Abstract
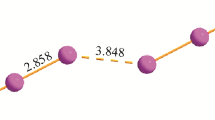
Analysis of iodine-derived chalcogenazolo(ino)quinolinium mono- and triiodides shows that the covalently bonded iodine atoms in the fragments C(sp2)–I and C(sp3)–I differ significantly in iodide–iodine halogen bonding ability. The local and integrated characteristics of kinetic, potential, and total electronic energy for C–I covalent bond have been examined. It has been found that both delocalization indices of iodine covalent bond and the total electronic energy density integrated over carbon–iodine interatomic surface can be used as quantitative criteria of iodine-derived cation ability to form the halogen bond with triiodide anion in crystals.







Similar content being viewed by others
References
Batalov VI, Dikhtiarenko A, Yushina ID, Bartashevich EV, Kim DG, García-Granda S (2014) Crystal structure of (E)-3-(1-iodoethylidene)-2,3-dihydro-[1,4]thiazino-[2,3,4-ij]quinolin-4-ium triiodide, C13H11I4NS. Zeitschrift für Kristallographie New Cryst Struct 229:195–196
Batalov VI, Kim DG, Dikhtiarenko A, Zakariae A, Bartashevich EV, García-Granda S (2014) Crystal structure of (E)-3-(iodomethylene)-2,3-dihydro-[1,4]oxazino-[2,3,4-ij]quinolin-4-ium triodide–iodine (2:1), [C12H9INO]I-3 center dot 0.5I(2), C12H9I5NO. Zeitschrift für Kristallographie New Cryst Struct 229:211–212
Batalov VI, Dikhtiarenko A, Yushina ID, Bartashevich EV, Kim DG, García-Granda S (2014) Crystal structure of 8,10-diiodo-3-(iodomethyl)-2,3-dihydro-[1,4]oxazino-[2,3,4-ij]quinolin-4-ium triiodide, [C12H9I3NO]center dot I-3, C12H9I6NO. Zeitschrift für Kristallographie New Cryst Struct 229:213–214
Batalov VI, Kim DG, Slepukhin PA (2013) Heterocyclization of 8-(2-methyl-prop-2-enylsulfanyl)quinoline using electrophilic reagents. Chem Heterocycl Compd 49:1092–1096. doi:10.1007/s10593-013-1348-4
Kim DG (2008) Synthesis and halocyclization of 2-alkenylthioquinolines. Chem Heterocycl Compd 44:1355–1358
Bartashevich EV, Yushina ID, Vershinina EA, Slepukhin PA, Kim DG (2014) Complex structure tri- and polyiodides of iodocyclization products of 2-allylthioquinoline. J Struct Chem 55:112–119
Kim DG, Vershinina EA (2014) Synthesis and properties of thiazolo- and oxazolo[3,2-a]quinolinium systems and their hydrogenated derivatives (review). Chem Heterocycl Compd 50:911–931
Desiraju GR, Ho PS, Kloo L, Legon AC, Marquardt R, Metrangolo P, Politzer PA, Resnati G, Rissanen K (2013) Definition of the halogen bond. Pure Appl Chem 85:1711–1713
Bartashevich EV, Yushina ID, Stash AI, Tsirelson VG (2014) Halogen bonding and other iodine interactions in crystals of dihydrothiazolo(oxazino)quinolinium oligoiodides from the electron-density viewpoint. Cryst Growth Des 14:5674–5684
Kilah NL, Wise MD, Beer PD (2011) Crystallographic implications for the design of halogen bonding anion receptors. Cryst Growth Des 11:4565–4571
Raatikainen K, Cavallo G, Metrangolo P, Resnati G, Rissanen K, Terraneo G (2013) In the pursuit of efficient anion-binding organic ligands based on halogen bonding. Cryst Growth Des 13:871–877
Tepper R, Schulze B, Jäger M, Friebe C, Scharf DH, Görls H, Schubert US (2015) Anion receptors based on halogen bonding with halo-1,2,3-triazoliums. J Org Chem. Article ASAP doi:10.1021/acs.joc.5b00028
Zapata F, Caballero A, Molina P, Alkorta I, Elguero J (2014) Open bis(triazolium) structural motifs as a benchmark to study combined hydrogen- and halogen-bonding interactions in oxoanion recognition processes. J Org Chem 79:6959–6969
Allen FH (2002) The Cambridge structural database: a quarter of a million crystal structures and rising. Acta Crystallogr B 58:380–388
Bondi A (1964) Van der Waals volumes and radii. J Phys Chem 68(3):441–451
Sakurai T, Sundaralingam M, Jeffrey GA (1963) A nuclear quadrupole resonance and X-ray study of the crystal structure of 2,5-dichloroaniline. Acta Crystallogr 16(5):354–363
Desiraju GR (1991) Crystal engineering: the design of organic solids. J Appl Cryst 24:265
Jentzsch AV, Matile S (2013) Transmembrane halogen-bonding cascades. J Am Chem Soc 135(14):5302–5303
Tsirelson VG, Zou P-F, Tang T-H, Bader RFW (1995) Topological definition of crystal structure: determination of the bonded interactions in solid molecular chlorine. Acta Crystallogr A 51:143–153
Bertolotti F, Shishkina AV, Forni A, Gervasio G, Stash AI, Tsirelson VG (2014) The intermolecular bonding features in solid iodine. Cryst Growth Des 14:3587–3595
Bartashevich EV, Tsirelson VG (2014) Interplay between non-covalent interactions in complexes and crystals with halogen bonds. Russ Chem Rev 83(12):1181–1203
Shields Z, Murray JS, Politzer P (2010) Directional tendencies of halogen and hydrogen bonds. Int J Quantum Chem 110(15):2823–2832
Politzer P, Murray JS, Clark T (2010) Halogen bonding: an electrostatically-driven highly directional noncovalent interaction. Phys Chem Chem Phys 12:7748–7757
Politzer P, Riley KE, Bulat FA, Murray JS (2012) Perspectives on halogen bonding and other σ-hole interactions: lex parsimoniae (Occam’s Razor). Comput Theor Chem 998:2–8
Clark T, Hennemann M, Murray JS, Politzer P (2007) Halogen bonding: the σ-hole. J Mol Model 13:291. doi:10.1007/s00894-006-0130-2
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) Properties of atoms in molecules: atomic volumes. J Am Chem Soc 109:7968–7979
Bankiewicz B, Palusiak M (2013) The shape of the halogen atom—anisotropy of electron distribution and its dependence on basis set and method used. Struct Chem 24:1297–1306
Nyburg SC, Faerman CH (1985) A revision of van der Waals atomic radii for molecular crystals: N, O, F, S, Cl, Se, Br and I bonded to carbon. Acta Crystallogr B 41:274–279
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Tsirelson VG (2014) Quantum chemistry. Molecules, molecular systems and solids, 3rd edn. Binom Publ, Moscow
Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. Wiley-VCH Verlag GmbH & Co, KGaA
Cremer D, Kraka E (1984) A description of the chemical bond in terms of local properties of electron density and energy. Croat Chem Acta 57:1259–1281
Bader RFW, Beddall PM (1972) Virial field relationship for molecular charge distributions and the spatial partioning of molecular properties. J Chem Phys 56:3320–3329
Bader RFW (2009) Confined atoms treated as open quantum systems in advances in quantum chemistry. Adv Quantum Chem 57:285–318
Bader RFW, Austen MA (1997) Properties of atoms in molecules: atoms under pressure. J Chem Phys 107:4271–4285
Bader RFW (2000) Atomic force microscope as an open system and the Ehrenfest force. Phys Rev B 61:7795
Bader RFW, Tang TH, Tal Y, Bieglier-Konig FW (1982) Molecular structure and its change: hydrocarbons. J Am Chem Soc 104(4):940–945
Exner K, PvR Schleyer (2001) Theoretical bond energies—a critical evaluation. J Phys Chem A 105:3407–3417
Howard ST (2003) An atoms-in-molecules model of bond energy distributions in polyatomic molecules. Phys Chem Chem Phys 5(15):3113–3119
Platts JA (2005) Properties of interatomic surfaces: Relation to bond energies. Phys Chem Chem Phys 7:3805–3810
Bader RFW, Stephens ME (1975) Spatial localization of the electronic pair and number distributions in molecules. J Am Chem Soc 97(26):7391–7399
Fradera X, Austen MA, Bader RFW (1999) The Lewis model and beyond. J Phys Chem A 103:304–314
Wang Y-G, Werstiuk NH (2003) A practical and efficient method to calculate AIM localization and delocalization indices at post-HF levels of theory. J Comput Chem 24(3):379–385
Keith TA (2013) AIMALL, Version 13.10.19. Professional. http://aim.tkgristmill.com
Granovsky AA. Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html
Dunitz JD, Gehrer H, Britton D (1972) The crystal structure of diiodacetylene: an example of pseudosymmetry. Acta Cryst B 28:1989–1994
Angelina EL, Duarte DJR, Peruchena NM (2013) Is the decrease of the total electron energy density a covalence indicator in hydrogen and halogen bonds? J Mol Mod 19(5):2097–2106
Stevens WJ, Fink WH (1987) Frozen fragment reduced variational space analysis of hydrogen bonding interactions. Application to the water dimer. Chem Phys Lett 139:15–22
Slepukhin PA, Personal communications
Acknowledgments
We thank Dr. P.A. Slepukhin for X-ray diffraction structural analysis of 1-iodomethyl-1,2-dihydro [1, 3] thiazolo[3,2-a]quinolinium monoiodide (CCDC 1055418) which was crystallized by E.A. Vershinina. This work was supported by the Russian Foundation for Basic Research, Grant 13-03-00767a and Grant 14-03-00961.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to the memory of Professor Oleg Shishkin
Rights and permissions
About this article
Cite this article
Bartashevich, E.V., Nasibullina, S.E., Bol’shakov, O.I. et al. Exploring heterocyclic cations ability to form the iodide–iodine halogen bond: case study of chalcogenazolo(ino)quinolinium crystals. Struct Chem 27, 305–313 (2016). https://doi.org/10.1007/s11224-015-0714-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0714-5