Abstract
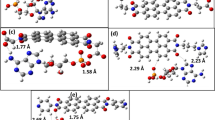
The compound 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-9-ol (9-hydroxyeucaliptol) has been prepared and characterized by single-crystal X-ray diffraction analysis, infrared, Raman, and UV-visible spectroscopies. The molecular geometry of the title compound was also investigated theoretically by density functional theory (DFT) calculations to compare with the experimental data. The substance crystallizes in the trigonal crystal system, space group P32 with Z = 9 molecules per unit cell. There are three independent molecules in the crystal asymmetric unit having the same chirality and showing some differences in the orientation of the H-atom of the hydroxyl group. The crystal structure of 9-hydroxyeucaliptol shows that the hydroxyl group presents an anti-conformation with respect to the O-atom of the ether group. The crystal packing of 9-hydroxyeucaliptol is stabilized by intermolecular O-H···O hydrogen bonds involving the hydroxyl groups of different molecules, which play a decisive role in the preferred conformation adopted in solid state. The intermolecular interactions observed in solid state were also studied through the Hirshfeld surface analysis and quantum theory of atoms in molecules (QTAIM) approaches. Energy framework calculations have also been carried out to analyze and visualize the topology of the supramolecular assembly, and the results indicate a significant contribution from electrostatic energy over the dispersion.
Graphical abstract









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Schaffarczyk M, Balaban TS, Rychlik M, Buettner A (2013) Syntheses of chiral 1,8-cineole metabolites and determination of their enantiomeric composition in human urine after ingestion of 1,8-cineole-containing capsules. ChemPlusChem 78:77–85
Bitteur SM, Baumes RL, Bayonove CL, Versini G, Martin CA, Dalla Serra A (1990) 2-exo-Hydroxy-1,8-cineole: a new component from grape var. sauvignon. J Agric Food Chem 38(5):1210–1213
Santos FA, Rao VSN (2000) Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res 14(4):240–244
Jun HJ, Hoang M-H, Yeo S-K, Jia Y, Lee S-J (2013) Induction of ABCA1 and ABCG1 expression by the liver X receptor modulator cineole in macrophages. Bioorg Med Chem Lett 23(2):579–583
Azerad R (2014) 1,8-Cineole: chemical and biological oxidation reactions and products. ChemPlusChem 79(5):634–655
Weerakkody NS, Caffin N, Lambert LK, Turner MS, Dykes GA (2011) Synergistic antimicrobial activity of galangal (Alpinia galanga), rosemary (Rosmarinus officinalis) and lemon iron bark (Eucalyptus staigerana) extracts. J Sci Food Agric 91(3):461–468
Tommasi L, Negro C, Miceli A, Mazzotta F (2009) Antimicrobial activity of essential oils from aromatic plants grown in the Mediterranean area. J Essent Oil Res 21(2):185–189
Miyazawa M, Hashimoto Y (2002) Antimicrobial and bactericidal activities of esters of 2-endo-hydroxy-1,8-cineole as new aroma chemicals. J Agric Food Chem 50(12):3522–3526
Villecco MB, Catalán JV, Vega MI, Garibotto FM, Enriz RD, Catalán CAN (2008) Synthesis and antibacterial activity of highly oxygenated 1,8-cineole derivatives. Nat Prod Commun 3(3):303–312
Horst K, Rychlik M (2010) Quantification of 1,8-cineole and of its metabolites in humans using stable isotope dilution assays. Mol Nutr Food Res 54(10):1515–1529
CrysAlisPro, Oxford Diffraction Ltd., version 1.171.33.48 (release 15-09-2009 CrysAlis171.NET)
Sheldrick GM (2015) SHELXT–integrated space-group and crystal-structure determination. Acta Cryst A 71:3–8
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122
McKinnon JJ, Spackman MA, Mitchel AS (2004) Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crysta B 60:627–668
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11(1):19–32
Spackman MA (1992) Molecular electric moments from x-ray diffraction data. Chem Rev 92(8):1769–1797
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. University of Western Australia
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc, Wallingford
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Bauzá A, Quiñonero D, Deyà PM, Frontera A (2013) Is the use of diffuse functions essential for the properly description of noncovalent interactions involving anions? J Phys Chem A 117:2651–2655
Ahmed MN, Yasin KA, Aziz S, Khan SU, Tahir MN, Gil DM, Frontera A (2020) Relevant π-hole tetrel bonding interactions in ethyl 2-triazolyl-2-oxoacetate derivatives: Hirshfeld surface analysis and DFT calculations. CrystEngComm 22:3567–3578
Madni M, Ahmed MN, Hafeez M, Ashfaq M, Tahir MN, Gil DM, Galmés B, Hameed S, Frontera A (2020) Recurrent π-π stacking motifs in three new 4,5-dihydropyrazolyl-thiazole-coumarin hybrids: X-ray characterization, Hirshfeld surface analysis and DFT calculations. New J Chem 44:14592–14603
Thakur S, Gil DM, Frontera A, Chattopadhyay (2020) Exploration of Br···O halogen bonding interactions in dinuclear vanadium(V) complexes with Schiff base ligands. Polyhedron 187:114676
Grimme S, Anthony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Hujo W, Grimme S (2011) Comparison of the performance of dispersion-corrected density functional theory for weak hydrogen bonds. Phys Chem Chem Phys 13:13492–13950
Farrugia LJ (1997) ORTEP-3 for Windows-a version of ORTEP-III with a graphical user interface (GUI). J Appl Crystallogr 30(5):565
Bond AD, Davies JE (2001) Crystal structure of eucalyptol at 265 K. Aust J Chem 54(11):683–684
Collins GE, Unterweger B, Dumsday GJ, Forsyth CM (2017) Structural elucidation of a hydroxy–cineole product obtained from cytochrome P450 monooxygenase CYP101J2 catalysed transformation of 1,8-cineole. Acta Cryst E 73(8):1242–1245
Farlow AJ, Bernhardt PV, De Voss JJ (2013) Synthesis of oxygenated cineole derivatives from cineole: utility of cytochrome P450cin as an enantioselective catalyst. Tetrahedron Asymmetry 24:324–333
Venkatesan P, Cerón M, Thamotharan S, Robles F, Percino MJ (2018) Quantitative analysis of weak non-covalent interactions in (Z)-3-(4-halophenyl)-2-(pyridin-2/3/4-yl) acrylonitriles. CrystEngComm 20(19):2681–2697
Thamontharan S, Kothandapani J, Selva Ganesan S, Venkataramanan NS, Madan Kumar S, Byrappa K, Percino J, Robles F (2018) Quantitative analysis of intermolecular interactions in 2,2′-((4-bromophenyl)methylene)bis(3-hydroxy-5,5-dimethylcyclohex-2-en-1-one): insights from crystal structure, PIXEL, Hirshfeld surfaces and QTAIM analysis. J Chem Sci 130:20
Matta CF, Hernandez-Trujillo J, Tang T-H, Bader RFW (2003) Hydrogen–hydrogen bonding: a stabilizing interaction in molecules and crystals. Chem Eur J 9(9):1940–1951
Koch U, Popelier PLA (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99(24):9747–9754
Saeed A, Erben MF, Bolte M (2012) Twisted imide bond in noncyclic imides. Synthesis and structural and vibrational properties of N,N-Bis(furan-2-carbonyl)-4-chloroaniline. J Org Chem 77(10):4688–4695
Funding
The authors thanks ANPCyT (PICT 2016-0226), SCAIT (Projects D683 and 26/D636), CONICET (Grant PIP 11220130100651CO), and UNLP (Grants 11/X709 and 11/X673) of Argentina for financial support. D.M.G., G.A.E., and O.E.P. are research fellows of CONICET. M.R. is post-doctoral fellow of CONICET.
Author information
Authors and Affiliations
Contributions
C. Galvez: formal analysis, investigation. M. Rocha: formal analysis, investigation. M.B Villecco: methodology, visualization, investigation. G.A. Echeverría: crystal data collection. O.E. Piro: crystal data collection, formal analysis. M.H. Loandos: conceptualization, supervision. D.M. Gil: conceptualization, supervision, writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gustavo A. Echeverría, Oscar E. Piro, and Diego M. Gil are Members of the research career of CONICET.
Supplementary Information
ESM 1
(DOCX 360 kb)
Rights and permissions
About this article
Cite this article
Galvez, C.E., Rocha, M., Villecco, M.B. et al. Role of hydrogen bonds and weak non-covalent interactions in the supramolecular assembly of 9-hydroxyeucaliptol: crystal structure, Hirshfeld surface analysis, and DFT calculations. J Mol Model 27, 13 (2021). https://doi.org/10.1007/s00894-020-04633-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04633-9