Abstract
Somatic embryogenesis is the most common regeneration method for the application of new genomic techniques like cisgenesis/intragenesis, genome editing, and RNAi. However, some local important genotypes show recalcitrance to this morphogenetic strategy, which represents an obstacle for the application of genetic engineering techniques. Whole flowers, stamens, and pistils of three different Italian Vitis vinifera L. cultivars (Ancellotta, Glera, and Lambrusco Salamino), and four hybrid rootstocks (110 Richter, 17.37, SO4, Star 50) have been tested in several culture media with changing basal salts (NN and MS), different combinations of growth regulators (BAP, 2,4-D, NOA, PIC, and NAA), and gelling agents, to initiate somatic embryogenesis. The formation of embryogenic calli was observed mainly from whole flowers cultured on PIV medium (NN salts, B5 vitamins, 3 g L−1 gelrite, 60 g L−1 sucrose, 8.9 µM BAP, and 4.5 µM 2,4-D), and stamens on MS1 medium (MS salts and vitamins, 7 g L−1 plant agar, 20 g L−1 sucrose, 4.5 µM BAP, and 5 µM 2,4-D), in the cv. Ancellotta, Lambrusco Salamino, and all the rootstocks, except for Star 50, which showed the best embryogenetic response from pistils placed on MS1. In a recalcitrant cv. as Glera, pistils placed on MS medium supplemented with 1 µM BAP, 5 µM 2,4-D, and gelrite as gelling agent, showed the highest percentage of embryogenesis. In addition, a two-step protocol was efficiently optimized for further induction of secondary embryo production for the above-listed grapevine genotypes, which guaranteed the long-term maintenance of embryogenic cultures from clusters or single somatic embryos.
Key message
Different types of explants, induction media combinations and concentrations influence the efficiency of regeneration via somatic embryogenesis in recalcitrant Vitis cultivars and rootstocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of an efficient in vitro regeneration protocol is the basis for the application of each biotechnological approach and can be used to speed up genetic improvement programs (Sabbadini et al. 2019a, b).
Somatic embryogenesis is an efficient regeneration model system for functional studies as well as for large-scale plant propagation in many crops, including grapevine (Von Arnold et al. 2002; Correia et al. 2019). Research on somatic embryogenesis processes in grapevine has been largely described in terms of culture improvement or protocol adaptation to various genotypes, although the molecular basis of this phenomenon has been addressed by few studies (Marsoni et al. 2008; Dal Santo et al. 2021). Somatic embryogenesis consists of the formation of somatic embryos with a structure very similar to a zygotic embryo but derived from the morphogenesis of cells without fertilization (Martinelli and Gribaudo 2009). Although somatic embryogenesis was proved to be a successful tool for grapevine in vitro regeneration and optimized for many Vitis species, this technique is strongly genotype-dependent, and its efficiency varies according to the starting explant chosen (Vidal et al. 2009). Different patterns of gene expression are observed during the acquisition of embryogenic competence, which makes induction and maintenance of this process particularly difficult (Schellenbaum et al. 2008; Yang and Zhang 2010; Dal Santo et al. 2021). The entire morphogenetic process relies on a unique developmental pathway characterized by various phases, that can be summarised as follows: (i) induction of callogenesis and acquisition of embryogenic competence; (ii) culture and development of embryogenic calli and characterization of embryo potential; (iii) long term culture of embryogenic calli and embryos proliferation; (iv) somatic embryos conversion in whole plantlets (Martinelli and Gribaudo 2009).
During the first step of culture initiation, somatic cells must undergo an internal cell reprogramming of gene expression patterns leading to a reversion of differentiation state followed by a polarized cell division (Corredoira et al. 2019; Fehér 2019). The obtainment of somatic embryos is possible through direct or indirect developmental ways that are often not well distinguishable. The indirect somatic embryogenesis starts with the development of an embryogenic callus followed by the emergence of pro embryogenic masses (PEMs), from which cell clusters or single cells form new somatic embryos (Horstman et al. 2017). The direct somatic embryogenesis occurs directly from the explant without the callus development phase, through the proliferation of already formed pre-embryogenic determined cells. In many woody plant species, direct somatic embryogenesis is preferred for clonal mass propagation rather than indirect somatic embryogenesis, which is characterized by a high incidence of somaclonal variation (Ghadirzadeh-Khorzoghi et al. 2019). Somatic embryos are widely used in grapevine breeding programs, and the most employed initial explants are anthers, ovaries, leaves, petioles, tendrils, and nodal sections (Catalano et al. 2021). Although the mineral composition of the culture medium is noteworthy, the type and concentrations of plant growth regulators (PGRs) have been demonstrated to be crucial in the embryogenic callus induction phase (Corredoira et al. 2019). Somatic embryogenesis can be considered as a stress-related plant reaction, and one, although not the only source of stress can be associated with the exogenous addition of PGRs (Zavattieri et al. 2010). Plant organs in vitro regeneration are generally controlled by PGRs, normally auxins and cytokinins, with the first ones that exert an important role in both early-stage embryo patterning and somatic embryos induction (Nic-Can and Loyola-Vargas 2016). In particular, the use of the synthetic herbicide 2,4-Dichlorophenoxyacetic acid (2,4-D) has been reported in several studies involving the stimulation of somatic embryogenesis, being able to modulate the auxin content and cause oxidative stress in plant tissues (Maillot et al. 2009; Ricci et al. 2020). Other factors such as pH and photoperiod conditions, carbonic sources, gelling agents, and the presence of antioxidant compounds can also influence embryogenic responses (Martinelli and Gribaudo 2009; Martínez et al. 2019). It has been demonstrated that the regeneration ability is genotype- and tissue-specific, and the process that leads the cell to form an entire new organism is not straightforward, hence requiring the ability to overcome numerous barriers both at the genetic and physiological level (Sugimoto et al. 2019). Depending on the regeneration efficiency of each genotype, somatic embryos can arise from the starting explants following a one-step process in which induction and expression of genes involved in the embryogenic competence occur in the same medium, or a two-step process, in which, after being cultured on an induction medium, the calli are transferred to an expression medium with very low or no concentration of the cytokinins-auxins (Corredoira et al. 2019). The maintenance of embryogenic activity is commonly achieved by the frequent subculture of PEMs that are continuously produced through their culture on PGRs free medium, or by inducing secondary embryogenesis (Vannini et al. 2005). Somatic embryos mainly develop randomly because not all embryogenic calli differentiate at the same time. In this context, secondary embryogenesis is the favored morphogenetic process to ensure the establishment of cycling somatic embryos cultures, and to increase somatic embryo production in recalcitrant genotypes (Mazri et al. 2020). Secondary embryogenesis allows the simultaneous regeneration of multiple PEMs, increasing the number of somatic embryos produced (Maillot et al. 2009). High plant regeneration rates through secondary embryos are also useful for the application of genetic transformation-based techniques because this system can prevent the development of chimerism, allowing the regeneration of completely transformed secondary embryos emerging from only the transformed part of the original primary embryos when cultured in selective condition (Eudes et al. 2003; Pérez-Núñez et al. 2006).
The use of somatic embryogenesis as a regeneration system for the application of new genomic techniques aimed at genetic improvement of grapevine is strictly dictated by the genotype and initial starting type of tissue, thus it is important to study and develop the most efficient protocols for inducing high production of primary and/or secondary somatic embryos for all major grapevine and table grape cultivars and rootstocks.
In view of developing highly efficient regeneration protocols for important grapevine cultivars and rootstocks, floriferous explants of widely cultivated Italian cultivars Ancellotta (4343 Ha), Glera (18,255 Ha), Lambrusco Salamino (5003 Ha), and the hybrid rootstocks 110 Richter (Vitis berlandieri Rességuier n. 2 × Vitis rupestris Martin), 17.37 (Vitis berlandieri x Vitis rupestris), SO4 (Selection Oppenheim #4) (Vitis berlandieri Planchon x Vitis riparia Michaux), and Star 50 (Vitis berlandieri x Vitis riparia) were used for the induction of somatic embryogenesis. A two-step protocol was optimized for further induction of secondary embryo production for the above-listed grapevine genotypes.
Materials and methods
Obtainment of starting explants
One-year lignified cuttings of the Vitis vinifera cv. Ancellotta, Glera, Lambrusco Salamino, and the rootstocks, Vitis berlandieri x Vitis rupestris (110 Richter, 17.37), and Vitis berlandieri x Vitis riparia (SO4, Star 50), were collected from the vineyard (Reggio-Emilia, Emilia Romagna region, North-Western Italy) and the center of cultivation and pre-multiplication of Centro Attività Vivaistiche (CAV) (Faenza, RA), during the 2020–2021 winter seasons, and then stored at 4 °C, preserving the dehydration of vegetal material. At the appropriate time, not exceeding 6 months of storage, cuttings of the different genotypes were surface sterilized with a solution containing 50% of commercial bleach (NaClO 1%), for 5 min, and then rinsed several times using tap water until the cuttings are free from the slipperiness of the bleach. The branches were cut at their distal ends (about 2 cm) and transferred into a vessel filled halfway with sterile distilled water, respecting their original geotropism, and maintained in a growth chamber with white fluorescent lights (70 µmol m−2 s−1 and 16 h photoperiod) at 24 °C ± 1. Inflorescences emerged from cuttings of each genotype after 3 to 5 weeks, and the unopened flowers of about 3 to 5 mm were used for different starting explants (whole flowers, stamens, and pistils) for the induction of somatic embryos.
Callus induction media for the initiation of the embryogenic process
Ancellotta, Lambrusco Salamino, 110 Richter, 17.37, SO4, and Star 50 starting explants were cultured in 100 mm × 15 mm Petri dishes containing either of the two different callus induction media, PIV (Franks et al. 1998) or MS1 (Dhekney et al. 2009), and therefore subjected to two different cytokinin/auxin ratios. PIV (Franks et al. 1998) (Table 1, Medium A) is composed of Nitsch and Nitsch (NN) basal salts (Nitsch and Nitsch 1969), B5 vitamins (Gamborg et al. 1968), 3 g L−1 gelrite, 60 g L−1 sucrose, 8.9 µM N6-benzylaminopurine (BAP), and 4.5 µM 2,4-dichlorophenoxyacetic acid (2,4-D). MS1 (Table 1, Medium B) contains Murashige and Skoog basal salts and vitamins (Murashige and Skoog 1962), 7 g L−1 plant agar (Duchefa Biochemie), 20 g L−1 sucrose, 4.5 µM BAP, and 5 µM 2,4-D (Duchefa Biochemie).
For the Glera grapevine cultivar, in addition to the callus induction media previously described (PIV and MS1), the induction of somatic embryos has been carried out also using other combinations of basal salt, PGRs, and gelling agents (Table 1, A–O medium).
The callus induction medium used to regenerate somatic embryos in Glera consists of MS basal salts and vitamins for media B, G, H, J–O with different combinations of PGRs. Induction medium A (PIV) and I contain a combination of NN mineral salts and B5 vitamins, while induction medium C corresponds to NB2 (Dhekney et al. 2016), and it contains NN mineral salts. Induction media D, E, F are composed of half-strength MS basal salts and vitamins. All media were adjusted to a pH of 5.8 using 1 N KOH before autoclaving (120 °C for 21 min) and then poured halfway into 100 mm × 15 mm Petri dishes.
Initiation of somatic embryos from whole flowers, stamens, and pistils
Flowers were excised and observed under a stereomicroscope to identify the right developmental stage of whole flowers, stamens, and pistils. Based on the appearance of explants, stamens and pistils have been selected at stages II and III, and whole flowers at stages IV and V are considered as the most suitable for somatic embryogenesis induction (Dhekney et al. 2009; Gribaudo et al. 2004). The inflorescences (Fig. 1a) were dissected before their immersion for 1 min in ethanol (70% v/v) and surface sterilized in 25% NaClO solution with a few drops of Tween 20 in continuous agitation for 10 min, followed by three 5-min washing steps in sterile distilled water. Stamens were collected removing flower calyptra and including their filaments and anthers (Fig. 1b), were placed in the center of the Petri dishes, while the pistils were arranged in their entirety at the perimeter of each Petri dish, five flowers were cultured in each petri dish. Whole unopened flowers (Fig. 1c) were dissected from the inflorescence, retaining their pedicels, and placed horizontally on the induction medium with each Petri dish holding 20 whole flowers. All these explants were cultured from 5 up to 7 weeks in dark conditions at 25 °C and checked regularly after being transferred to mid light conditions (30 µmol m−2 s−1 and 16 h photoperiod) at 24 °C ± 1.
Examples of different grapevine explants used for pre-embryogenic callus induction: a inflorescence, b anther, and c the whole flower at the right stage of development of Ancellotta cultivar, d embryogenic calli that developed from whole flowers on MS1 induction medium. (bars = 2 mm in figure a and d; 1 mm in figures b and c)
A total of 20 whole flowers, or stamens and pistils (coming from the disruption of five flowers), were cultured in each Petri dish (at least 10 Petri dishes for each starting explant) containing different callus induction media described in Table 1.
Embryogenic calli (EC) that gradually emerged from flowers, stamens, or pistils were transferred to hormone-free X6 medium (Li et al. 2001; Dhekney et al. 2009, 2016), consisting of a modified MS medium without glycine and supplemented with 3.033 g L−1 KNO3 and 0.364 g L−1 NH4Cl as the only nitrogen sources, 60.0 g L−1 sucrose, 1.0 g L−1 Myo-inositol, 7.0 g L−1 plant agar (Duchefa), and 0.5 g L−1 activated charcoal. Pro-embryogenic masses (PEMs) and somatic embryos (SE) were grown at low light conditions (15 µmol m−2 s−1 and 16 h photoperiod), to maintain high embryogenic competence and sub-cultured every 2 weeks on fresh medium.
Induction of secondary embryogenesis
A single cluster of SEs and type II separate somatic embryos at the mid-cotyledonary stage as described by Zhou and colleagues, were stimulated to induce secondary embryogenesis (Zhou et al. 2014) for the genotypes Ancellotta, Lambrusco Salamino, Glera, and 110 Richter. The starting explants for induction of secondary embryogenesis were selected among the embryos regenerating on X6 medium and were cultured for one month in dark conditions on E96 medium (Maillot et al. 2006), which is composed of MS salts and vitamins, with a half concentration of major salts, 60.0 g L−1 sucrose, 7 g L−1 plant agar, 9 μM 2,4-D and 4.5 μM BAP. All the explants were transferred to a different medium named Medium A (Maillot et al. 2006) for embryogenic callus growth and development, incubated under light (15 µmol m−2 s−1 and 16 h photoperiod), and sub-cultured to a fresh medium every 4 weeks. At each subculture, well-developed embryogenic calli were transferred to a fresh X6 medium for embryos development. The obtained somatic embryos were maintained and proliferated by transferring them on an X6 culture medium every fourteen days under the same low light condition.
Data acquisition and statistical analysis
The explants for regeneration via somatic embryogenesis were acquired using cuttings of the different genotypes harvested in two consecutive years (2020 and 2021). Data on the number of embryogenic calli regenerating from whole flowers, stamens, and pistils of each genotype were acquired at 5, 10, and 15 weeks after culture initiation. Embryogenic efficiency (%) was reported as the [(number of explants that produced at least one embryogenic callus/total number of explants cultured) × 100] after 15 weeks of culture. Due to a slower-growing process, embryogenic efficiency (%) of whole flowers, stamens, and pistils of Glera was calculated for a total of 30 weeks after culture initiation, using the method above-mentioned. This calculation was applied to each genotype and culture medium with different PGRs tested as described in Table 1.
Regeneration data were subjected to analysis of variance (ANOVA). The mean comparison between the frequency of non-embryogenic calli recorded in each regeneration medium was determined using the Student–Newman–Keuls t-test at p ≤ 0.05. All analyses were performed with the Statistica 7 software (Statsoft Tulsa, CA, USA). Data on the percentage of regeneration were transformed by the arcsine square root transformation, ARSIN [SQRT (X)] before analysis.
Results
Embryogenic efficiency using floral-derived starting explants in different grapevine genotypes
This study aimed to individuate the best floral-derived starting explant and medium combination for the induction of primary somatic embryogenesis on Ancellotta and Lambrusco Salamino cultivars, and the hybrid rootstocks 110 Richter, 17.37, SO4, and Star 50, using MS1 and PIV as induction culture media. A variable number of stamens, pistils, and whole flowers of each genotype were cultured on each culture medium listed in Table 1, based on the availability of branches capable of differentiating inflorescences. The number of embryogenic calli was recorded at 5, 10, and 15 weeks after culture initiation, and the embryogenic efficiency for each genotype was calculated at the end of the observations. The results obtained (Table 2) highlight that, for all the genotypes tested, PIV seems to be the most suitable induction medium when whole flowers are used as starting explant (from 0.63 up to 8.1% of embryogenic efficiency), whereas MS1 is most suitable for induction in stamens (more than 1% of embryogenic efficiency was observed in all genotypes except for SO4 rootstock).
The highest embryogenic efficiency was recorded when whole flowers were used as starting explants for almost all the genotypes and the culture media tested (Table 2). The highest percentage of embryogenic calli formation (8.1%) was observed for 110 Richter whole flowers cultured on the PIV medium. Although in almost all the genotypes (apart from Glera) the first embryogenic calli (Supplementary Fig. S1) regenerated after the first month of culture, the emergence of new embryogenic tissues was still observed also after 6 months of culture, especially from Lambrusco Salamino whole flowers.
The number of embryogenic calli of Ancellotta and Lambrusco Salamino identified during 15 weeks of culture demonstrated a high regeneration potential of all the starting explants in the culture condition adopted for these two genotypes. Although for the above-mentioned cultivars the embryogenic response occurred mainly from whole flower explants (from 2.3 up to 5.5%), a massive embryogenic callus induction was also detected at the base of the stamen filaments and pistils (from 0.8 up to 3.8%), and also from the ovaries (up to 2.5%) in Ancellotta on PIV medium. The rootstock Star 50 is the only genotype for which pistils were the most suitable starting explants in both culture media tested (from 2.2 up to 6.67%). Embryogenic calli obtained were subsequently moved to X6 medium for the development of somatic embryos, which passed through each stage of the somatic embryo differentiation process (globular, heart, torpedo, cotyledonary) (data not shown).
Initiation of somatic embryogenesis from stamens, pistils, and whole flowers of Glera using different basal salts and growth regulators
In this study, somatic embryogenesis efficiency of the grapevine Italian cultivar Glera was evaluated using stamens, pistils, and whole flowers as starting explants. The explants were placed on induction media consisting of different basal salts (NN and MS), and different combinations of growth regulators (BAP, 2,4-D, NOA, PIC, and NAA) (Table 1). There was no embryogenic calli formation on all the explants (stamens, pistils, and whole flowers) in all the NN-based media (A, C, I) tested (data not shown). In particular, the explants had a limited aptitude for regeneration as they shriveled and dried up. Embryogenic calli were obtained from explants placed on media with MS full strength or MS half-strength (media E, G, and H, Table 3). These media had a common cytokinin-to-auxin ratio ranging from 0.2 (medium H) to 0.5 (medium E). In particular, the highest embryogenic potential was observed from pistils cultured on media G and H, while on medium E, whole flowers were the most capable to produce embryogenic calli. Appearance of the first pre-embryogenic calli started gradually and well beyond the period of culture in dark conditions (4 weeks), reaching the highest efficiency after 15 to 30 weeks of culture. During almost 8 months of culture, the emergence of embryogenic calli has been reported for almost all the assayed starting explants, although the embryogenic frequency varied with the explant type (Fig. 2 and Table 3).
Considering the data collected from all the induction media at 5, 10, 15, and 30 weeks after culture initiation, the most responsive starting explants were pistils initiated on medium H, which makes this medium the most suitable for the production of embryogenic calli for Glera, a very recalcitrant grapevine cultivar.
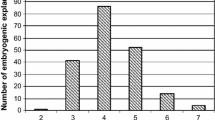
Systematically, in order to make a more complete assessment to guide the choice of the best induction medium for Glera embryogenesis, it was necessary to also evaluate the percentage of calli that didn’t produce any embryogenic callus after 30 weeks in initiation culture also referred to as non-embryogenic calli (NEC). Indeed, a high percentage of the calli that formed from Glera explants in all the media was NEC. The highest percentage of NEC was observed on medium J which also had a statistically significant difference compared with the other media (Fig. 3). Media O, K, and M showed a high percentage of NEC production with no significant difference, while media F and L were not capable to regenerate calli. The rest of the media combinations had low percentages of calli formation.
Induction of secondary embryogenesis
Embryogenic cultures obtained as previously described from various types of explants for the cultivars Ancellotta, Lambrusco Salamino, Glera, and the rootstock 110 Richter were used to induce secondary embryogenesis. The unaltered cluster of SEs or SEs at the mid-cotyledonary stage (type II) for each genotype were cultured on E96 medium to induce secondary embryogenesis, for the purposes of increasing the availability of embryos thus, providing higher number and continuously renewed embryo production, this is in line with Maillot and co-authors (Maillot et al. 2006). After 4 weeks of culture, single somatic embryos (Fig. 4a) and the clusters of somatic embryos (Fig. 4b) developed different types of calluses, including embryogenic calli (Fig. 4a, b). After their transfer onto medium A, cream-colored non-embryogenic calli stopped growing and progressively turned brown. Clusters of secondary embryos appeared gradually on some calli within an additional culture period of 1–4 months on medium A, the calli were subcultured on fresh medium every 4 weeks. Embryogenic structures, composed of several translucent embryos (Fig. 4c) at different developmental stages (mainly at globular stages), were selected and transferred to X6 medium to germinate (Fig. 4d). Both clusters of SEs or type II SEs explants were successfully tested in all cultivars and 110 Richter rootstock, for the establishment of new cultures of proliferating somatic embryos.
Initiation of secondary embryogenesis from a somatic embryo at mid-cotyledonary stage (type II) and b embryo clusters of 110 Richter: a, b development of embryogenic calli and different types of calli on E96 medium after 1 month of culture, c embryogenic callus growth and development of embryogenic aggregates starting from 1 month on culture medium A, d stable continuous production of SE proliferated on PGR-free X6 medium (bars = 2 mm)
Discussion
Somatic embryogenesis is a unique developmental process that gives rise to the expression of cellular totipotency, confirming that somatic cells contain all the genetic information to stimulate plant development, not necessarily requiring sexual fertilization (Correia et al. 2016). Proteomics studies performed on somatic embryogenesis processes have highlighted differences between embryogenic and non-embryogenic calli, allowing researchers to refine the culture conditions to increase the production of embryogenic tissues (Marsoni et al. 2008; Correia et al. 2016). Many successful reports have highlighted evidence that the genotype is one of the most relevant factors which influence initiation of the embryogenic process, and especially in Vitis vinifera, various embryogenic competencies have been observed in different cultivars (Gray 1995; Saporta et al. 2016). One of the main aims of this study was to determine the most suitable and efficient strategy (explant and media compositions) for the induction of somatic embryogenesis in the grapevine cultivars Ancellotta, Lambrusco Salamino, Glera, and the rootstocks 110 Richter, 17.37, SO4, and Star 50. In particular, we evaluated somatic embryogenesis competence for two consecutive years, to limit the annual variation in embryogenic responses that could arise from a different physiological and nutritional stage of the donor plant, or changed conditions in the removal and manipulation of the explant (Kikkert et al. 2005; Vidal et al. 2009).
The results obtained showed that whole flowers, stamens, and pistils had similar efficiency in SE induction for almost all genotypes tested in the different culture media. However, whole flowers were the best performing explants, especially when cultured on PIV culture medium, and they are recommendable explants for establishing grapevine embryogenic cultures in comparison with stamens and pistils, due to their ease of collection and excision thus, enabling one to start the in vitro culture with a high number of explants in a relatively short time (Gambino et al. 2007).
Also for the V. berlandieri x rupestris rootstocks (110 Richter and 17.37), which showed a strong similarity in embryogenic response, whole flowers were confirmed to be the best starting explants. Similar results have been described by other researchers who obtained somatic embryogenesis on SO4 rootstock, using a combination of Thidiazuron (TDZ) and 2,4-D as PGRs in the induction medium (Perrin et al. 2001; Oláh et al. 2009), however, in our study, we demonstrated that the use of BAP also gives good results in the initiation of embryogenic culture. Pistils are still considered a valuable starting explant too for somatic embryogenesis in grapevine rootstocks (Martinelli et al. 2003), as demonstrated in this study from the embryogenic efficiency obtained in Star 50 (greater than 6.5%).
The embryogenic competence of the recalcitrant cultivar Glera has also been fostered in this research, hence increasing embryogenic efficiency based on the scientific literature on the same genotype, this was achieved by initiating different explants on culture media having multiple basal salts and PGRs combinations (Forleo et al. 2021). The results determined that all the explants were able to regenerate somatic embryos to a different extent according to the induction medium chosen. Our study demonstrated that full or half-strength MS basal salts worked best for Glera as there was no calli formation on the PIV medium, in contrast with findings from recent similar research performed on the same genotype, where the induction of somatic embryogenesis in various local Italian varieties was obtained using PIV medium (Gambino et al. 2007; Forleo et al. 2021). Embryogenic calli formation was nil in twelve of the fifteen media tested, and there is no absolute determining factor to explain the induction of embryogenic calli in Glera as the calli formation was somewhat random (embryogenic calli formed on media E, G, and H); the common factor in these three media was the presence of BAP and 2,4-D, in which, the concentration of auxin was double that of cytokinins. Our results determined that Glera flower explants showed higher embryogenic potential when cultured on the MS-optimized media, compared to NN-based media. Recent findings from the study by Forleo et al. (2021) performed on the same genotype, reported whole flowers as the best explant for somatic embryogenesis induction when initiated on PIV medium. While the optimized composition of the induction media assayed in this study led to individuate pistils as the most responsive starting explant on culture media G and H, enhancing the embryogenic potential of this cultivar.
The novel achievement of this study is the description of an efficient procedure for the regeneration of the rootstocks 17.37 and Star 50 via somatic embryogenesis, which, to our knowledge, is reported for the first time in this research. Similarly, somatic embryogenesis induction in Ancellotta and Lambrusco Salamino has not been previously reported in scientific literature, although the in vitro propagation of these two cultivars has been successfully attempted by Gatti et al. (2017). While for the cultivar Glera, known for ‘Prosecco’ wine, the regeneration via somatic embryogenesis has been described recently by only one study (Forleo et al. 2021). Thus, the additional results obtained in our research will enhance future attempts in genetic engineering approaches of this important Italian cultivar, well-known for its recalcitrance to both in vitro regeneration and genetic transformation.
Furthermore, we proved that two possibilities are feasible for obtaining a long-term culture of somatic embryos readily available for the application of the New Genomic Techniques (Limera et al. 2017): (i) the new induction of embryogenic calli starting from floriferous explants; (ii) the somatic embryos proliferation through the induction of secondary somatic embryogenesis using cluster or single SEs (Fig. 5).
Long-term culture of embryogenic calli and somatic embryos proliferation: Embryogenic calli and pro-embryogenic masses produced during the induction step were proliferated in an adequate PGR-free medium and SEs are converted into whole plantlets after the acclimatization step. The perpetuation of embryo cultures is possible through the new induction from floriferous starting explants, or by inducing secondary embryogenesis, following a two-step protocol that allows the establishment of new stable embryos production. (bars = 2 mm)
However, further future studies can be useful to increase the embryogenetic efficiency obtained for the described genotypes. Indeed, researchers have shown that the embryogenic competence by somatic cells in woody species is not only influenced by the genotype or the different type of starting tissue, but also by the correct developmental stage of the starting explant (Isah 2016), and the age of the explant source (Molina et al. 2002; Youssef et al. 2012). For example, studies carried out on date palm and coffee plants revealed that the responsiveness in the production of embryogenic calli was influenced by the inflorescence explant age, or the time of excision of the starting tissue (Molina et al. 2002; Abul-Soad 2012).
Additionally, the source of explant and the condition of the donor plant can influence the initiation of somatic embryogenesis process. Stamp and Meredith (1988) reported an increased number of explants forming somatic embryos in Vitis vinifera cultivars Thompson Seedless, White Riesling, and the hybrid rootstock Ganzin No. 1 (V. vinifera x V. rupestris), when the anthers were isolated from unopened flowers of greenhouse growing plants, compared to those collected from the vineyard (Stamp and Meredith 1988).
In this study, the choice to take woody canes in the winter season and store them at 4 °C in moist and dark conditions, was motivated by the fact that cuttings of different genotypes can be forced to produce inflorescences in a scalar way, and therefore to obtain a flowering period more deferred in time than in the open field, which is usually climatic-dependent and concentrated to a couple of weeks. In this way, the environmental condition can be controlled in the growth chamber from the bud break to the period before anthesis, reducing the exposure of the growing shoot to fungi and bacteria, which could be a source of contamination in the subsequent in vitro cultivation steps. However, it remains fundamental to also evaluate other parameters, such as explants from different years, or to compare the performance of flowers obtained by donor plants placed at different conditions (from cuttings forced in the growth chamber or from plants in the field), to evaluate their influence on embryogenetic competence of important Italian recalcitrant cultivars.
Conclusion
In our experiments, the establishment of an embryogenic culture has been successfully achieved from all genotypes tested in one or more years on the standard and new-optimized initiation media. Generation and continuous proliferation of somatic embryos of two Italian cultivars (Lambrusco Salamino and Ancellotta) and two hybrid rootstocks (17.37 and Star 50) have been reported for the first time, and different floriferous explants sources were used for embryogenic callus induction. Even though no medium was found to be optimal for all the genotypes and plant flower parts, we highlighted the best combination of the type of floriferous explant-induction medium for each cultivar/rootstock. All the parameters tested including basal salts, PGRs, and starting explants, had a strong influence on the percentage of embryogenesis. Efficient induction media have been studied and selected as suitable for the obtainment of actively proliferating somatic embryos starting from floral explants of the recalcitrant Italian cultivar Glera, and the other cultivars, as well as rootstocks, assayed.
The embryogenic cultures have been maintained for a long period through the induction of secondary embryogenesis, starting from clusters of SEs or single embryos at the mid-cotyledonary stage, this allowed the increase of the availability of material with high competence to regeneration which can be used for the application of classical biotechnological approaches and New Genomic Techniques (Capriotti et al. 2020).
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Abul-Soad A (2012) Influence of inflorescence explant age and 2,4-D incubation period on somatic embryogenesis of date palm. Emir J Food Agric 24(5):434–443
Capriotti L, Baraldi E, Mezzetti B et al (2020) Biotechnological approaches: gene overexpression, gene silencing, and genome editing to control fungal and oomycete diseases in grapevine. Int J Mol Sci 21:5701. https://doi.org/10.3390/ijms21165701
Catalano C, Abbate L, Motisi A et al (2021) Autotetraploid emergence via somatic embryogenesis in Vitis vinifera induces marked morphological changes in shoots, mature leaves, and stomata. Cells. https://doi.org/10.3390/CELLS10061336/S1
Corredoira E, Merkle SA, Martínez MT et al (2019) Non-zygotic embryogenesis in hardwood species. Crit Rev Plant Sci 38:29–97. https://doi.org/10.1080/07352689.2018.1551122
Correia SI, Alves AC, Veríssimo P, Canhoto JM (2016) Somatic embryogenesis in broad-leaf woody plants: what we can learn from proteomics. In: Germana MA, Lambardi M (eds) Methods in molecular biology. Humana Press Inc., Totowa, pp 117–129
Correia S, Alhinho AT, Casimiro B et al (2019) NEP-TC a rRNA methyltransferase involved on somatic embryogenesis of tamarillo (Solanum betaceum cav.). Front Plant Sci 10:438. https://doi.org/10.3389/fpls.2019.00438
Dal Santo S, De Paoli E, Pagliarani C et al (2021) Stress responses and epigenomic instability mark the loss of somatic embryogenesis competence in grapevine. Plant Physiol. https://doi.org/10.1093/PLPHYS/KIAB477
Dhekney SA, Li ZT, Compton ME, Gray DJ (2009) Optimizing initiation and maintenance of Vitis embryogenic cultures. HortScience 44:1400–1406. https://doi.org/10.21273/hortsci.44.5.1400
Dhekney SA, Li ZT, Grant TNL, Gray DJ (2016) Somatic embryogenesis and genetic modification of Vitis. In: Germana MA, Lambardi M (eds) Methods in molecular biology. Humana Press Inc., Totowa, pp 263–277. https://doi.org/10.1007/978-1-4939-3061-6_11
Eudes F, Acharya S, Laroche A et al (2003) A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tissue Organ Cult 73:147–157. https://doi.org/10.1023/A:1022800512708
Fehér A (2019) Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front Plant Sci 10:536. https://doi.org/10.3389/FPLS.2019.00536
Forleo LR, D’amico M, Basile T et al (2021) Somatic embryogenesis in Vitis for genome editing: optimization of protocols for recalcitrant genotypes. Horticulturae 7:511. https://doi.org/10.3390/HORTICULTURAE7110511
Franks T, He DG, Thomas M (1998) Regeneration of transgenic Vitis vinifera L. Sultana plants: genotypic and phenotypic analysis. Mol Breeding 4:321–333. https://doi.org/10.1023/A:1009673619456
Gambino G, Ruffa P, Vallania R, Gribaudo I (2007) Somatic embryogenesis from whole flowers, anthers and ovaries of grapevine (Vitis spp.). Plant Cell Tissue Organ Cult 90:79–83. https://doi.org/10.1007/s11240-007-9256-x
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gatti E, Imazio SA, Sgarbi E (2017) In vitro propagation of Italian cultivars of Vitis vinifera and evaluation of genetic stability by SSRs markers. Acta Hort 1155:165–172. https://doi.org/10.17660/ACTAHORTIC.2017.1155.23
Ghadirzadeh-Khorzoghi E, Jahanbakhshian-Davaran Z, Seyedi SM (2019) Direct somatic embryogenesis of drought resistance pistachio (Pistacia vera L.) and expression analysis of somatic embryogenesis-related genes. S Afr J Bot 121:558–567. https://doi.org/10.1016/j.sajb.2019.01.023
Gray DJ (1995) Somatic embryogenesis in grape. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Forestry sciences. Springer, Dordrecht, pp 191–217. https://doi.org/10.1007/978-94-011-0491-3_12
Gribaudo I, Gambino G, Vallania R (2004) Somatic embryogenesis from grapevine anthers: the optimal developmental stage for collecting explants. Am J Enol Viticult 55:427–430
Horstman A, Bemer M, Boutilier K (2017) A transcriptional view on somatic embryogenesis. Regeneration 4:201–216. https://doi.org/10.1002/reg2.91
Isah T (2016) Induction of somatic embryogenesis in woody plants. Acta Physiol Plant 38:1–22. https://doi.org/10.1007/S11738-016-2134-6
Kikkert JR, Striem MJ, Vidal JR et al (2005) Long-term study of somatic embryogenesis from anthers and ovaries of 12 grapevine (Vitis spp.) genotypes. In Vitro Cell Dev Biol 41:232–239. https://doi.org/10.1079/IVP2004609
Li Z, Jayasankar S, Gray DJ (2001) Expression of a bifunctional green fluorescent protein (GFP) fusion marker under the control of three constitutive promoters and enhanced derivatives in transgenic grape (Vitis vinifera). Plant Sci 160:877–887. https://doi.org/10.1016/S0168-9452(01)00336-3
Limera C, Sabbadini S, Sweet JB, Mezzetti B (2017) New biotechnological tools for the genetic improvement of major woody fruit species. Front Plant Sci 8:1418. https://doi.org/10.3389/fpls.2017.01418
Maillot P, Kieffer F, Walter B (2006) Somatic embryogenesis from stem nodal sections of grapevine. Vitis 45:185–189
Maillot P, Lebel S, Schellenbaum P et al (2009) Differential regulation of SERK, LEC1-like and pathogenesis-related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol Biochem 47:743–752. https://doi.org/10.1016/j.plaphy.2009.03.016
Marsoni M, Bracale M, Espen L et al (2008) Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Rep 27:347–356. https://doi.org/10.1007/s00299-007-0438-0
Martinelli L, Gribaudo I (2009) Strategies for effective somatic embryogenesis in grapevine: an appraisal. Grapevine molecular physiology and biotechnology, 2nd edn. Springer, Dordrecht, pp 461–493
Martinelli L, Gribaudo I, Semenzato M et al (2003) Ovary as valuable explant for somatic embryogenesis induction in grapes (Vitis spp.). Acta Hort. https://doi.org/10.17660/ActaHortic.2003.603.65
Martínez MT, San-José MDC, Arrillaga I et al (2019) Holm oak somatic embryogenesis: current status and future perspectives. Front Plant Sci 10:239. https://doi.org/10.3389/FPLS.2019.00239/BIBTEX
Mazri MA, Naciri R, Belkoura I (2020) Maturation and conversion of somatic embryos derived from seeds of olive (Olea europaea L.) cv. Dahbia: occurrence of secondary embryogenesis and adventitious bud formation. Plants (basel, Switzerland) 9:1–14. https://doi.org/10.3390/PLANTS9111489
Molina DM, Aponte ME, Cortina H, Moreno G (2002) The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell Tissue Organ Cult 71:117–123. https://doi.org/10.1023/A:1019965621041
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nic-Can GI, Loyola-Vargas VM (2016) The role of the auxins during somatic embryogenesis. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Somatic embryogenesis: fundamental aspects and applications. Springer, Cham, pp 171–182. https://doi.org/10.1007/978-3-319-33705-0_10
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87. https://doi.org/10.1126/science.163.3862.85
Oláh R, Zok A, Pedryc A et al (2009) Somatic embryogenesis in a broad spectrum of grape genotypes. Sci Hortic 120:134–137. https://doi.org/10.1016/J.SCIENTA.2008.10.003
Pérez-Núñez MT, Chan JL, Sáenz L et al (2006) Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In Vitro Cell Dev Biol 42:37–43. https://doi.org/10.1079/IVP2005722
Perrin M, Martin D, Joly D et al (2001) Medium-dependent response of grapevine somatic embryogenic cells. Plant Sci 161:107–116. https://doi.org/10.1016/S0168-9452(01)00385-5
Ricci A, Sabbadini S, Prieto H et al (2020) Genetic transformation in peach (Prunus persica L.): challenges and ways forward. Plants 9:971. https://doi.org/10.3390/PLANTS9080971
Sabbadini S, Capriotti L, Limera C et al (2019a) A plant regeneration platform to apply new breeding techniques for improving disease resistance in grapevine rootstocks and cultivars. BIO Web Conf 12:01019. https://doi.org/10.1051/bioconf/20191201019
Sabbadini S, Capriotti L, Molesini B et al (2019b) Comparison of regeneration capacity and Agrobacterium-mediated cell transformation efficiency of different cultivars and rootstocks of Vitis spp. via organogenesis. Sci Rep 9:582. https://doi.org/10.1038/s41598-018-37335-7
Saporta R, San Pedro T, Gisbert C (2016) Attempts at grapevine (Vitis vinifera L.) breeding through genetic transformation: the main limiting factors. Electronico 55:173–186. https://doi.org/10.5073/VITIS.2016.55.173-186
Schellenbaum P, Jacques A, Maillot P et al (2008) Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27:1799–1809. https://doi.org/10.1007/s00299-008-0588-8
Stamp JA, Meredith CP (1988) Somatic embryogenesis from leaves and anthers of grapevine. Sci Hortic 35:235–250. https://doi.org/10.1016/0304-4238(88)90117-3
Sugimoto K, Temman H, Kadokura S, Matsunaga S (2019) To regenerate or not to regenerate: factors that drive plant regeneration. Curr Opin Plant Biol 47:138–150. https://doi.org/10.1016/J.PBI.2018.12.002
Vannini C, Bracale M, Croce P et al (2005) An easy and convenient method for maintenance of embryogenic cultures of Vitis vinifera. Vitis 44:197–198
Vidal JR, Rama J, Taboada L et al (2009) Improved somatic embryogenesis of grapevine (Vitis vinifera) with focus on induction parameters and efficient plant regeneration. Plant Cell Tissue Organ Cult 96:85–94. https://doi.org/10.1007/s11240-008-9464-z
Von Arnold S, Sabala I, Bozhkov P et al (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249. https://doi.org/10.1023/A:1015673200621
Yang X, Zhang X (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29:36–57. https://doi.org/10.1080/07352680903436291
Youssef M, James A, Mayo-Mosqueda A et al (2012) Influence of genotype and age of explant source on the capacity for somatic embryogenesis of two Cavendish banana cultivars (Musa acuminata Colla, AAA). Afr J Biotech 9:2216–2223. https://doi.org/10.4314/ajb.v9i15
Zavattieri MA, Frederico AM, Lima M et al (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol 13:12–13. https://doi.org/10.4067/S0717-34582010000100012
Zhou Q, Dai L, Cheng S et al (2014) A circulatory system useful both for long-term somatic embryogenesis and genetic transformation in Vitis vinifera L. cv. Thompson seedless. Plant Cell Tissue Organ Cult 118:157–168. https://doi.org/10.1007/s11240-014-0471-y
Acknowledgements
We gratefully acknowledge financial support from Enotria Nursery, Cavasagra di Vedelago (Treviso); Ampelos Italian Viticulture Consortium, Faenza, (Ravenna), and from the provincial phytosanitary consortium of Modena and Reggio Emilia.
Funding
Open access funding provided by Università Politecnica delle Marche within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
SS and BM conceived and conceptualized the manuscript; LC wrote and prepared the original draft. LC, CL, SS, AR conducted the experiments. LC analyzed data. SS, BM, CL, AR contributed to reviewing and critically revising the manuscript. BM and SS visualized and carefully supervised the work and the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Klaus Eimert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIFF 85395 KB)
Embryogenic calli and single embryos obtained during the induction phase on Ancellotta (a), SO4 (e), and Star 50 (f) from whole flowers, on Lambrusco Salamino from stamens (b), on 110 Richter (c) and 17.37 (d) from pistils. (bars= 2 mm on a), b), e), and f), 1 mm on c) and d).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Capriotti, L., Limera, C., Mezzetti, B. et al. From induction to embryo proliferation: improved somatic embryogenesis protocol in grapevine for Italian cultivars and hybrid Vitis rootstocks. Plant Cell Tiss Organ Cult 151, 221–233 (2022). https://doi.org/10.1007/s11240-022-02346-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02346-w