Abstract
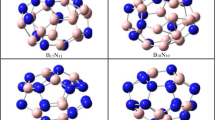
The structures and stability of C-doped boron fullerenes with the three-dimensional arrangement of non-classical pentacoordinated quasi-flat carbon centers were studied using the density functional theory (DFT) B3LYP/6-311+G(d,p) method. The doping with carbon atoms in apical positions above the five-membered rings stabilizes the spherical boron fullerene forms due to multicenter interactions of pz-orbitals of the carbons and adjacent boron atoms. Increasing in the size of the fullerene cluster is accompanied by change in the bonding pattern and by flattening of the hypercoordinated carbon centers. Endohedral metal atoms significantly affect on the structure and stability of the fullerene systems with hypercoordinated carbon centers.




Similar content being viewed by others
References
van’t Hoff JH (1874) Sur les formules de structure dans l’espace. Arch Neerl Sci Exactes Nat 9:445–454
LeBel JA (1874) Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull Soc Chim Fr 22:337–347
Olah GA, Prakash GKS, Williams RE, Field LD, Wade K (1987) Hypercarbon chemistry. Wiley-Interscience, New York
Minkin VI, Minyaev RM, Zhdanov YA (1987) Nonclassical structures of organic compounds. Mir Publishers, Moscow
Minkin VI, Minyaev RM, Hoffmann R (2002) Nonclassical structures of organic compounds: Non-standard stereochemistry and hypercoordination. Russ Chem Rev 71:989–1015
Jemmis ED, Jayasree EG, Parameswaran P (2006) Hypercarbon in polyhedral structures. Chem Soc Rev 35:157–168
Minyaev RM, Minkin VI (2008) Nonclassical carbon: from theory to experiment. Russ J Gen Chem 78:732–749
Minyaev RM, Gribanova TN, Minkin VI (2013) In: Reedijk J, Poeppelmeier K (eds) Comprehensive Inorganic Chemistry II (Second Edition): From elements to applications, vol 9. Elsevier, Amsterdam, pp. 109–132
Yang LM, Ganz E, Chen Z, Wang ZX, Schleyer PR (2015) Four decades of the chemistry of planar hypercoordinate compounds. Angew Chem Int Ed 54:9468–9501
Olah GA (1974) In: Klopman G (ed) Chemical reactivity and reaction paths. Wiley, London
Olah GA, Schlosberg RH (1968) Chemistry in super acids. I. Hydrogen exchange and polycondensation of methane and alkanes in FSO3H-SbF5 (“magic acid”) solution. Protonation of alkanes and the intermediacy of CH5 + and related hydrocarbon ions. The high chemical reactivity of “paraffins” in ionic solution reactions. J Am Chem Soc 90:2726–2727
Olah GA, Prakash GKS, Sommer J (1985) Superacids. Wiley, New York
Olah GA, Laali KK, Wang Q, Prakash GKS (1998) Onium Ions. Wiley, New York
Rasul G, Prakash GKS, Olah GA (1998) Calculated 1B–13C NMR chemical shift relationship in hypercoordinate methonium and boronium ions. Proc Natl Acad Sci 95:7257–7259
Olah GA, Rasul G (1997) From Kekulé’s tetravalent methane to five-, six-, and seven-coordinate protonated methanes. Acc Chem Res 30:245–250
Olah GA, Rasul G (1996) Comparison and search for CH5 3+ and CH6 4+ and their isoelectronic boron analogues BH5 2+ and BH6 3+. J Am Chem Soc 118:12922–12924
Lammertsma K, Olah GA (1982) Diprotonated methane, CH6 2+, and diprotonated ethane, C2H8 2+. J Am Chem Soc 104:6851–6853
Rasul G, Prakash GKS, Olah GA (2002) Ab initio GIAO-MP2-calculated structures and 11B-13C NMR chemical shift relationship in hypercoordinate onium-carbonium dications and isoelectronic onium-boronium cations. Proc Natl Acad Sci 99:9635–9638
Lammertsma K, Barzaghi M, Olah GA, Pople JA, Schleyer PR, Simonetta M (1983) Structure and stability of diprotonated methane, СH6 2+. J Am Chem Soc 105:5258–5263
Olah GA, Rasul G (1996) Triprotonated methane, СH7 3+: the parent heptacoordinate carbonium ion. J Am Chem Soc 118:8503–8504
Olah GA (2001) 100 Years of carbocations and their significance in chemistry. J Org Chem 66:5943–5957
Rasul G, Olah GA (1997) Hexa-, hepta-, and octacoordinate boronium ions: BH6 +, BH7 2+, and BH8 3+. Inorg Chem 36:1278–1281
Talrose VL, Ljubimova AK (1952) Secondary processes in the ion source of a mass spectrometer. Doklady AN (USSR) 86:909–912
Schreiner PR (2000) Does CH5 + have (a) “structure?” A tough test for experiment and theory. Angew Chem Int Ed Engl 39:3239–3241
Marx D, Parrinello M (1999) CH5 +: The cheshire cat smiles. Science 284:59–61
Thompson KC, Crittenden DL, Jordan MJT (2005) СH5 +: chemistry’s chameleon unmasked. J Am Chem Soc 127:4954–4958
Scherbaum F, Grohmann A, Huber B, Krüger C, Schmidbaur H (1988) “Aurophilicity” as a consequence of relativistic effects: the hexakis (triphenylphosphaneaurio) methane dication [(Ph3PAu)6C]2+. Angew Chem Int Ed Engl 27:1544–1546
Scherbaum F, Grohmann A, Müller G, Schmidbaur H (1989) Synthesis, structure, and bonding of the cation [{(C6H5)3PAu}5C]+. Angew Chem Int Ed Engl 28:463–465
Schmidbaur H (1993) Some new concepts in the chemistry of the p-block elements. Pure Appl Chem 65:691–698
Häberlen OD, Schmidbaur H, Rösch N (1994) Stability of main-group element-centered gold cluster cations. J Am Chem Soc 116:8241–8248
Görling A, Rösch N, Ellis DE, Schmidbaur H (1991) Electronic structure of main-group-element-centered octahedral gold clusters. Inorg Chem 30:3986–3994
Williams RE (1971) Carboranes and boranes; polyhedra and polyhedral fragments. Inorg Chem 10:210–214
Stohrer WD, Hoffmann R (1972) Bond-stretch isomerism and polytopal rearrangements in (CH)5 +, (CH)5 -, and (CH)4CO. J Am Chem Soc 94:1661–1668
Masamune S, Sakai M, Ona H (1972) Nature of the (CH)5 + species. I. Solvolysis of 1, 5-dimethyltricyclo[2.1.0.02,5]pent-3-yl benzoate. J Am Chem Soc 94:8955–8956
Masamune S, Sakai M, Ona H, Jones AJ (1972) Nature of the (CH)5 + species. II. Direct observation of the carbonium ion of 3-hydroxyhomotetrahedrane derivatives. J Am Chem Soc 94:8956–8958
Hehre WJ, Schleyer PR (1973) Cyclopentadienyl and related (CH)5 + cations. J Am Chem Soc 95:5837–5839
Minkin VI, Minyaev RM (2002) Pyramidane and pyramidal cations. Dokl Chem 385:502–507
Schleyer PvR, Boldyrev AI (1991) A new, general strategy for achieving planar tetracoordinate geometries for carbon and other second row periodic elements. J Chem Soc Chem Commun 21:1536–1538
Boldyrev AI, Wang LS (2001) Beyond classical stoichiometry: experiment and theory. J Phys Chem A 105:10759–10775
Minyaev RM, Gribanova TN (2000) Stabilization of nonclassical types of valence bond orientation at the carbon atom in organoboron compounds. Russ Chem Bull Int Ed 49:783–793
Exner K, Schleyer PR (2000) Planar hexacoordinate carbon: A viable possibility. Science 290:1937–1940
Wang ZX, Schleyer PR (2001) Construction principles of “Hyparenes”: Families of molecules with planar pentacoordinate carbons. Science 292:2465–2469
Minyaev RM, Gribanova TN, Starikov AG, Minkin VI (2002) Heptacoordinated carbon and nitrogen in a planar boron ring. Dokl Chem 382:41–45
Minyaev RM, Gribanova TN, Starikov AG, Minkin VI (2001) Octacoordinated main-group element centres in a planar cyclic B8 environment: An ab initio study. Mendeleev Commun 6:213–214
Gribanova TN, Minyaev RM, Minkin VI (2008) Theoretical design of planar systems with hypercoordinate p elements of the second period in a nonmetallic environment. Russ J Gen Chem 78:750–768
Wang LM, Huang W, Averkiev BB, Boldyrev AI, Wang LS (2007) CB7 -: Experimental and theoretical evidence against hypercoordinate planar carbon. Angew Chem Int Ed 46:4550–4553
Averkiev BB, Zubarev DY, Wang LM, Huang W, Wang LS, Boldyrev AI (2008) Carbon avoids hypercoordination in CB6 -, CB6 2-, and C2B5 - planar carbon-boron clusters. J Am Chem Soc 130:9248–9250
Averkiev BB, Wang LM, Huang W, Wang LS, Boldyrev AI (2009) Experimental and theoretical investigations of CB8 -: towards rational design of hypercoordinated planar chemical species. Phys Chem Chem Phys 11:9840–9849
Minkin VI, Minyaev RM (2004) Hypercoordinate carbon in polyhedral organic structures. Mendeleev Commun 14:43–46
Minyaev RM, Minkin VI, Gribanova TN (2004) A quantum-chemical study of carbon sandwich compounds. Mendeleev Commun 14:96–98
Minyaev RM, Gribanova TN (2005) Carbon, nitrogen, and oxygen hypercoordination in half-sandwich and sandwich structures. Russ Chem Bull 54:533–546
Minyaev RM, Minkin VI, Gribanova TN, Starikov AG (2006) Sandwich compounds with central hypercoordinate carbon, nitrogen, and oxygen: A quantum-chemical study. Heteroatom Chem 17:464–474
Minyaev RM, Gribanova TN, Minkin VI (2004) Hexacoordinated carbon in an organoboron cage. Dokl Chem 396:122–126
Minyaev RM, Minkin VI, Gribanova TN, Starikov AG (2004) A hydrocarbon dication with nonplanar hexacoordinated carbon. Mendeleev Commun 14:47–48
Gribanova TN, Gapurenko OA, Minyaev RM, Minkin VI (2005) Stabilization of octacoordinate carbon center in metal-containing derivatives of orthocarbonic acid. Russ Chem Bull 54:1989–1998
Minyaev RM, Gribanova TN, Starikov AG, Gapurenko OA, Minkin VI (2005) Octacoordinated carbon in a boron-carbon cage. Dokl Chem 404:193–198
Minyaev RM, Minkin VI, Gribanova TN, Starikov AG, Gapurenko OA (2006) Hypercoordinated carbon in endohedral hydrocarbon cage complexes C@C20H20 4- and C@C20H20•Li4. Dokl Chem 407:47–50
Gribanova TN, Minyaev RM, Minkin VI (2006) Sandwich compounds of second-row elements: a quantum chemical study. Russ Chem Bull 55:1893–1903
Gapurenko OA, Gribanova TN, Minyaev RM, Minkin VI (2007) Octacoordinate carbon atom in tetra(metalloamino)methanes CN 4M4 (M = Be, Mg, Ca): quantum-chemical investigation. Russ J Org Chem 43:685–690
Gapurenko OA, Gribanova TN, Minyaev RM, Minkin VI (2007) Hypercoordinate atoms of second-row elements in dodecahedrane endohedral complexes. Russ Chem Bull 56:856–862
Gribanova TN, Minyaev RM, Minkin VI (2008) Nonclassical systems with two hypercoordinate atoms in a polyhedral cage. Dokl Chem 418:10–14
Gribanova TN, Gapurenko OA, Starikov AG, Minyaev RM, Minkin VI (2008) Hypercoordination of first-row elements in heteroanalogues of prismanes and propellanes. Dokl Chem 422:255–259
Gribanova TN, Minyaev RM, Minkin VI (2009) Sandwich and multidecker sandwich derivatives of first-row elements (Be, C, N). Dokl Chem 424:1–6
Gribanova TN, Minyaev RM, Minkin VI (2016) Structure and stability of the С-doped boron fullerenes B60С12 and B80C12 with quasi-planar pentacoordinated cage carbon atoms: a quantum-chemical study. Mendeleev Commun 26:485–487
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Foresman JB, Frisch E (1996) Exploring chemistry with electronic structure methods. Gaussian Inc, Pittsburg
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam MJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Revision D.01. Gaussian Inc, Wallingford
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Weinhold F, Landis CR (2012) Discovering chemistry with Natural Bond Orbitals. Wiley, Hoboken, NJ
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis R, Weinhold F. (2013) NBO 6.0 Theoretical Chemistry Institute, University of Wisconsin, Madison
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Bader RFW (1998) A bond path: A universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Keith TA (2011) AIMAll (Version 11.06.19) TK Gristmill Software, Overland Park, KS (http://aim.tkgristmill.com/)
Zhurko GA (2016) ChemCraft software version 1.8. (http://www.chemcraftprog.com)
Emsley J (1991) The elements. Clarendon Press, Oxford
Shaik S, Danovich D, Silvi B, Lauvergnat DL, Hiberty PC (2005) Charge-shift bonding - A class of electron-pair bonds that emerges from valence bond theory and is supported by the electron localization function approach. Chem Eur J 11:6358–6371
Shaik S, Danovich D, Wu W, Hiberty PC (2009) Charge-shift bonding and its manifestations in chemistry. Nat Chem 1:443–449
Bushmarinov IS, Lyssenko KA, Antipin MY (2009) Atomic energy in the “Atoms in Molecules” theory and its use for solving chemical problems. Russ Chem Rev 78:283–302
Wu W, Gu J, Song J, Shaik S, Hiberty PC (2009) The inverted bond in [1.1.1] propellane is a charge-shift bond. Angew Chem Int Ed 48:1407–1410
Shaik S, Danovich D, Braida B, Wu W, Hiberty PC (2016) New landscape of electron-pair bonding: covalent, ionic, and charge-shift bonds. Struct Bond 170:169–212
Rzepa SH (2010) The rational design of helium bonds. Nat Chem 2:390–393
Acknowledgments
The work was supported by the Russian Science Foundation (Grant 16-13-10050).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Gribanova, T.N., Minyaev, R.M. & Minkin, V.I. Hypercoordinated carbon in C-doped boron fullerenes: a quantum chemical study. Struct Chem 28, 357–369 (2017). https://doi.org/10.1007/s11224-016-0886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0886-7