Abstract
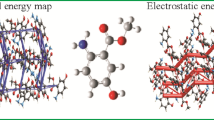
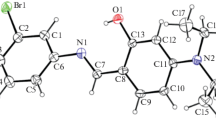
The synthesis and crystal structure of 2-bromo-4,6-bis(dibromoacetyl)resorcinol, I, was reported. In the title compound, I, crystalized in the triclinic crystal system with Pī space group. The two carbon and the oxygen atom of the acetyl groups (atoms C7, C8, O3, and C9, C10, O4) are nearly co-planar with the central phenyl ring. Intramolecular O–H···O, C–H···Br, and intermolecular C–H···Br/O interactions, two non-bonded contacts (Br5···Br3 and O4···C8) and π-π stacking interaction are stabilized the crystal packing of the title compound. Intermolecular interactions that exist in the title compound, I, are quantified with the aid of PIXEL and Hirshfeld surface (HS) analysis and the decomposed fingerprint (FP) plots. The FP plot reveals that the Br···Br contacts are comparably higher than the other contacts in the title crystal structure. Furthermore, the theoretical density functional theory (DFT) calculations were performed at the M062X/cc-PVTZ level of theory. The experimental geometry parameters of the title molecule are compared with the geometry of the optimized molecule in the gas phase. The chemical reactivity and charge transfer properties of the title compound were calculated from the HOMO and LUMO energy. In addition, the molecular electrostatic potential map was generated at their crystal structure geometry and quantitatively analyzed.











Similar content being viewed by others
Availability of data and materials
Data and materials will be made available on request.
References
Ali TE, Ibrahim MA, El-Gendy ZM, El-Amin EM (2013) 4,6-Diacetylresorcinol in heterocyclic synthesis, part I: synthesis and biological evaluation of some new linearly and angularly substituted pyrano[3,2-g] chromenes via Vilsmeier-Haack formylation of 4,6-diacetylresorcinol, Its Schiff Bases, and Hydrazones. Synth Commun 43:3329–3341. https://doi.org/10.1080/00397911.2013.783074
Abdel-Rahman RM, Salem MAI, Ali TE, Ibrahim MA (2015) Utility of 4,6-diacetylresorcinol in heterocyclic synthesis. Chem Heterocycl Comp 51:299–309. https://doi.org/10.1007/s10593-015-1699-0
Ashok D, Shravani D (2009) Microwave-assisted solvent free synthesis of some 4, 6-dicinnamoyl resorcinols. Asian J Chem 808–10. https://asianjournalofchemistry.co.in/User/ViewFreeArticle.aspx?ArticleID=21_1_103
Salavati-Niasari M, Salimi Z, Bazarganipour M, Davar F (2009) Synthesis, characterization and catalytic oxidation of cyclohexane using a novel host (zeolite-Y)/guest (binuclear transition metal complexes) nanocomposite materials. Inorganica Chim Acta 362:3715–3724. https://doi.org/10.1016/j.ica.2009.04.028
Emara AAA, El-Sayed BA, Ahmed E-SAE (2008) Syntheses, spectroscopic characterization and thermal behavior on novel binuclear transition metal complexes of hydrazones derived from 4,6-diacetylresorcinol and oxalyldihydrazine. Spectrochim Acta- A: Mol Biomol 69:757–769. https://doi.org/10.1016/j.saa.2007.05.028
Krishna Murthy KS, Rajitha B, Kanakalingeswara Rao M, Raja Komuraiah T, Reddy SM (2002) Facile synthesis of biologically active linear bisaroyl benzodifurans by PTC and solvent free microwave irradiation. Heterocycl Comm 8:179–186. https://doi.org/10.1515/HC.2002.8.2.179
Makode J, Bhadange J, Aswar A (2003) Structural, thermal, biological and semiconducting properties of Mn (II), Fe (II), Co (II), Ni (II), Cu (II), Zn (II), Cd (II) and VO (IV) complexes of Schiff base derived from resdiacetophenone and S-benzyldithiocarbazate. Pol J Chem 77:855–65. https://www.infona.pl/resource/bwmeta1.element.baztech-article-BUJ1-0021-0046
Borisenko KB, Hargittai I (1993) Intramolecular hydrogen bonding and molecular structure of 2-nitroresorcinol from gas-phase electron diffraction. J Phy Chem 97:4080–4084. https://doi.org/10.1021/j100118a025
Bock CW, Hargittai I (1994) Geometrical consequences of resonance-assisted intramolecular hydrogen-bond formation from Ab initio MO calculations on 2-nitroresorcinol. Struct Chem 5:307–312. https://doi.org/10.1007/BF02281221
Kovács A, Hargittai I (1998) Hydrogen bonding in 2-trifluoromethylresorcinol and 2,6-bis(trifluoromethyl)phenol and its geometrical consequences. J Mol Struct Theochem 455:229–238. https://doi.org/10.1016/S0166-1280(98)00101-8
Borisenko KB, Bock CW, Hargittai I (1997) Molecular structure and intramolecular hydrogen bonding in 4,6-dinitroresorcinol and 2,5-dinitrohydroquinone from ab initio molecular orbital calculations. J Mol Struct Theochem 393:121–126. https://doi.org/10.1016/S0166-1280(96)04859-2
Hattori K, Ishiuchi S-I, Fujii M, Howard DL, Kjaergaard HG (2007) Vibrational OH-stretching overtone spectroscopy of jet-cooled resorcinol and hydroquinone rotamers. J Phys Chem A 111:6028–6033. https://doi.org/10.1021/jp071677c
Onawole AT, Abdul Halim M, Ullah N, Al-Saadi AA (2018) Structural, spectroscopic and docking properties of resorcinol, its -OD isotopomer and dianion derivative: a comparative study. Struct Chem 29:403–414. https://doi.org/10.1007/s11224-017-1037-5
Loganathan K, Ali KS, Purushothaman M, Silambarasan S, Nasser AJA (2015) Synthesis and characterization of Azo derivatives of diacetylresorcinol. J Chem Pharm Res 7:1452–55. https://www.jocpr.com/abstract/synthesis-and-characterization-of-azo-derivatives-of-diacetylresorcinol-4725.html
Purushothaman M, Loganathan K, Sithick A (2012) Synthesis, characterization, and biological importance of aminocyanopyridines. Int J Chem Tech Res 4:479–483. https://sphinxsai.com/2012/chemAJ/CHEM/CT=02(479-483)AJ12.pdf
Purushothaman M, Loganathan K, Ali KS, Selvin JA (2014) An efficient and facile synthesis of coumarin derivatives as potent antimicrobial agents. Int J Chem Tech Res 6:538–546. https://sphinxsai.com/2014/ChemTech/JM14CT51_100/CT=64(538-546)JM14.pdf
Gavezzotti A (2002) Calculation of intermolecular interaction energies by direct numerical integration over electron densities. I. Electrostatic and polarization energies in molecular crystals. J Phys Chem B 106:4145–4154. https://doi.org/10.1021/jp0144202
Gavezzotti A (2003) Calculation of intermolecular interaction energies by direct numerical integration over electron densities. 2. An Improved polarization model and the evaluation of dispersion and repulsion energies. J Phys Chem B 107:2344–2353. https://doi.org/10.1021/jp022288f
Gavezzotti A (2011) Efficient computer modeling of organic materials. The atom-atom, Coulomb-London-Pauli (AA-CLP) model for intermolecular electrostatic-polarization, dispersion and repulsion energies. New J Chem 35:1360–1368. https://doi.org/10.1039/C0NJ00982B
Dey D, Mohan TP, Vishalakshi B, Chopra D (2014) Computational study of the formation of short centrosymmetric N-H···S supramolecular synthon and related weak interactions in crystalline 1,2,4-triazoles. Cryst Growth Des 14:5881–5896. https://doi.org/10.1021/cg501103c
Kaur G, Panini P, Chopra D, Roy Choudhury A (2012) Structural investigation of weak intermolecular interactions in fluorine substituted isomeric N-benzylideneanilines. Cryst Growth Des 12:5096–5110. https://doi.org/10.1021/cg3010294
Khan I, Panini P, Khan SU-D, Rana UA, Andleeb H, Chopra D, Hameed S, Simpson J (2016) Exploiting the role of molecular electrostatic potential, deformation density, topology, and energetics in the characterization of S···N and Cl···N supramolecular motifs in crystalline triazolothiadiazoles. Cryst Growth Des 16:1371–1386. https://doi.org/10.1021/acs.cgd.5b01499
Panini P, Bhandary S, Chopra D (2016) Quantitative investigation of polymorphism in 3-(trifluoromethyl)-N-[2-(trifluoromethyl)phenyl]benzamide. Cryst Growth Des 16:2561–2572. https://doi.org/10.1021/acs.cgd.5b01638
Shukla R, Chopra D (2015) Crystallographic and computational investigation of intermolecular interactions involving organic fluorine with relevance to the hybridization of the carbon atom. Cryst Eng Comm 17:3596–3609. https://doi.org/10.1039/C4CE02391A
Panini P, Chopra D (2013) Quantitative insights into energy contributions of intermolecular interactions in fluorine and trifluoromethyl substituted isomeric N-phenylacetamides and N-methylbenzamides. Cryst Eng Comm 15:3711–3733. https://doi.org/10.1039/C3CE40111A
Shukla R, Saeed A, Simpson J, Chopra D (2017) Quantitative investigation of C-H···π and other intermolecular interactions in a series of crystalline N-(substituted phenyl)-2-naphthamide derivatives. Cryst Eng Comm 19:5473–5491. https://doi.org/10.1039/C7CE01310H
Panini P, Mohan TP, Gangwar U, Sankolli R, Chopra D (2013) Quantitative crystal structure analysis of 1,3,4-thiadiazole derivatives. Cryst Eng Comm 15:4549–4564. https://doi.org/10.1039/C3CE40278A
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis Cryst Eng Comm 11:19–32. https://doi.org/10.1039/B818330A
McKinnon JJ, Spackman MA, Mitchell AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr B: Struct Sci Cryst Eng Mater 60:627–668. https://doi.org/10.1107/S0108768104020300
Parkin A, Barr G, Dong W, Gilmore CJ, Jayatilaka D, McKinnon JJ, Spackman MA, Wilson CC (2007) Comparing entire crystal structures: structural genetic fingerprinting. Cryst Eng Comm 9:648–652. https://doi.org/10.1039/B704177B
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. Cryst Eng Comm 4:378–392. https://doi.org/10.1039/B203191B
Spackman MA, McKinnon JJ, Jayatilaka D (2008) Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. Cryst Eng Comm 10:377–388. https://doi.org/10.1039/B715227B
Turner MJ, McKinnon JJ, Jayatilaka D, Spackman MA (2011) Visualisation and characterisation of voids in crystalline materials. Cryst Eng Comm 13:1804–1813. https://doi.org/10.1039/C0CE00683A
Wood PA, McKinnon JJ, Parsons S, Pidcock E, Spackman MA (2008) Analysis of the compression of molecular crystal structures using Hirshfeld surfaces. Cryst Eng Comm 10:368–376. https://doi.org/10.1039/B715494A
Kathiravan P, Balakrishnan T, Venkatesan P, Ramamurthi K, Percino MJ, Thamotharan S (2016) Crystal structure and Hirshfeld surface analysis of 1-carboxy-2-(3,4-dihydroxyphenyl)ethan-1-aminium bromide 2-ammonio-3-(3,4-dihydroxyphenyl) propanoate. Acta Crystallogr E: Crystallogr Commun 72:1544–1548. https://doi.org/10.1107/S2056989016015425
Kathiravan P, Balakrishnan T, Venkatesan P, Ramamurthi K, Percino MJ, Thamotharan S (2016) Crystal structure and Hirshfeld surface analysis of 1-carboxy-2-(3,4-dihydroxyphenyl)ethan-1-aminium chloride 2-ammonio-3-(3,4-dihydroxyphenyl) propanoate: a new polymorph of l-dopa HCl and isotypic with its bromide counterpart. Acta Crystallogr E: Crystallogr Commun 72:1628–1632. https://doi.org/10.1107/S2056989016016789
Percino J, Cerón M, Venkatesan P, Ceballos P, Bañuelos A, Rodríguez O, Siegler MA, Robles F, Chapela VM, Soriano-Moro G, Pérez-Gutiérrez E, Bonilla-Cruz J, Thamotharan S (2017) Two different emissions of (2Z)-2-(4-bromophenyl)-3-[4-(dimethylamino)phenyl]prop-2-enenitrile due to crystal habit and size: synthesis, optical, and supramolecular characterization. Cryst Growth Des 17:1679–1694. https://doi.org/10.1021/acs.cgd.6b01670
Udayakumar M, Jagatheeswaran K, Ganesan SS, Venkataramanan NS, Madan Kumar S, Byrappa K, Thamotharan S (2017) Investigation of 9-(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-one: crystal structure, AIM and NBO analysis. J Mol Struct 1133:510–518. https://doi.org/10.1016/j.molstruc.2016.11.082
Venkatesan P, Thamotharan S, Kumar RG, Ilangovan A (2015) Invariant and variable intermolecular interactions in functionalized malonic acid half-esters: X-ray, Hirshfeld surface and PIXEL energy analyses. Cryst Eng Comm 17:904–915. https://doi.org/10.1039/C4CE02125H
Venkatesan P, Thamotharan S, Ilangovan A, Liang H, Sundius T (2016) Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino] prop-2-enoic acid. Spectrochim Acta-A: Mol Biomol 153:625–663. https://doi.org/10.1016/j.saa.2015.09.002
Venkatesan P, Rajakannan V, Venkataramanan NS, Ilangovan A, Sundius T, Thamotharan S (2016) Structural investigation of (2E)-2-(ethoxycarbonyl)-3-[(4-methoxyphenyl)amino]prop-2-enoic acid: X-ray crystal structure, spectroscopy and DFT. J Mol Struct 1119:259–268. https://doi.org/10.1016/j.molstruc.2016.04.090
Clegg W, Watson DG (2008) Structure reports online: major changes in response to a huge success. Acta Crystallogr E: Crystallogr Commun 64:e15–e17. https://doi.org/10.1107/S1600536808002778
Sheldrick G (2015) Crystal structure refinement with SHELXL. Acta Crystallogr: C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Spek A (2009) Structure validation in chemical crystallography. Acta Crystallogr: D 65:148–155. https://doi.org/10.1107/S090744490804362X
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457. https://doi.org/10.1107/S002188980600731X
Turner M, McKinnon J, Wolff S, Grimwood D, Spackman P, Jayatilaka D, Spackman M (2017) Crystal Explorer 17, University of Western Australia
Gavezzotti A (2008) Non-conventional bonding between organic molecules. The ‘halogen bond’ in crystalline systems. Mol Phy 106:1473–1485. https://doi.org/10.1080/00268970802060674
Udayakumar M, Cerón M, Ceballos P, Percino MJ, Thamotharan S (2019) Interplay of weak noncovalent interactions in two conjugated positional isomers: a combined X-ray, optical properties and theoretical investigation. J Mol Struct 1195:32–42. https://doi.org/10.1016/j.molstruc.2019.05.109
Venkatesan P, Cerón M, Ceballos P, Pérez-Gutiérrez E, Thamotharan S, Percino MJ (2019) Experimental study and DFT calculation for the strength of intermolecular interactions in Schiff base with the phenylarsonic acid scaffold. J Mol Struct 1196:306–322. https://doi.org/10.1016/j.molstruc.2019.06.073
Venkatesan P, Cerón M, Thamotharan S, Robles F, Percino MJ (2018) Quantitative analysis of weak non-covalent interactions in (Z)-3-(4-halophenyl)-2-(pyridin-2/3/4-yl)acrylonitriles. Cryst Eng Comm 20:2681–2697. https://doi.org/10.1039/C7CE02096A
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J Mol Model 16:1679–1691. https://doi.org/10.1007/s00894-010-0692-x
Groom CR, Bruno IJ, Lightfoot MP, Ward SC (2016) The Cambridge Structural Database. Acta Crystallogr: B Struct Sci Cryst Eng Mater 72:171–179. https://doi.org/10.1107/S2052520616003954
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr: B Struct Sci Cryst Eng Mater 58:389–397. https://doi.org/10.1107/S0108768102003324
Riwar L-J, Trapp N, Kuhn B, Diederich F (2017) Substituent effects in parallel-displaced π–π stacking interactions: distance matters. Angew Chem Int Ed 56:11252–11257. https://doi.org/10.1002/anie.201703744
Bader RFW (1994) Atoms in molecules: a quantum theory. Clarendon Press
Todd AK (2017) AIMALL Version 16.05.18
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Blagojević JP, Veljković DŽ, Zarić SD (2017) Stacking interactions between hydrogen-bridged and aromatic rings: study of crystal structures and quantum chemical calculations. CrystEngComm 19:40–46. https://doi.org/10.1039/C6CE02045C
Blagojević JP, Janjić GV, Zarić SD (2016) Very strong parallel interactions between two saturated acyclic groups closed with intramolecular hydrogen bonds forming hydrogen-bridged rings. Crystals 6:34. https://doi.org/10.3390/cryst6040034
Venkatesan P, Thamotharan S, Percino MJ, Ilangovan A (2021) Intramolecular resonance assisted N–H⋅⋅⋅O=C hydrogen bond and weak noncovalent interactions in two asymmetrically substituted geminal amido-esters: crystal structures and quantum chemical exploration. J Mol Struct 1246:131210. https://doi.org/10.1016/j.molstruc.2021.131210
Venkatesan P, Thamotharan S, Percino MJ, Ilangovan A (2021) Crystal packing modulation of the strength of resonance-assisted hydrogen bonds and the role of resonance-assisted pseudoring stacking in geminal amido esters: study based on crystallography and theoretical calculations. Cryst Growth Des 21:779–798. https://doi.org/10.1021/acs.cgd.0c01010
Gatti C (2005) Chemical bonding in crystals: new directionsZeitschrift für Kristallographie - Crystalline Materials 220:399–457. https://doi.org/10.1524/zkri.220.5.399.65073
Blagojević JP, Zarić SD (2015) Stacking interactions of hydrogen-bridged rings – stronger than the stacking of benzene molecules. Chem Commun 51:12989–12991. https://doi.org/10.1039/C5CC04139B
Lieberman HF, Davey RJ, Newsham DMT (2000) Br···Br and Br···H interactions in action: polymorphism, hopping, and twinning in 1,2,4,5-tetrabromobenzene. Chem Mater 12:490–494. https://doi.org/10.1021/cm991123p
Acknowledgements
The authors PV and MJP wish to express their gratitude to VIEP-BUAP (grant pv19-ID00375, and grant 00110-VIEP-202). Also, the authors thankfully acknowledge the computer resources and technical support provided by the Laboratorio Nacional de Supercómputo del Sureste de México. KL and MP wish to thank the Jamal Mohamed College management for providing the necessary facilities.
Funding
The authors PV and JP have received research support from VIEP-BUAP (grant pv19-ID00375, and grant 00110-VIEP-202).
Author information
Authors and Affiliations
Contributions
K. Loganathan: conceptualization, chemical synthesis, characterization; A. Anandan: conceptualization, investigation, crystal structure analysis, characterization, writing—original draft; M. Purushothaman: conceptualization, chemical synthesis, characterization, writing—review and editing; P. Daniel Jebaraj: chemical synthesis; K. Thanigaimani: data curation, review; M. Judith Percino: resources, data curation, writing—review and editing; P. Venkatesan: conceptualization, investigation, supervision, writing—original draft—review and editing.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Competing interests
Authors PV and JP have research funding from VIEP-BUAP (grant pv19-ID00375, and grant 00110-VIEP-202), and other authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
K. Loganathan and A. Anandan contributed equally to this work as the first authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loganathan, K., Anandan, A., Purushothaman, M. et al. Synthesis, crystal structure, Hirshfeld surface analysis, and DFT studies of 2-bromo-4,6-bis(dibromoacetyl)resorcinol. Struct Chem 35, 627–640 (2024). https://doi.org/10.1007/s11224-023-02221-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02221-0