng J J, Tie Y, et al. Preliminary studies about the biogenesis mechanism of half-tRNAs. The Fifth National Symposium on Ribonucleic Acid (RNA), Shanghai, China
Thompson D M, Lu C, Green P J, et al. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA, 2008, 14(10): 2095–2103 18719243, 10.1261/rna.1232808, 1:CAS:528:DC%2BD1cXht1ektLfI
Adam R D. Biology of Giardia lamblia. Clin Microbiol Rev, 2001, 14(3): 447–475 11432808, 10.1128/CMR.14.3.447-475.2001, 1:CAS:528:DC%2BD3MXlslWlsLw%3D
Luján H D, Mowatt M R, Nash T E. Mechanisms of Giardia lamblia differentiation into cysts. Microbiol Mol Biol Rev, 1998, 61(3): 294–304
Morrison H G, McArthur A G, Gillin F D, et al. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science, 2007, 317(5846): 1921–1926 17901334, 10.1126/science.1143837, 1:CAS:528:DC%2BD2sXhtVOlt73L
Li Y, Luo J, Zhou H, et al. Stress-induced tRNA-derived RNAs: a novel class of small RNAs in the primitive eukaryote Giardia lamblia. Nucleic Acids Res, 2008, 36, 6048–6055 18820301, 10.1093/nar/gkn596, 1:CAS:528:DC%2BD1cXhtlGgs73E
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (Grant Nos. 30870530 and 30570398) and the National Key Basic Research Program of China (Grant No. 2005CB724600)
Rights and permissions
About this article
Cite this article
Li, Y., Zhou, H. tRNAs as regulators in gene expression. SCI CHINA SER C 52, 245–252 (2009). https://doi.org/10.1007/s11427-009-0039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-009-0039-y