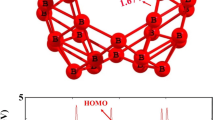
Abstract
A combined approach (endohedral doping and exohedral environment) to stabilization of boron clusters with classical fullerene structures has been studied. The boron clusters with classical fullerene structures are stable when heteroatomic part of the complex (endohedral atom and exohedral environment) donates in total 18 electrons to the composite system, stability of which depends on the coordination capabilities and donor ability of the endohedral and surrounding atoms. The most effective stabilization is achieved in the case of the endohedral transition metals atoms, whereas the most effective environment is given by the lithium surrounding.








Similar content being viewed by others
References
Sattler KD (2010) Handbook of nanophysics: clusters and fullerenes. Taylor & Francis, New York,
Langa F, Nierengarten JF (2011) Fullerenes: principles and applications. RSC Publishing, Cambridge,
Szwacki NG, Sadrzadeh A, Yakobson BI (2007) B80 fullerene: an ab initio prediction of geometry, stability, and electronic structure. Phys Rev Lett 98:166804
Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, Piazza ZA, Tian WJ, Lu HG, Wu YB, Mu YW, Wei GF, Liu ZP, Li J, Li SD, Wang LS (2014) Observation of an all-boron fullerene. Nat Chem 6:727–731
Zubarev DY, Boldyrev AI (2007) Comprehensive analysis of chemical bonding in boron clusters. J Comput Chem 28:251–268
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS (2006) All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord Chem Rev 250:2811–2866
Sergeeva AP, Popov IA, Piazza ZA, Li WL, Romanescu C, Wang LS, Boldyrev AI (2014) Understanding boron through size selected clusters: structure, chemical bonding, and fluxionality. Acc Chem Res 47:1349–1358
Boustani I (1995) Structure and stability of small boron clusters. A density functional theoretical study. Chem Phys Lett 240:135–140
Boustani I (1997) Systematic ab initio investigation of bare boron clusters: determination of the geometry and electronic structures of Bn (n=2-14). Phys Rev B Condens Matter Mater Phys 55:16426–16438
Kato H, Yamashita K, Morokuma K (1992) Ab initio MO study of neutral and cationic boron clusters. Chem Phys Lett 190:361–366
Zhai HJ, Wang LS, Alexandrova AN, Boldyrev AI (2002) Electronic structure and chemical bonding of B5 − and B5 by photoelectron spectroscopy and ab initio calculations. J Chem Phys 117:7917–7924
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang L-S, Steiner E, Fowler PW (2003) Structure and bonding in B6 − and B6: planarity and antiaromaticity. J Phys Chem A 107:1359–1369
Zhai HJ, Wang LS, Alexandrova AN, Boldyrev AI, Zakrzewski VG (2003) Photoelectron spectroscopy and ab initio study of B3 − and B4 − anions and their neutrals. J Phys Chem A 107:9319–9328
Zhai HJ, Alexandrova AN, Birch KA, Boldyrev AI, Wang LS (2003) Hepta- and octacoordinate boron in molecular wheels of eight- and nine-atom boron clusters: observation and confirmation. Angew Chem Int Ed 42:6004–6008
Zhai HJ, Kiran B, Li J, Wang LS (2003) Hydrocarbon analogues of boron clusters planarity, aromaticity and antiaromaticity. Nature Mater 2:827–833
Alexandrova AN, Boldyrev AI, Zhai HJ, Wang LS (2004) Electronic structure, isomerism, and chemical bonding in B7 − and B7. J Phys Chem A 108:3509–3517
Sergeeva AP, Zubarev DY, Zhai HJ, Boldyrev AI, Wang LS (2008) A photoelectron spectroscopic and theoretical study of B16 and B16 2−: an all-boron naphthalene. J Am Chem Soc 130:7244–7246
Huang W, Sergeeva AP, Zhai HJ, Averkiev BB, Wang LS, Boldyrev AI (2010) A concentric planar doubly π-aromatic B19 cluster. Nat Chem 2:202–206
Romanescu C, Harding DJ, Fielicke A, Wang LS (2012) Probing the structures of neutral boron clusters using infrared/vacuum ultraviolet two color ionization: B11, B16, and B17. J Chem Phys 137:014317
Cheng L (2012) B14: an all-boron fullerene. J Chem Phys 136:104301
Kiran B, Bulusu S, Zhai HJ, Yoo S, Zeng XC, Wang LS (2005) Planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc Nat Acad Sci USA 102:961–964
An W, Bulusu S, Gao Y, Zeng XC (2006) Relative stability of planar versus double-ring tubular isomers of neutral and anionic boron cluster B20 and B20 −. J Chem Phys 124:154310
Pham HT, Duong LV, Pham BQ, Nguyen MT (2013) The 2D-to-3D geometry hopping in small boron clusters: the charge effect. Chem Phys Lett 577:32–37
Tai TB, Tam NM, Nguyen MT (2012) Structure of boron clusters revisited, Bn with n = 14-20. Chem Phys Lett 530:71–76
Marques MAL, Botti S (2005) The planar-to-tubular structural transition in boron clusters from optical absorption. J Chem Phys 123:014310
Mukhopadhyay S, He H, Pandey R, Yap YK, Boustani I (2009) Novel spherical boron clusters and structural transition from 2D quasi-planar structures to 3D double-rings. J Phys Conf Ser 176:012028
Wang LS (2016) Photoelectron spectroscopy of size-selected boron clusters: from planar structures to borophenes and borospherenes. Int Rev Phys Chem 35:69–142
Boustani I, Rubio A, Alonso JA (1999) Ab initio study of B32 clusters: competition between spherical, quasiplanar and tubular isomers. Chem Phys Lett 311:21–28
Lau KC, Deshpande M, Pati R, Pandey R (2005) A theoretical study of electronic and vibrational properties of neutral, cationic, and anionic B24 clusters. Int J Quant Chem 103:866–874
Chacko S, Kanhere DG, Boustani I (2003) Ab initio density functional investigation of B24 clusters: rings, tubes, planes, and cages. Phys Rev B Condens Matter Mater Phys 68:354141–3541411
Piazza ZA, Hu HS, Li WL, Zhao YF, Li J, Wang LS (2014) Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat Commun 5:3113–3117
Li WL, Chen Q, Tian WJ, Bai H, Zhao YF, Hu HS, Li J, Zhai HJ, Li SD, Wang LS (2014) The B35 cluster with a double-hexagonal vacancy: a new and more flexible structural motif for borophene. J Am Chem Soc 136:12257–12260
Boustani I (1997) New quasi-planar surfaces of bare boron. Surf Sci 370:355–363
Boustani I (1997) New Convex and spherical structures of bare boron clusters. J Solid State Chem 133:182–189
Boustani I, Quandt A (1997) Nanotubules of bare boron clusters: ab initio and density functional study. Europhys Lett 39:527–532
Tian FY, Wang YX (2008) The competition of double-, four-, and three-ring tubular B3n (n = 8–32) nanoclusters. J Chem Phys 129:024903
Tai TB, Nguyen MT (2015) Electronic structure and photoelectron spectra of Bn with n = 26-29: an overview of structural characteristics and growth mechanism of boron clusters. Phys Chem Chem Phys 17:13672–13679
Zhao J, Huang X, Shi R, Liu H, Su Y, King RB (2015) B28: the smallest all-boron cage from an ab initio global search. Nano 7:15086–15090
Wang YJ, Zhao YF, Li WL, Jian T, Chen Q, You XR, Ou T, Zhao XY, Zhai HJ, Li SD, Li J, Wang LS (2016) Observation and characterization of the smallest borospherene, B28 − and B28. J Chem Phys 144:064307
Tai TB, Duong LV, Pham HT, Mai DTT, Nguyen MT (2014) A disk-aromatic bowl cluster B30: toward formation of boron buckyballs. Chem Commun 50:1558–1560
Pham HT, Duong LV, Tam NM, Pham-Ho MP, Nguyen MT (2014) The boron conundrum: bonding in the bowl B30 and B36, fullerene B40 and triple ring B42 clusters. Chem Phys Lett 608:295–302
Wang K, Li DZ, Li R, Feng LY, Wang YJ, Zhai HJ (2016) Concentric dual π aromaticity in bowl-like B30 cluster: an all-boron analogue of corannulene. Phys Chem Chem Phys 18:23304–23311
Li H, Shao N, Shang B, Yuan LF, Yang J, Zeng XC (2010) Icosahedral B12-containing core–shell structures of B80. Chem Commun 46:3878–3880
Wang L, Zhao J, Li F, Chen Z (2010) Boron fullerenes with 32-56 atoms: irregular cage configurations and electronic properties. Chem Phys Lett 501:16–19
Zhao J, Wang L, Li F, Chen Z (2010) B80 and other medium-sized boron clusters: Core-shell structures, not hollow cages. J Phys Chem A 114:9969–9972
Zdoğan CÖ, Mukhopadhyay S, Hayami W, Güvenc ZB, Pandey R, Boustani S (2010) The unusually stable B100 fullerene, structural transitions in boron nanostructures, and a comparative study of α- and γ-boron and sheets. J Phys Chem C 114:4362–4375
Quarles KD, Kah CB, Gunasinghe RN, Musin RN, Wang XQ (2011) Filled pentagons and electron counting rule for boron fullerenes. J Chem Theor Comput 7:2017–2020
De S, Willand A, Amsler M, Pochet P, Genovese L, Oedecker S (2011) Energy landscape of fullerene materials: a comparison of boron to boron nitride and carbon. Phys Rev Lett 106:225502
Li F, Jin P, Jiang DE, Wang L, Zhang SB, Zhao J, Chen Z (2012) B80 and B101-103 clusters: remarkable stability of the core-shell structures established by validated density functionals. J Chem Phys 136:074302
Tai TB, Nguyen MT (2016) A new chiral boron cluster B44 containing nonagonal holes. Chem Commun 52:1653–1656
Lv J, Wang Y, Zhu L, Ma Y (2014) B38: an all-boron fullerene analogue. Nano 6:11692–11696
Tai TB, Nguyen MT (2015) Comment on “B38: an all-boron fullerene analogue” by J. Lv, Y. Wang, L. Zhu and Y. Ma, Nanoscale, 6, 11692. Nano 7:316–3317
Szwacki NG (2008) Boron fullerenes: a first-principles study. Nanoscale Res Lett 3:49–54
Gribanova TN, Minyaev RM, Minkin VI (2016) Structure and stability of the С-doped boron fullerenes B60С12 and B80C12 with quasi-planar pentacoordinated cage carbon atoms: a quantum-chemical study. Mendeleev Commun 26:485–487
Gribanova TN, Minyaev RM, Minkin VI (2017) Hypercoordinated carbon in C-doped boron fullerenes: a quantum chemical study. Struct Chem 28:357–369
Tam NM, Pham HT, Duong LV, Pham-Ho MP, Nguyen MT (2015) Fullerene-like boron clusters stabilized by an endohedrally doped iron atom: BnFe with n = 14, 16, 18 and 20. Phys Chem Chem Phys 17:3000–3003
Lv J, Wang Y, Zhang L, Lin H, Zhao J, Ma Y (2015) Stabilization of fullerene-like boron cages by transition metal encapsulation. Nano 7:10482–10489
Chen Q, Li HR, Miao CQ, Wang YJ, Lu HG, Mu YW, Ren GM, Zhai HJ, Li SD (2016) Endohedral Ca@B38: stabilization of a B38 2− borospherene dianion by metal encapsulation. Phys Chem Chem Phys 18:11610–11615
Chen Q, Li HR, Tian WJ, Lu HG, Zhai HJ, Li SD (2016) Endohedral charge-transfer complex Ca@B37 −: stabilization of a B37 3− borospherene trianion by metal-encapsulation. Phys Chem Chem Phys 18:14186–14190
Tian WJ, Chen Q, Li HR, Yan M, Mu YW, Lu HG, Zhai HJ, Li SD (2016) Saturn-like charge-transfer complexes Li4&B36, Li5&B36 +, and Li6&B36 2+: exohedral metalloborospherenes with a perfect cage-like B36 4− core. Phys Chem Chem Phys 18:9922–9926
Minyaev RM, Gribanova TN (2000) Stabilization of nonclassical types of valence bond orientation at the carbon atom in organoboron compounds. Russ Chem Bull 49:783–793
Minyaev RM, Minkin VI, Starikov AG, Gribanova TN (2001) Induced aromaticity. Russ Chem Bull 50:2325–2335
Gribanova TN, Minyaev RM, Minkin VI (2001) Stabilization of planar hexacoordinated boron: an ab initio study. Rus J Inorg Chem 46:1207–1210
Minyaev RM, Minkin VI, Gribanova TN (2004) A quantum-chemical study of carbon sandwich compounds. Mendeleev Commun 14:96–98
Minyaev RM, Gribanova TN (2005) Carbon, nitrogen, and oxygen hypercoordination in half-sandwich and sandwich structures. Russ Chem Bull 54:533–546
Minyaev RM, Minkin VI, Gribanova TN, Starikov AG, Gapurenko OA (2006) Hypercoordinated carbon in endohedral hydrocarbon cage complexes C@C 20H20 4− and C@C20H 20·Li4. Dokl Chem 407:47–50
Minyaev RM, Minkin VI, Gribanova TN, Starikov AG (2006) Sandwich compounds with central hypercoordinate carbon, nitrogen, and oxygen: a quantum-chemical study. Heteroatom Chem 17:464–474
Gribanova TN, Minyaev RM, Minkin VI (2008) Theoretical design of planar systems with hypercoordinate p-elements of the second period in a nonmetallic environment. Russ J Gen Chem 78:750–768
Gapurenko OA, Gribanova TN, Minyaev RM, Minkin VI (2007) Hypercoordinate atoms of second-row elements in dodecahedrane endohedral complexes. Russ Chem Bull 56:856–862
Minyaev RM, Gribanova TN, Minkin VI (2013) in Comprehensive inorganic chemistry II (Second Edition): From elements to applications. Reedijk J, Poeppelmeier K (Eds) Elsevier, Amsterdam, 9: 109–132
Olson JK, Boldyrev AI (2012) Electronic transmutation: boron acquiring an extra electron becomes ‘carbon’. Chem Phys Lett 523:83–86
Alexandrova AN, Birch KA, Boldyrev AI (2003) Flattening the B6H6 2− octahedron. Ab initio prediction of a new family of planar all-boron aromatic molecules. J Am Chem Soc 125:10786–10787
Grünbaum B (1967) ConVex polytopes. Wiley-Interscience, New York,
Domene MC, Fowler P, Mitchell D, Seifert G, Zerbetto F (1997) Energetics of C20 and C22 fullerene and near-fullerene carbon cages. J Phys Chem A 101:8339–8344
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Foresman JB, Frisch E (1996) Exploring chemistry with electronic structure methods. Gaussian Inc, Pittsburg,
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Revision D.01. Gaussian Inc, Wallingford,
Medvedev MG, Bushmarinov IS, Sun J, Perdew JP, Lyssenko KA (2017) Density functional theory is straying from the path toward the exact functional. Science 355:49–52
Hammes-Schiffer S (2017) A conundrum for density functional theory. DFT studies may sometimes get the right results for the wrong reasons. Science 355:28–29
Borden WT (2011) Current applications of computational chemistry in JACS - molecules, mechanisms, and materials. J Am Chem Soc 133:14841–14843
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Weinhold F, Landis CR (2012) Discovering chemistry with natural bond orbitals. John Wiley & Sons, Hoboken,
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis R, Weinhold F. (2013) NBO 6.0 Theoretical Chemistry Institute, University of Wisconsin, Madison
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford,
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Keith TA (2011) AIMAll (Version 11.06.19) TK Gristmill Software, Overland Park, KS (http://aim.tkgristmill.com/)
Zhurko GA ChemCraft software, version 1.8. (http://www.chemcraftprog.com)
Bushmarinov IS, Lyssenko KA, Antipin MY (2009) Atomic energy in the “atoms in molecules” theory and its use for solving chemical problems. Russ Chem Rev 78:283–302
Shaik S, Danovich D, Silvi B, Lauvergnat DL, Hiberty PC (2005) Charge-shift bonding - a class of electron-pair bonds that emerges from valence bond theory and is supported by the electron localization function approach. Chem Eur J 11:6358–6371
Shaik S, Danovich D, Wu W, Hiberty PC (2009) Charge-shift bonding and its manifestations in chemistry. Nat Chem 1:443–449
Ziolkowski M, Grabowski SJ, Leszczynski J (2006) Cooperativity in hydrogen-bonded interactions: ab initio and “atoms in molecules” analyses. J Phys Chem A 110:6514–6521
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Acknowledgments
The work was supported by the Russian Science Foundation (Grant 16-13-10050).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 6294 kb)
Rights and permissions
About this article
Cite this article
Gribanova, T.N., Minyaev, R.M. & Minkin, V.I. Stabilization of boron clusters with classical fullerene structures by combined doping effect: a quantum chemical study. Struct Chem 29, 327–340 (2018). https://doi.org/10.1007/s11224-017-1031-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-1031-y