Abstract
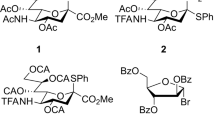
Supramer analysis was applied to explain the earlier discovered phenomenon of the transformation of nonselective glycosylation into stereospecific one upon changing the glycosyl donor concentration. The supramer analysis is a part of the supramer approach being developed by us and makes it possible to find concentration ranges separated by “critical” concentrations at which supramolecular aggregates (supramers) have different structures. As a result, the “critical” concentration for solutions of 2,3,5-tri-O-benzoyl-α-d-arabinofuranosyl bromide (1) in acetonitrile was found by polarimetry, and that for solutions of glycosyl bromide 1 in 1,2-dichloroethane (DCE) was found using static light scattering. The presence of nanosized supramers in solutions of 1 in acetonitrile and nano- and mesoscale supramers in solutions of 1 in DCE was demonstrated using dynamic light scattering. An analysis of the obtained experimental and earlier published data suggested a possibility of both formation of halogen bonds involving DCE molecules and halogen bond-mediated supramolecular aggregation of molecules of the dissolved substance in this solvent.
Similar content being viewed by others
References
D. A. Ahiadorme, N. M. Podvalnyy, A. V. Orlova, A. O. Chizhov, L. O. Kononov, Russ. Chem. Bull., 2016, 65, 2776–2778; DOI: https://doi.org/10.1007/s11172-016-1654-y.
L. O. Kononov, K. G. Fedina, A. V. Orlova, N. N. Kondakov, P. I. Abronina, N. M. Podvalnyy, A. O. Chizhov, Carbohydr. Res., 2017, 437, 28–35; DOI: https://doi.org/10.1016/j.carres.2016.11.009.
M. O. Nagornaya, A. V. Orlova, E. V. Stepanova, A. I. Zinin, T. V. Laptinskaya, L. O. Kononov, Carbohydr. Res., 2018, 470, 27–35; DOI: https://doi.org/10.1016/j.carres.2018.10.001.
A. V. Orlova, T. V. Laptinskaya, N. N. Malysheva, L. O. Kononov, J. Solution Chem., 2020, 49, 629–644; DOI: https://doi.org/10.1007/s10953-020-00977-1.
L. O. Kononov, RSC Adv., 2015, 5, 46718–46734; DOI: https://doi.org/10.1039/c4ra17257d.
L. O. Kononov, D. E. Tsvetkov, A. V. Orlova, Russ. Chem. Bull., 2002, 51, 1337–1338; DOI: https://doi.org/10.1023/a:1020981320040.
L. O. Kononov, N. N. Malysheva, E. G. Kononova, A. V. Orlova, Eur. J. Org. Chem., 2008, 3251–3255; DOI: https://doi.org/10.1002/ejoc.200800324.
L. O. Kononov, N. N. Malysheva, A. V. Orlova, A. I. Zinin, T. V. Laptinskaya, E. G. Kononova, N. G. Kolotyrkina, Eur. J. Org. Chem., 2012, 1926–1934; DOI: https://doi.org/10.1002/ejoc.201101613.
A. V. Orlova, R. R. Andrade, C. O. da Silva, A. I. Zinin, L. O. Kononov, ChemPhysChem, 2014, 15, 195–207; DOI: https://doi.org/10.1002/cphc.201300894.
A. V. Orlova, A. I. Zinin, L. O. Kononov, Russ. Chem. Bull., 2014, 63, 295–297; DOI: https://doi.org/10.1007/s11172-014-0429-6.
A. V. Orlova, L. O. Kononov, RENSIT, 2020, 12, 95–106; DOI: https://doi.org/10.17725/rensit.2020.12.095.
A. V. Orlova, T. V. Laptinskaya, N. V. Bovin, L. O. Kononov, Russ. Chem. Bull., 2017, 66, 2173–2179; DOI: https://doi.org/10.1007/s11172-017-1999-x.
A. V. Orlova, T. V. Laptinskaya, L. O. Kononov, Russ. Chem. Bull., 2019, 68, 1462–1464; DOI: https://doi.org/10.1007/s11172-019-2580-6.
M. Sedlák, J. Phys. Chem. B, 2006, 110, 13976–13984; DOI: https://doi.org/10.1021/jp061919t.
D. Rak, M. Sedlák, J. Phys. Chem. B, 2019, 123, 1365–1374; DOI: https://doi.org/10.1021/acs.jpcb.8b10638.
P. Politzer, J. S. Murray, T. Clark, Phys. Chem. Chem. Phys., 2010, 12, 7748–7757; DOI: https://doi.org/10.1039/c004189k.
P. Politzer, J. S. Murray, ChemPhysChem, 2013, 14, 278–294; DOI: https://doi.org/10.1002/cphc.201200799.
G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati, G. Terraneo, Chem. Rev., 2016, 116, 2478–2601; DOI: https://doi.org/10.1021/acs.chemrev.5b00484.
T. Clark, M. Hennemann, J. S. Murray, P. Politzer, J. Mol. Model., 2007, 13, 291–296; DOI: https://doi.org/10.1007/s00894-006-0130-2.
G. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, G. Terraneo, Cryst. Growth Des., 2014, 14, 2697–2702; DOI: https://doi.org/10.1021/cg5001717.
P. Politzer, J. S. Murray, T. Clark, Top. Curr. Chem., 2015, 358, 19–42; DOI: https://doi.org/10.1007/128_2014_568.
A. Bauzá, T. J. Mooibroek, A. Frontera, ChemPhysChem, 2015, 16, 2496–2517; DOI: https://doi.org/10.1002/cphc.201500314.
H. Wang, W. Wang, W. J. Jin, Chem. Rev., 2016, 116, 5072–5104; DOI: https://doi.org/10.1021/acs.chemrev.5b00527.
K. E. Riley, P. Hobza, J. Chem. Theory Comput., 2008, 4, 232–242; DOI: https://doi.org/10.1021/ct700216w.
T. Clark, P. Politzer, J. S. Murray, Wiley Interdiscip. Rev. Comput. Mol. Sci., 2015, 5, 169–177; DOI: https://doi.org/10.1002/wcms.1210.
P. Politzer, J. S. Murray, T. Clark, J. Mol. Model., 2015, 21, 52; DOI: https://doi.org/10.1007/s00894-015-2585-5.
J. Zurita, V. Rodriguez, C. Zambrano, J. R. Mora, L. Rincón, F. J. Torres, Molecules, 2020, 25, 530; DOI: https://doi.org/10.3390/molecules25030530.
J.-W. Zou, Y.-J. Jiang, M. Guo, G.-X. Hu, B. Zhang, H.-C. Liu, Q.-S. Yu, Chem. Eur. J., 2005, 11, 740–751; DOI: https://doi.org/10.1002/chem.200400504.
P. Politzer, P. Lane, M. C. Concha, Y. Ma, J. S. Murray, J. Mol. Model., 2007, 13, 305–311; DOI: https://doi.org/10.1007/s00894-006-0154-7.
A. Bundhun, P. Ramasami, J. S. Murray, P. Politzer, J. Mol. Model., 2013, 19, 2739–2746; DOI: https://doi.org/10.1007/s00894-012-1571-4.
K. Eskandari, H. Zariny, Chem. Phys. Lett., 2010, 492, 9–13; DOI: https://doi.org/10.1016/j.cplett.2010.04.021.
W. Kunz, K. Holmberg, T. Zemb, Curr. Opin. Colloid Interface Sci., 2016, 22, 99–107; DOI: https://doi.org/10.1016/j.cocis.2016.03.005.
T. Buchecker, S. Krickl, R. Winkler, I. Grillo, P. Bauduin, D. Touraud, A. Pfitzner, W. Kunz, Phys. Chem. Chem. Phys., 2017, 19, 1806–1816; DOI: https://doi.org/10.1039/c6cp06696h.
R. K. Ness, H. G. Fletcher, Jr., J. Am. Chem. Soc., 1958, 80, 2007–2010; DOI: https://doi.org/10.1021/ja01541a058.
N. M. Podvalnyy, A. I. Zinin, B. Venkateswara Rao, L. O. Kononov, in Carbohydrate Chemistry: Proven Synthetic Methods, Eds R. Roy, S. Vidal, CRC Press-Taylor & Francis Group, Boca Raton, FL, 2015, Vol. 3, pp. 147–154.
W. L. F. Armarego, Purification of Laboratory Chemicals, 8th ed., Butterworth-Heinemann, Oxford, 2017, 1198 pp.
W. Schärtl, Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st ed., Springer-Verlag, Berlin—Heidelberg, 2007, 191 pp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences O. M. Nefedov on the occasion of his 90th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2214–2219, November, 2021.
This paper does not contain descriptions of studies on animals or humans.
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Orlova, A.V., Ahiadorme, D.A., Laptinskaya, T.V. et al. Supramer analysis of 2,3,5-tri-O-benzoyl-α-d-arabinofuranosyl bromide solutions in different solvents: supramolecular aggregation of solute molecules in 1,2-dichloroethane mediated by halogen bonds. Russ Chem Bull 70, 2214–2219 (2021). https://doi.org/10.1007/s11172-021-3335-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3335-8