Abstract
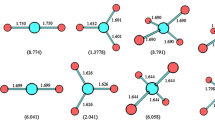
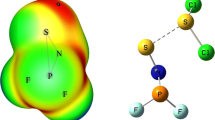
The computational investigations are carried out on the heterodimers containing CF2Cl2 with isoelectronic and isostructure (linear triatomics) species of N2O and CO2 through MP2/aug-cc-pV(D+d)Z, MP2/aug-cc-pV(T+d)Z//MP2/aug-cc-pV(D+d)Z, and CCSD(T)/aug-cc-pV(D+d)Z//MP2/aug-cc-pV(D+d)Z levels. Five and twelve heterodimers are located on the potential energy surface of CF2Cl2–CO2 and CF2Cl2–N2O systems, respectively. Binding energies of heterodimers in the CF2Cl2–CO2 and CF2Cl2–N2O systems corrected with BSSE are in the ranges of 1.30–5.79 and 1.30–6.85 kJ/mol at the MP2/aug-cc-pV(T+d)Z//MP2/aug-cc-pV(D+d)Z level, respectively. The calculated results reveal that the five most stable heterodimers among all heterodimers obtained for the CF2Cl2–CO2 and CF2Cl2–N2O systems belong to CF2Cl2–N2O system. Therefore, CF2Cl2–N2O system has more key role than CF2Cl2–CO2 one in the atmosphere.



Similar content being viewed by others
References
Alkorta I, Grabowski SJ (2012) Comput Theor Chem 998:1
Tachikawa H, Abe S (2003) Inorg Chem 42:2188
Solimannejad M, Gharabaghi M, Alkorta I (2012) Struct Chem. doi:10.1007/s11224-012-0099-7
Yang Y (2009) Int J Quantum Chem 109:266
Scheiner S (2009) J Phys Chem B 113:10421
Müller-Dethlefs K, Hobza P (1999) Chem Rev 100:143
Sloss LL (1992) Nitrogen oxides control technology fact book. William Andrew Publishing, Norwich, p 6. ISBN 978-0-8155-1294-3
US Emissions Inventory 2010: Inventory of US Greenhouse Gas Emissions and Sinks 1990–2008
Rowland FS, Molina M (1974) Nature 249:810
Farman JD, Gardiner BG, Shanklin JD (1985) Nature 315:207
Wallington TJ, Schneider WF, Sehested J, Nielsen OJ (1995) J Chem Soc Faraday Discussion 100:55
Xavier ES, Rocha WR, Da Silva JCS, Dos Santos HF, De Almeida WB (2007) Chem Phys Lett 448:164
Macalady DL (1998) Perspectives in Environmental Chemistry. Oxford University Press, Oxford 240
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision B 03). Gaussian. Inc, Pittsburgh
Møller C, Plesset MS (1934) Phys Rev 46:618
Dunning TH (1989) J Chem Phys 90:1007
Dunning TH, Peterson KA, Wilson AK (2001) J Chem Phys 114:9244
Boys SF, Bernardi F (1970) Mol Phys 19:553
Bader RFW (1990) In: Halpen J, Green MLH (eds) The international series of monographs of chemistry. Clarendon Press, Oxford
Biegler-Konig F, Schonbohm J (2002) AIM2000 Program Package, Ver. 2.0. University of Applied Sciences, Bielefeld
Curtiss L, Pochatko DG, Reed AE, Weinhold F (1985) J Chem Phys 82:2679
Reed AE, Weinhold F (1985) J Chem Phys 83:1736
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211
Diao K-S, Wang F, Wang H-j (2009) J Mol Struct Theochem 913:195
Ziółkowski M, Grabowski SJ, Leszczynski J (2006) J Phys Chem A 110:6514
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Seif, A., Ebrahimi, S., Vessally, E. et al. Comparative study on the stabilities and properties of heterodimers containing the intermolecular interactions of CF2Cl2 with the isoelectronic and isostructure species of N2O and CO2 . Struct Chem 24, 1737–1745 (2013). https://doi.org/10.1007/s11224-013-0218-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0218-0