Abstract
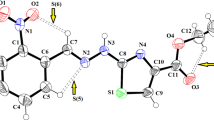
The title compound with the molecular formula C8H9NO3 is synthesized by refluxing 2-amino-5-hydroxybenzoic acid in methanol. The molecular structure of the compound is determined by single crystal X-ray diffraction. Methyl 2-amino-5-hydroxybenzoate crystallizes in the orthorhombic space group P212121 with a = 4.973(2) Å, b = 10.923(5) Å, c = 14.074(6) Å, Z = 4 and V = 764.4(6) Å3. DFT is used to compute HOMO–LUMO energy levels, to predict the reactivity of substituents (NH2 and OH), and to determine the nucleophilic character of these two groups. The orientation and nature of substituents on benzene favors the formation of a stable six-membered ring via hydrogen bonding which plays a key role in the properties of the investigated compound. The natural bond orbital (NBO) population analysis demonstrates that the hyperconjugative effect between the donor lone pairs located on the carbonyl oxygen atom and the N–H group, via the lp O → σ*(N–H) 1,6-remote interaction, is responsible for the preferred conformation. The molecular electrostatic potential (MEP) surface shows the electrical neutrality in the molecule. To get an insight to the intermolecular interactions in the crystal a Hirshfeld surface analysis is also carried out.













Similar content being viewed by others
REFERENCES
S. Ferrere, A. Zaban, and B. A. Gregg. J. Phys. Chem. B, 1997, 101, 4490-4493. https://doi.org/10.1021/jp970683d
A. W. H. Lam, W. T. Wong, S. Gao, G. Wen, and X. X. Zhang. Eur. J. Inorg. Chem., 2003, 2003, 149-163. https://doi.org/10.1002/ejic.200390021
A. Young and T. R. Sweet. J. Am. Chem. Soc., 1958, 80, 800-803. https://doi.org/10.1021/ja01537a013
Z. Aibibuli, Y. Wang, H. Tu, X. Huang, and A. Zhang. Molecules, 2012, 17, 3181-3201. https://doi.org/10.3390/molecules17033181
L. Z. Wei, J. S. Cho, and S. Y. Lee. Proc. Natl. Acad. Sci., 2019, 116, 10749-10756. https://doi.org/10.1073/pnas.1903875116
C. S. McKay and M. G. Finn. Angew. Chem., 2016, 128, 12833-12839. https://doi.org/10.1002/ange.201602797
W. P. Appel, M. M. Nieuwenhuizen, M. Lutz, B. F. De Waal, A. R. Palmans, and E.W. Meijer. Chem. Sci., 2014, 5, 3735-3745. https://doi.org/10.1039/C4SC00871E
S. Hinsberger, K. Hüsecken, M. Groh, M. Negri, J. Haupenthal, and R. W. Hartmann. J. Med. Chem., 2013, 56, 8332-8338. https://doi.org/10.1021/jm400485e
B. Lippa, G. S. Kauffman, J. Arcari, T. Kwan, J. Chen, W. Hungerford, and S. Steyn. Bioorg. Med. Chem. Lett., 2007, 17, 3081-3086. https://doi.org/10.1016/j.bmcl.2007.03.046
A. Kamal, N. Shankaraiah, K. L. Reddy, and V. Devaiah. Tetrahedron Lett., 2006, 47, 4253-4257. https://doi.org/10.1016/j.tetlet.2006.04.025
A. Witt and J. Bergman. J. Org. Chem., 2001, 66, 2784-2788. https://doi.org/10.1021/jo001696h
Z. W. Luo, J. S. Cho, and S. Y. Lee. Proc. Natl. Acad. Sci., 2019, 116, 10749-10756. https://doi.org/10.1073/pnas.1903875116
Bruker SMART and SAINT. Madison, Wisconsin, USA: Bruker AXS Inc., 2002.
G. M. Sheldrick. SADABS. Göttingen, Germany: University of Göttingen, 2004.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112-122. https://doi.org/10.1107/S0108767307043930
L. J. Farrugia. J. Appl. Crystallogr., 2012, 45, 849-854. https://doi.org/10.1107/S0021889812029111
F. L. Hirshfeld. Theor. Chim. Acta, 1977, 44, 129-138. https://doi.org/10.1007/BF00549096
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone,B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi,M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma,V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09, Revision C.1. Wallingford, CT: Gaussian Inc., 2009.
J. D. Chai and M. Head-Gordon. J. Chem. Phys., 2008, 128, 084106. https://doi.org/10.1063/1.2834918
M. E. Hachim, K. Sadik, S. Byadi, and A. Aboulmouhajir. J. Mol. Model., 2020, 26, 1-16. https://doi.org/10.1007/s00894-020-04430-4
A. E. Reed, L. A. Curtiss, and F. Weinhold. Chem. Rev., 1988, 88, 899-926. https://doi.org/10.1021/cr00088a005
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3-8. https://doi.org/10.1107/s2053229614024218
I. Alkorta and J. Elguero. Int. J. Mol. Sci., 2003, 4, 64-92. https://doi.org/10.3390/i4030064
C. G. Zhan, J. A. Nichols, and D. A. Dixon. J. Phys. Chem. A, 2003, 107, 4184-4195. https://doi.org/10.1021/jp0225774
I. V. Alabugin, M. Manoharan, S. Peabody, and F. Weinhold. J. Am. Chem. Soc., 2003, 125, 5973-5987. https://doi.org/10.1021/ja034656e
L. Aguilar-Castro, M. Tlahuextl, L. H. Mendoza-Huizar, A. R. Tapia-Benavides, and H. Tlahuext. ARKIVOC, 2008, 210-226. https://doi.org/10.3998/ark.5550190.0009.517
A. Saeed, A. Khurshid, J. P. Jasinski, C. G. Pozzi, A. C. Fantoni, and M. F. Erben. Chem. Phys., 2014, 431, 39-46. https://doi.org/10.1016/j.chemphys.2014.01.009
J. E. Del Bene, S. A. Perera, and R. J. Bartlett. J. Am. Chem. Soc., 2000, 122, 3560/3561. https://doi.org/10.1021/ja994312h
E. Scrocco and J. Tomasi. Adv. Quantum Chem., 1978, 11, 115-193. https://doi.org/10.1016/S0065-3276(08)60236-1
M. A. Spackman and D. Jayatilaka. CrystEngComm, 2009, 11, 19-32. https://doi.org/10.1039/B818330A
M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka, and M. A. Spackman. CrystalExplorer17. The University of Western Australia, 2017.
P. Venkatesan, S. Thamotharan, A. Ilangovan, H. Liang, and T. Sundius. Spectrochim. Acta, Part A, 2016, 153, 625-636. https://doi.org/10.1016/j.saa.2015.09.002
J. J. McKinnon, D. Jayatilaka, and M. A. Spackman. Chem. Commun., 2007, 37, 3814-3816. https://doi.org/10.1039/b704980c
V. R. Hartwar, M. Sist, M. R. V. Jorgensen, A. H. Mamakhel, X. Wang, C. M. Hoffmann, K. Sugimoto, J. Overgaard, and B. B. Iversen. IUCrJ, 2015, 2, 563-574. https://doi.org/10.1107/S2052252515012130
M. J. Turner, S. Grabowsky, D. Jayatilaka, and M. A. Spackman. J. Phys. Chem. Lett., 2014, 5, 4249-4255. https://doi.org/10.1021/jz502271c
M. J. Turner, S. P. Thomas, M. W. Shi, D. Jayatilaka, and M. A. Spackman. Chem. Commun., 2015, 51, 3735-3738. https://doi.org/10.1039/C4CC09074H
C. F. Mackenzie, P. R. Spackman, D. Jayatilaka, and M. A. Spackman. IUCrJ, 2017, 4, 575-587. https://doi.org/10.1107/S205225251700848X
A. D. Becke. J. Chem. Phys., 1993, 98, 1372-1377. https://doi.org/10.1063/1.464304
C. Lee, W. Yang, and R. G. Parr. Phys. Rev. B, 1998, 37, 785. https://doi.org/10.1103/PhysRevB.37.785
A. Abbas, H. Gökce, and S. Bahçeli. Spectrochim. Acta, Part A, 2016, 152, 596-607. https://doi.org/10.1016/j.saa.2015.01.099
G. Yuan, K. Z. Shao, D. Y. Du, X. L. Wang, Z. M. Su, and J. F. Ma. CrystEngComm, 2012, 14, 1865-1873. https://doi.org/10.1039/c1ce06178j
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 11, pp. 1857-1869.https://doi.org/10.26902/JSC_id83819
Supplementary material
Rights and permissions
About this article
Cite this article
Saeed, A., Shabir, G., Channar, P.A. et al. COMPUTATIONAL INVESTIGATIONS, HIRSHFELD SURFACE ANALYSIS, INTERACTION ENERGY CALCULATIONS, AND ENERGY FRAMEWORK CRYSTAL STRUCTURE OF METHYL 2-AMINO-5-HYDROXYBENZOATE. J Struct Chem 62, 1745–1758 (2021). https://doi.org/10.1134/S0022476621110111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621110111