Abstract
The bleak Alburnus alburnus is a medium body-size leuciscid fish that is naturally distributed across central European and western Asian fresh waters. However, during the last two decades A. alburnus has been widely introduced elsewhere in Europe and in northern Africa, mostly as a forage species for game fishes. Given its relatively recent history of invasion in non-native Eurasian waters, where it can become highly abundant, A. alburnus poses a serious risk to native communities where introduced. This study provides a review and meta-analysis of the biological traits of A. alburnus coupled with insights into its invasiveness. In its native range, A. alburnus has a moderate lifespan, inhabiting lakes or still waters in medium-to-large rivers, where it feeds mainly on zooplankton. However, non-native A. alburnus populations display high phenotypic plasticity in their biological attributes. Thus, growth, reproductive and/or dietary traits have adapted to local environmental conditions, with the species also invading lotic (stream) ecosystems. Feeding changes to benthic invertebrates, plant material and detritus when zooplankton is scarce. Such plasticity, including broad physiological tolerance, is likely to facilitate the species' adaptation and invasion of new habitats in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are considered one of the main threats to global biodiversity, with freshwater ecosystems being particularly sensitive to introduced invasive species (Clavero 2011). Invasive fishes can alter aquatic communities by causing changes in food webs and/or the decline, displacement and disappearance of native species (Cucherousset and Olden 2011), although not all introduced species become invasive (Copp et al. 2005a, 2005b; Gozlan 2008). The establishment and spread of non-native fish can be facilitated by degradation of water quality and hydro-morphological alterations (Moyle and Light 1996; Bunn and Arthington 2002; Kennard et al. 2005; Leprieur et al. 2008), which explains why some fish species, such as the bleak Alburnus alburnus, become widespread and exert impacts when introduced outside their native range intentionally and/or unintentionally by humans (Gehrke and Harris 2001; Marchetti and Moyle 2001; Filipe et al. 2004).
The genus Alburnus belongs to the Family Leuciscidae, comprising small minnow species of which ≈45 are recognised for and distributed across a vast geographic range, extending from western Europe to the northern parts of southwest Asia. Alburnus species are commonly known as ‘bleaks’ (Buj et al. 2010), though a group of them is known as ‘shemayas’ (Özuluğ and Freyhof 2007). The name ‘bleak’ originates from the late Middle English name “bleke”, which means ‘pale’. Historically, the scales of A. alburnus were used to produce the “Essence d'Orient”—a coating for artificial pearls (von Wagner et al. 1903; Hugh 1911). Turkey is the centre of the genus' radiation, extending throughout the Palearctic Ecozone (Özuluğ and Freyhof 2007). Alburnus alburnus is naturally distributed across Europe and Asia, but during the last decades this species has been introduced to several regions in the south and east of its native range (Froese and Pauly 2021). Although A. alburnus is of no interest to the aquarium trade, it is considered a valuable species in recreational fishing for which it is used mainly as bait or as a forage fish that is stocked into waters to promote piscivorous fish populations. For this reason, A. alburnus has been introduced widely as a forage fish for piscivores such as the northern pike Esox lucius, largemouth (a.k.a. black) bass Micropterus salmoides, and pikeperch Sander lucioperca (Elvira and Almodóvar 2001). These are the main target species (trophy fishes) of recreational anglers and have consequently led to the rapid invasion by A. alburnus of Iberian river catchments (Vinyoles et al. 2007).
The aim of the present study is to review the available literature on the biological traits of A. alburnus in its native and introduced distribution ranges, highlighting differences thereof in particular. This review encompasses all aspects of A. alburnus' environmental biology, including morphology, distribution, habitat use, population structure and dynamics, ontogeny and growth, reproduction, trophic ecology, physiology, behaviour, pathogens and parasites, and genetic traits. Moreover, a comprehensive overview is provided on the species' potential invasiveness and its adverse impacts on native species and ecosystems in invaded regions, ranging from hybridisation, parasite transmission, resource competition to foodweb changes. Management recommendations are also provided.
Review
Morphology
Alburnus alburnus has a fusiform body that is laterally compressed. Mean size is 150 mm total length (TL), with maximum length and weight of 250 mm TL and 60 g, respectively (Billard 1997). Body pigmentation is green- or blue-tinted with silvery flanks, and yellowish paired anal fins (Keith and Allardi 2001). Sexual dimorphism is said not to occur in A. alburnus (Interesova and Chakimov 2015), however, males in a population of the River Ob basin (Siberia, Russia) have been found to have significantly longer anal-fin base, ventral rays and anal fin rays (Interesova and Chakimov 2015). In the Rybinsk Reservoir (Russia), sexual differences were observed in trunk-muscle lipid composition during the pre-spawning period (Khalko 2018). In aggregated samples, lipid content was found to be higher in females than in males, the former demonstrating a bimodal distribution in trunk muscles and the latter a modal distribution (Khalko 2018).
Previous studies have suggested that A. alburnus exhibits some phenotypical plasticity due to both ecological and geographical influences (Gąsowska 1974; Baruš et al. 1998; Masó et al. 2016). For instance, A. alburnus morphology is influenced by waterbody type whereby riverine A. alburnus show a deeper body, larger head, shorter caudal peduncle length and a smaller number of lateral line scales than lacustrine A. alburnus populations (Gąsowska 1974; Golub et al. 2019). , riverine A. alburnus populations have longer pelvic and pectoral fins than A. alburnus in lakes, probably due to water current. Variability observed in A. alburnus meristic characters (e.g. number of scales in the lateral line or number of vertebrae) has also been attributed to habitat type, geography and climatic conditions (Gąsowska 1974; Rafikov and Boznak 2021). For instance, lake-dwelling A. alburnus were found to have a higher mean number of lateral line scales (n = 50.7) than riverine A. alburnus (n = 49.3) and estuarine/firth A. alburnus (n = 48.8) (Gąsowska 1974). However, despite several descriptive studies that evaluated A. alburnus morphological characteristics at a regional scale (.g. Gąsowska 1974; Baruš et al. 1998; Interesova and Chakimov 2015; Rafikov and Boznak 2021), no studies have systematically compared morphological traits among native and introduced populations.
Geographical distribution
Native range
The oldest Leuciscid fossils have been recovered in central Anatolia and date from around the Oligocene–Miocene boundary (Özuluğ and Freyhof 2007; Perea et al. 2010). The first colonisation of Central Europe by leuciscids was possible after the connection of Eurasia and Afro-Arabia (20 mya) through the Gomphotherium landbridge (Perea et al. 2010). The first fossils of Alburnus sp. in central Europe, which date from about 18–19 mya, were found in Czechia. These paleontological records fit well with the beginning of diversification of Alburnus lineages, 19.7 mya (Perea et al. 2010). Alpine orogeny may have also played an important role in isolating Iberian and Italian ichthyofaunas, thereby preventing the arrival of A. alburnus to these regions (Zardoya and Doadrio 1999; Levy et al. 2009; Perea et al. 2010).
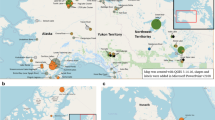
The native range of A. alburnus (Fig. 1) extends from the Ural Mountains in Russia and the River Emba in Kazakhstan in the East (Balzani et al. 2020) to the eastern side of England (Great Britain) in the West (Dodd et al. 2019). The latitudinal distribution of A. alburnus extends from 36°N to 65°N (Blanc and Lamoroux 2007), ranging in the north from southern Scandinavia (Rask et al. 2000), southward to the northern slopes of the Pyrénées, across to the Swiss side of the Alps, and continuing uninterrupted to Austria (Gerdeaux et al. 2006). The extent of A. alburnus southernmost distribution appears to be the tributary rivers of the southern Caspian Sea in eastern Iran (Kiabi et al. 1999), though translocated populations of A. alburnus exist in other parts of Iran (Coad 2006).
Non-native range
From its original distribution range, A. alburnus has been introduced in the last decades to several areas in both Europe and Africa, with records of non-native A. alburnus extending from Russia (Siberia) to Cyprus (Welcomme 1988; Zogaris et al. 2012) as well as in Portugal and Spain (Vinyoles et al. 2007; Sousa-Santos et al. 2018), Italy (Nocita 2007; Balzani et al. 2020), Algeria (Kara 2012; Attou and Arab 2019) and Morocco (Clavero et al. 2015) (Fig. 1). In Great Britain, A. alburnus have been translocated to all other River Basin Districts (sensu European Union 2000) from the species' native distribution in England, which ranges from the River Thames to the Humber Estuary (Wheeler 1977; Dodd et al. 2019).
The non-native distributional range of A. alburnus has recently expanded by natural dispersal through the River Ob basin in Russia (Interesova 2016; Reshetnikov et al. 2017), where the species was first reported in 1933 (Berg 1933), but whose presence was subsequently questioned (Ioganzen 1947). In the early 1990s, A. alburnus was recorded in the River Tobol (Terent'eva and Mukhachev 2006), spreading in the following years throughout rivers of Siberia, mainly the River Tom—a tributary of the Upper Ob basin- by the end of the 1990s (Yurakova and Petlina 2001), the River Ishim by 2000 (Kolomin 2006), and the rivers Om and Miass by 2007 (Zinov'ev and Baklanov 2007). Currently, A. alburnus inhabits Novosibirsk Reservoir and most of the rivers and lakes in the upper and middle sections of the River Ob, where the species is highly abundant and still increasing in number and distribution (Yadrenkina 2012; Babkina et al. 2013; Interesova and Chakimov 2015; Romanov et al. 2017; Yevseyeva et al. 2019).
In the Iberian Peninsula, A. alburnus have been accidentally or intentionally introduced into reservoirs due to their use as bait by anglers targeting non-native piscivorous fishes, spreading rapidly to other water bodies by natural dispersal (Elvira and Almodóvar 2001; Amat-Trigo et al. 2019). The first record of A. alburnus was in June 1992, for the River Noguera-Ribagorzana (a tributary of the River Ebro) (Elvira 1995), after which the species quickly spread to other river basins in Spain and Portugal (Vinyoles et al. 2007; Maceda-Veiga et al. 2010; Martelo et al. 2021). Alburnus alburnus was detected in basins of the Eastern Pyrénées in 1997, in the River Muga in 1999 (Cardona et al. 2002), in the River Tormes (River Duero basin) and in the Campo Maior Reservoir (River Guadiana basin) in 2003 (Pérez-Bote et al. 2004; Velasco et al. 2005), as well as in the basins of the rivers Segura (Andreu-Soler et al. 2004) and Tagus in 2004 (Vinyoles et al. 2007). Expansion of the species’ range in the Ebro basin and other Mediterranean rivers (i.e. Jucar, Mijares and Turia) has also been documented (Doadrio 2001). Currently, A. alburnus is very abundant and present in all the main river systems of the Iberian Peninsula, particularly those with nearby reservoirs (Masó et al. 2016; Latorre et al. 2018; Matono et al. 2018; Martelo et al. 2021).
Although A. alburnus has been reported for Italy (Nocita 2007; Balzani et al. 2020), the congeneric arborella Alburnus arborella, which is endemic to the Padano-Venetian district, has been alternatively considered a sub-species of A. alburnus (e.g. Tirelli et al. 2012). In this regard, the scientific name of the congeneric A. arborella has been formally validated (Ketmaier et al. 2009). Overall, owing to the similarity between these two congeners and their morphological features, further research is needed to assess whether or not available A. alburnus records confirm the species' presence in Italy.
In the African continent, the first official record of A. alburnus was in 2003 for the River Kebir, Algeria (Oum Toub, Skikda Province)—a tributary of the Guenitra Dam and the River Guebil (Tandjir and Djebar 2010). However, A. alburnus bones were found in the spraints of Eurasian otter Lutra lutra in east Algeria (El-Kala National Park) in 1997 (Libois et al. 2015). Alburnus alburnus was subsequently recorded in the Hamiz Reservoir in 2006 following the species' introduction as a contaminant of carp species (i.e. common carp Cyprinus carpio, bighead carp Aristichthys nobilis, silver carp Hypophthalmichthys molitrix) consignments imported from Hungary (Attou and Arab 2013). Speciments of Alburnus alburnus were also collected in Keddara Reservoir in May 2008, which is connected to Hamiz Reservoir by a water transfer canal (Attou and Arab 2013, 2019), in the Sebaou Basin (Great Kabylia) from 2012 to 2013. More recently, A. alburnus has been found in Taksebt Reservoir, probably as part of a 2005–2006 introduction of common carp (Lounaci-Daoudi et al. 2016). In Morocco, A. alburnus was first recorded in 2013 in the River Ghir basin, probably originating from the Djorf Torba Reservoir, Algeria (Clavero et al. 2015).
Habitat use
In both their native and non-native distribution ranges, A. alburnus populations are found mainly in lotic and semi-lotic environments (Mann 1996; Wolter and Bischoff 2001; Mehner et al. 2005; Latorre et al. 2016). In its native range, A. alburnus inhabits eutrophic and mesotrophic water bodies (Říha et al. 2013) at altitudes of up to 1800 m above sea level (Stefanov 2007); this reflects the species’ wide oxygen tolerance, i.e. ability to inhabit waters in which oxygen concentration can drop periodically down to 1.5–3.0 mg L−1 (Blanck et al. 2007).
In the native range, established populations of A. alburnus can be found in riverine systems, generally adjacent to annexes of the main channel, characterised mainly by semi-lotic habitats that range from artificial (Williams 1965), rehabilitated (Grift et al. 2003) and near-natural side-channels (Copp and Peňáz 1988; Copp 1992; Roux and Copp 1996). In this respect, A. alburnus has been identified as a ‘functional describer’ (in terms of ecological succession) of natural and regulated riverine ecosystems (Copp 1989; Copp et al. 1991). In riverine environments, A. alburnus appears to use connected waterways that are subject to periodic inundation for spawning (Hohausová et al. 2003; Penczak et al. 2004; Scharbert and Borcherding 2013). For example, a field study of fish movements between the main channel of the River Morava (Czechia) and a reconnected, rehabilitated former meander reported older A. alburnus (≥ 1 + years, i.e. standard lengths (SL) of 100–150 mm), moving in May between dusk and dawn, and exclusively from the former meander to the main channel (Hohausová et al. 2003). The larvae of A. alburnus have been found in shallow, still waters, whereas juveniles in shallow, low-velocity habitats (Copp 1992; Grift et al. 2003), such as river side-channels, where lateral movements of young-of-the-year (0 +) juveniles have been observed to take place as an anti-predator behavioural response to predator threats (Copp 1992). Alburnus alburnus eggs and larvae are carried downstream by river currents (Copp et al. 2002), and from flood plains to their shallow nurseries at the channel banks (Černý et al. 2003; Scharbert and Borcherding 2013). Riverine habitats that contain refuge habitats (i.e. and crevices or submerged roots) are also inhabited by A. alburnus, as revealed in a study of a heavily-modified stream tributary of the River Danube that had undergone rehabilitation (Pander and Geist 2010). Specifically, artificial dead-wood fascines (i.e. bank reinforcement with overhanging riparian wood) were found to provide an excellent winter habitat for smaller-bodied fishes, including A. alburnus (Pander and Geist 2010). Moreover, in a recently-constructed artificial fishway on the River Segura (southeast of Iberian Peninsula), A. alburnus was the dominant species both in frequency of occurrence and abundance, which exemplifies its ability to adapt to establish in heavily modified waters (Sánchez-Pérez et al. 2022).
In lacustrine habitats, including reservoirs, A. alburnus has been found to spawn preferentially in the faster-flowing waters of tributaries before returning to the main water body for foraging (Říha et al. 2013). Still-water populations are found in lakes, reservoirs, river floodplain channels and adjacent water bodies (e.g. oxbow lakes and abandoned side-channels) and marshlands (Bohl 1979; Copp 1989, 1992; Gozlan et al. 1998; Černý et al. 2003; Blanc and Lamoroux 2007; Navodaru et al. 2002; Pehlivanov et al. 2011; Balzani et al. 2020; Martelo et al. 2021). However, A. alburnus populations in ponds (sensu Biggs et al. 2005: i.e. from 1 m2 to 2 ha area) are uncommon, so references to small water bodies generally relate to shallow lakes or small reservoirs that have been mis-labelled as ‘ponds’ (e.g. Baruš et al. 1998).
In Mediterranean rivers, non-native A. alburnus populations inhabit high-velocity microhabitats such as run-type sections (Masó et al 2016; Muñoz-Mas et al. 2019), where the species is able to sustain relatively high swimming speeds (Cano-Barbacil et al. 2020). During summer, A. alburnus shoals perform daily horizontal migrations and become abundant in the shallow littoral zone during the day and in the pelagic zone at night where they feed (Bohl 1982; Kratochvíl et al. 2014). In Mediterranean rivers and reservoirs (e.g. River Guadiana, southwestern Iberian Peninsula), ontogenetic shifts in habitat use that partition/segregate A. alburnus populations spatially are difficult to identify due to greater variability in the transition amongst mesohabitat types across seasons in rivers relative to reservoirs (Almeida et al. 2017). Nevertheless, young and small A. alburnus (cf. juveniles) in Iberian reservoirs appear to be more restricted to the littoral zone than reported for lakes in the species' native range (Almeida et al. 2017); whereas, older/larger individuals (cf. adults) tend to occupy both littoral and pelagic zones (Bíró and Muskó 1995; Bogack-Kapusta and Kapusta 2007).
Population structure and dynamics
Information on A. alburnus population structure is relatively scarce for both native (Bíró and Muskó 1995) and non-native (Almeida et al. 2014; Amat-Trigo et al. 2019) areas, thus precluding a comprehensive comparison of its population traits. In the native distribution range, size, age structure and population dynamics of A. alburnus populations were found to change in response to both biotic and abiotic factors, such as in the shallow waters of Lake Balaton, Hungary (Bíró and Muskó 1995), where the species’ growth, mortality and production rates were particularly influenced by food availability (i.e. zooplankton and benthos) along the littoral zone. Previous studies revealed substantial differences in stock densities especially during the spawning period, where some populations had slower growth rates (Entz and Lukacsovics 1957; Bíró 1980, 1990). These differences probably resulted from density-dependent regulating mechanisms in fish (Elliott 1987) as well as from interspecific competition and predation within the A. alburnus populations (Latorre and Almeida 2019).
In terms of sex ratio, A. alburnus populations in Iberian waters generally contain a greater proportion of males than native-range populations (Masó et al. 2016; Latorre et al. 2018) (Table 1). A seasonal effect on sex ratio, with a strong bias toward males, both in lotic and lentic environments, was observed in the rivers Segura and Guadiana during spring, although females were found to be more abundant in lotic habitats during autumn (Almeida et al. 2014; Amat-Trigo et al. 2019). This pattern may be due to elevated predation pressure on females in spring, mediated by their higher ambulation rate (Almeida et al. 2014) when searching for spawning sites (Latorre et al. 2018), thus affecting sex ratio in favour of males. Sex ratio of non-native A. alburnus in the Keddara Reservoir (Algeria) was reported to be strongly influenced by environmental factors (Fouzia and Abdeslem 2012), whereby oxygen and conductivity favoured females, with males favoured by pH and conductivity.
Seasonal differences in A. alburnus size structure have been reported in lacustrine populations of the native range (Bíró and Muskó 1995) as well as in non-native populations between rivers and reservoirs from the southwest of the Iberian Peninsula (Almeida et al. 2017). Size structure in Iberian reservoirs showed a bimodal pattern in autumn and winter, whereas only one cohort was observed in spring, probably because of a high winter mortality of larger individuals due to low food resources availability (Almeida et al. 2017). These two cohorts were also observed in the river population in winter although they were less apparent, suggesting an effect of severe environmental conditions (e.g. lower temperatures and higher discharge rates) on the size structure of A. alburnus populations (Almeida et al. 2014). In the relatively uniform stream discharges of the River Stour, native A. alburnus demonstrated the typical temperate-zone growth pattern, which consists of rapid length and weight increases during summer with virtually no growth in winter, and this is apparent in ages 0 + , 1 + and 2 + (Mann 1991).
The dynamics of fish populations in their early stage of invasiveness rely on different life strategies compared with long-established populations (Ribeiro et al. 2008). In south-eastern Iberian populations, greater longevity and larger mature cohorts were found in sites with longer residence time, but still subject to a longitudinal gradient effect, with upstream populations showing higher growth rates and reproductive investment than in downstream populations (Amat-Trigo et al. 2019). Moreover, in the aforementioned study, A. alburnus abundance and growth were significantly dependent on ecological variables related to water discharge, such as discharge variability influenced A. alburnus abundance in a positive manner. Fast growth rates and high reproductive investment promote a rapid spread along highly regulated rivers, as reported for the River Segura, where A. alburnus colonised about 170 km of the river since its introduction in 2004 (Andreu-Soler et al. 2004; Amat-Trigo et al. 2019). Overall, a suite of factors mediate A. alburnus invasions of southern Iberian rivers, including habitat conditions and river discharge regulation along the rivers longitudinal course. This is apparent from variations in A. alburnus population structure among invaded rivers of the Iberian Peninsula (Masó et al. 2016; Latorre et al. 2018).
Ontogeny and growth
The SL of A. alburnus at the end of the free embryo stage (i.e. absorption of the yolk sac) is reported to be ≈6.5–7.0 mm (Pinder 2001), with larvae being 13 mm (TL ≈16 mm) at ≈20 days after fin formation, whereas the SL of 0 + A. alburnus towards the end of summer was ≈40 mm, but with some individuals only measuring 20 mm in the Międzyodrze wetland, Poland (Kompowski 2000). Young-of-the-year A. alburnus can represent nearly half of biomass increase (46.2%) in A. alburnus populations, such as reported for the River Thames in England (Mann 1991).
In Iberian populations, somatic growth is faster in the first two years of life than after maturity has been achieved, whichis characterised by decreased somatic growth, with annual growth increments becoming minimal after 6–7 years (Latorre et al. 2018). There are no reported differences in growth rate between males and females (Masó et al. 2016; Latorre et al. 2018). As for other cyprinids, ageing of A. alburnus specimens is typically based on scale analysis. Although highly variable, growth in A. alburnus is generally considered to be slow (e.g. Williams 1967; Bíró and Muskó 1995; Kompowski 2000; Britton 2007). Mean growth increment is around 30 mm SL in the first year, ≈18 mm between the first and second year, then decreasing to 6 mm between ages eight and nine years, which is the maximum recorded age for this species (Bíró and Muskó 1995). Based on length-at-age data from the native and introduced ranges (Tables 6 and 7; see also Appendix: Age and growth modelling), global growth in body length is asymptotic with an estimated SL∞ = 130.4 mm (Table 2) and is characterised by large variation within year classes (Fig. 2a). Alburnus alburnus populations in lotic environments were found to achieve larger sizes that those in lentic environments (Fig. 2b), and a similar pattern was apparent in continental populations compared with those located in temperate climate zones (Fig. 2c). No apparent relation is observed in the length–weight relationships for A. alburnus populations along a latitudinal gradient neither between native or non-native populations (Table 3).
Growth in length of A. alburnus, as described by the von Bertalanffy growth function fitted to: (a) global dataset, (b) habitat, (c) Köppen-Geiger climate class (C = temperate; D = continental). In the scatterplots, each point represents a single mean length-at-age value (see Table 7) and the shaded area for each curve indicates 95% bootstrapped confidence intervals. Points in the scatterplots (except for the global fit) are slightly jittered to improve visibility. Parameters in Table 2
Reproduction
Alburnus alburnus is a dioecious species, with external fertilisation. In its native range, size at maturity ranges 85–100 mm TL in males and 120 mm TL in females, with age at maturity being 2–3 years (Politou 1993). Spawning takes place at water temperatures of 14–28 °C (Alabaster and Lloyd 1980), though a lower limit of 17 °C has been reported (Mann 1996). Alburnus alburnus is a phyto-lithophilous species, scattering its eggs on submerged aquatic plants, alluvia and ligneous debris (Balon 1975). In A. alburnus eggs, yolk mass represents 30% of oocyte volume, with the remainder being perivitelline space (Winnicki and Korzelecka 1997). During egg development, a lateral position is taken pre-hatch by the blastodisc and the subsequent embryo and larva, and A. alburnus distinguishes itself from many teleost fishes by the absence of lipid droplets in the yolk mass. Despite their moderate embryonic respiratory organs, A. alburnus embryos hatch out late, are photophobic and possess cement glands with which to attach to the spawning substratum to avoid descent to the bottom (Balon 1975, 1990).
Reproductive traits show large variability both in the species' native (Rinchard and Kestemont 1996; Bonisławska et al. 2001) and non-native ranges and both in small and large rivers (Masó et al. 2016; Latorre et al. 2018). This reproductive variability is expressed mainly in terms of fecundity (Mackay and Mann 1969), oocyte diameter (Bonisławska et al. 2001) and energy investment in reproduction (Rinchard and Kestemont 1996; Amat-Trigo et al. 2019). In small Mediterranean rivers in the northeast of the Iberian Peninsula (Catalonia), a high variability was also observed in traits such as reproductive investment, length at maturity and age at maturity in different A. alburnus populations (Masó et al. 2016).
Greater breeding performance has been observed in the non-native range (Latorre et al. 2018), with reproduction rates changing in response to the prevailing environmental conditions. In a comparison of A. alburnus in the River Gévora and the Sierra Brava Reservoir (southwestern Spain), the proportion of smaller mature A. alburnus individuals was lower in the river than in the reservoir, and both males and females were larger and presented higher body condition and reproductive investment in the river (Almeida et al. 2014). Alburnus alburnus fecundity is highly variable in both its native (Politou 1993; Baruš and Prokeš 1993; Raikova-Petrova et al. 2009) and non-native ranges (Latorre et al. 2018), ranging from 1,707 to 12,284 spawned eggs in native populations (Raikova-Petrova et al. 2009) and from 1,829 to 8,069 eggs in non-native populations (Latorre et al. 2018). Relative fecundity by size class is equally variable elsewhere in the native range, ranging in Bulgaria from 104 to 788 in Lake Chepintsi, which is almost 2.1 × higher than in the Batak Dam (Table 6). The greatest variability across the species' native range has been reported for the Věstonice Reservoir, Czechia, where seasonal fecundity ranged from 3383 to 15,438 eggs, and relative fecundity reached 102.2–220.4 eggs g−1 of body weight (Baruš and Prokeš 1993). Fecundity rate in the River Thames (UK) was estimated at ≈6400 eggs in 5-year-old females (Mackay and Mann 1969), which is almost double the mean of ≈3800 eggs estimated in 4-year-old females from the River Sâone in France (Latorre et al. 2018). Egg diameter in the species' native range also varies, with a mean diameter of 1.48 mm from various water courses in Poland (Bonisławska et al. 2001) being greater than the mean (≈1.20 mm) observed in the River Sâone (Latorre et al. 2018). Outside the species’ native range, high variability in fecundity (1829–8069 eggs) and egg diameter (0.95–1.14 mm) was observed across large rivers of the Iberian Peninsula (Latorre et al. 2018). The high reproductive plasticity reported by Latorre et al. (2018) may be a mechanism for adapting successfully to newly invaded habitats, which can differ greatly in local conditions (e.g. water quality, available habitat) and in landscape character (e.g. topography, rainfall). These patterns suggest that A. alburnus in non-native Iberian populations may display greater reproductive capacity (i.e. ovary mass, fecundity and egg size) than in the species' native range; however, this requires further investigation due to the limited data from studies on non-native populations and to differences in the methodological approaches employed therein.
Trophic ecology
Prey
Alburnus alburnus feed on a wide range of food items, and for this reason it should be regarded as an omnivorous, opportunistic forager (Chappaz et al. 1987). In its native range, A. alburnus forages at the surface of open inland waters (preferably lentic habitats), and its diet is based primarily on zooplankton (Herzig 1994; Vinni et al. 2000; Vašek and Kubečka 2004), crustaceans and chironomids (Bíró and Muskó 1995; Latorre et al. 2016) and nektonic invertebrates, but it can include other drifting and terrestrial prey (e.g. flying and terrestrial arthropods that fall onto the water's surface). As with many omnivores, A. alburnus are known to adapt their diet in response to food availability (Chappaz et al. 1987; Almeida et al. 2017; Latorre et al. 2018) in both native (Chappaz et al. 1987) and non-native (Latorre et al. 2016, 2018, 2020b; Almeida et al. 2017) populations.
In shallow river stretches, the diet of A. alburnus consists of benthic invertebrates including insect nymphs, larvae and snails (Haberlehner 1988; Latorre et al. 2016; Almeida et al. 2017), with detritus and plant material being a frequent component (Vøllestad 1985; Bíró and Muskó 1995). The eggs of other fish species have also been reported in the diet of A. alburnus in the Želivka Reservoir (Czechia), where the species may become the dominant egg predator (Šmejkal et al. 2017)–though the effect of A. alburnus predation on native threatened or endemic species requires further investigation.
In reservoirs and lakes, A. alburnus forage more intensively during the first half of the day, preying on zooplankton in deeper waters (Politou et al. 1993), whereas night-time diet consists of terrestrial invertebrates that have fallen on the water's surface (Chappaz et al. 1987). The presence of predators can modify the species' feeding strategies, so A. alburnus foraging follows a nocturnal pattern when predators are abundant and a diurnal pattern when predators do not represent a threat (Politou et al. 1993). This may explain the mainly diurnal pattern observed for A. alburnus larvae in a side-channel of the River Rhine (Schröder 1979; see also Fig. 5 in Copp et al. 2005c) and for 0 + juvenile A. alburnus in a lentic side-channel of the River Danube. In the latter case, 0 + juvenile A. alburnus densities were negatively correlated with zooplankton densities, which increased in abundance overnight (Copp et al. 2005c). Additionally, changes in foraging strategy have been observed in relation to water transparency (Ivlev 1960). Similarly, foraging activity depends directly on water temperature, being related to the species' general mobility and seasonal dynamics (Politou et al. 1993). Depending on density, stocking of A. alburnus may play an important role for the food web (Bíró and Muskó 1995).
Diet composition in A. alburnus is not likely to be influenced by its body size, though there are slight shifts during early ontogeny (Politou et al. 1993; Bíró and Muskó 1995; Almeida et al. 2017), and the diet is primarily zooplankton (e.g. Nunn et al. 2007), with Bosmina sp. being the most frequent prey (Garner 1996). The variety of dietary items increases substantially with growth (Bogacka-Kapusta and Kapusta 2007), so adult diet includes insect nymphs, worms and algae. Although A. alburnus select higher energy prey items when occurring in habitats with little or scarce competition (Latli et al. 2019), in conditions of higher interspecific competition and lower trophic availability, juvenile individuals can diversify their diet and ingest lower energy resources (i.e. plants or algae). Consumption of algae seems to be secondary and depends on the availability of other food resources, but the energy content of algae is low (Latorre et al. 2016); similar to roach Rutilus rutilus (Mann 1997)—this could explain why A. alburnus with abundant algal remains in their gut have been observed to demonstrate reduced growth and fecundity (Chappaz et al. 1987).
In lakes and reservoirs, A. alburnus forage intensively during summer and at the beginning of autumn, and foraging activity declines in winter upon a temperature decrease (Politou et al. 1993). Foraging intensity increases from early February, although a decrease occurs in April, coinciding with gonad development (Politou et al. 1993). The relative position of A. alburnus in the water column also varies among seasons in response to food availability (Chappaz et al. 1987). Regarding diet composition, there are seasonal changes that follow zooplankton composition and structure during spring and summer, being substituted by chironomids during autumn (Bíró and Muskó 1995; Bogacka-Kapusta and Kapusta 2007), with food diversity increasing in summer in response to available food resources (Politou et al. 1993; Almeida et al. 2017).
Predators
Alburnus alburnus is a common prey for most freshwater piscivorous species throughout its distribution range. Predation of A. alburnus begins at an early age, with eggs preyed upon by European eel Anguilla anguilla (Mills 1991). Older A. alburnus are predated by piscivorous fishes, including Esox lucius (Vøllestad et al. 1986; Kangur and Kangur 1998; Mérő 2014) and Sander lucioperca (Bíró and Muskó 1995; Peltonen et al. 1996; Kangur and Kangur 1998), but also by Lutra lutra (Prigioni et al. 2006) and piscivorous birds such as European kingfisher Alcedo atthis (e.g. Reynolds and Hinge 1996; Vilches et al. 2012), grey heron Ardea cinerea (e.g. Jakubas and Mioduszewska 2005; Stolbunov et al. 2017) and great cormorant Phalacrocorax carbo (e.g. Gagliardi et al. 2007; Čech et al. 2008; Čech and Vejřík 2011; Russell et al. 2022). Use of A. alburnus as a prey (forage) species for piscivorous fishes has been the vector for introductions in the Mediterranean region (Vinyoles et al. 2007), such as Spain (Ruiz-Olmo and Jiménez 2009; Vilches et al. 2012; Ribeiro et al. 2021) and Italy (Prigioni et al. 2006; Gagliardi et al. 2007), where A. alburnus has been used by recreational anglers as bait.
Physiology
Ontogeny of swimming performance and metabolism
In fish and other aquatic organisms, swimming performance is considered to be a principal attribute for individual survival, reproductive success, and even invasiveness (Cano-Barbacil et al. 2020). Following an initial 8–10 days post-hatch in the substratum's interstices, when the small yolk sac has been absorbed and the swim bladder is functional, the A. alburnus larvae initiate swim-up behaviour (El-Fiky et al. 1987). Larval swimming behaviour is dominated by attempts to hold position in the water by undulating their body and fin folds. During this early period of development, young A. alburnus larvae demonstrate a relatively high propensity to drift, both in smaller (Copp et al. 2002) and larger water courses (Copp and Cellot 1988; Oesmann 2003; Zitek et al. 2004a, 2004b). The rate (density) of larval drift, and not size, was found to be negatively correlated with light intensity, the mean drift density of A. alburnus in the River Morava (Czechia) being more than 6 × greater at night than at twilight; this suggests that A. alburnus drift is not a passive displacement due to visual disorientation but instead a behavioural decision that is triggered by light levels (Reichard et al. 2002).
Swimming is almost entirely aerobic, being powered by the deep layers of muscle fibres, which exhibit strong activity of aerobic enzymes such as cytochrome oxidase (El-Fiky et al. 1987; El-Fiky and Wieser 1988). The superficial layer of red muscle fibres is the main respiratory organ for newly hatched A. alburnus, though gills are not yet functional (El-Fiky and Wieser 1988). Following the onset of exogenous feeding, A. alburnus larvae grow, the red layer of muscle fibres diminishes gradually in mass by contracting towards the lateral region of the body, while at the same time gill filaments and secondary lamellae increase rapidly in number (El-Fiky et al. 1987). In A. alburnus, the red layer represents about 20% of the total muscle mass after hatching and it is not until 20 days later that it begins to decrease in size (El-Fiky and Wieser 1988). Compared with other cyprinid species (e.g. Rutilus rutilus), which begin to swim freely 2–3 days after hatching, completion of the gill structure in A. alburnus is delayed due to the longer period of attachment of the larvae to the substratum (El-Fiky et al. 1987). In addition, the activities of the isoenzymes of lactate dehydrogenase, which are characteristic of fast glycolytic muscle fibres, increase more slowly in developing larvae of A. alburnus (El-Fiky et al. 1987; El-Fiky and Wieser 1988). Notably, this delay in development of the enzymes of anaerobic energy metabolism may compromise the ability of fish to sustain ultrafast movements (Wieser 1991).
Critical swimming speed (Ucrit), which represents the maximum aerobic swimming speed that a fish can attain, has been found to increase positively with body length in A. alburnus larvae (Abramiuk and Afanasyev 2017) and adults (Rubio-Gracia et al. 2020a). In the former study, however, velocity was gradually increased until larvae were no longer able to withstand the current, whereas the latter study relied on stepwise increases in water velocity (i.e. one body length s−1) with a 20 min time interval. In determining swimming speed, the duration of the step-test interval is important because shorter time steps will result in a higher critical swimming speed (Cano-Barbacil et al. 2020). As such, methodological differences between the above two studies would explain why some larvae exhibited higher critical swimming speed than small-bodied adults. Also, comparative studies have shown that not only can A. alburnus larvae and adults swim faster than other fish species of similar size, like Eurasian perch Perca fluviatilis (Abramiuk and Afanasyev 2017; Cano-Barbacil et al. 2020), but they can also exhibit lower mass-specific cost of transport (Rubio-Gracia et al. 2020a). This improved swimming performance and efficiency is consistent with the species' active exploratory behaviour throughout the water column during early ontogeny (da Silva et al. 2019).
Like swimming capacity, maximum metabolic rate (i.e. the highest rate of oxygen consumption) and standard metabolic rate (i.e. the basal metabolism of an animal to sustain basic life functions) are positively related with body size (measured as fresh weight) in A. alburnus. It has been shown that A. alburnus adults have lower standard metabolic rate at a comparable temperature than do other freshwater fish species, including some other cyprinids, centrarchids (e.g. pumpkinseed Lepomis gibbosus) and salmonids (e.g. Arctic char Salvelinus alpinus) (Rubio-Gracia et al. 2020a; Voutilainen et al. 2011).
The critical swimming speed of A. alburnus adults is enhanced by their more streamlined body, including a head shape adapted to reduce friction when swimming against the current (Gąsowska 1974), relatively low drag coefficient (Sagnes and Sfgtatzner 2009), estimated as fineness ratio (measured as SL/maximum body depth) and by the thickness of the caudal peduncle (estimated as caudal peduncle depth factor)–the latter being considered a key morphological trait to generate thrust (e.g. Fisher and Hogan 2007; Rubio-Gracia et al. 2020b). Moreover, the species’ standard metabolic rate is also influenced by body shape (Table 4). Similarly, deep-bodied fish have been found to attain lower standard metabolic rate than shallow-bodied fish (Pettersson and Brönmark 1999; Latorre et al. 2020a). Propulsive ratio (measured as propulsive body area/total body area), which represents the proportion of the fish's body able to be used for swimming (Fisher and Hogan 2007), can also increase markedly the variation in standard metabolic rate (Table 4). Therefore, elevated standard metabolic rate in A. alburnus can be largely based on the development of muscles and other features related to locomotion, as reported for other fishes (e.g. Nanami 2007).
Energy acquisition and allocation
The energy acquisition and allocation is particularly relevant to understand fish survival when facing novel environments, such as newly invaded areas, but also when coping with environmental change, e.g. resulting from global warming. Preferential allocation of energy to somatic growth is an essential feature of larvae since rapid growth rates favour survival and predator avoidance (Wieser 1991). However, only a few studies have attempted to determine the partitioning of ingested energy during the development of A. alburnus by measuring rates of food consumption, routine metabolism and growth (Keckeis and Schiemer 1990, 1992). Overall, consistent with the foraging patterns described here above, the pattern of energy partitioning depends on food availability. During the early ontogeny of A. alburnus, when food is unlimited, daily food consumption increases with age, e.g. at 22 days daily consumption was 0.60 mg day–1 and at 61 days 3.85 mg day–1 (Keckeis and Schiemer 1990). The relationship between daily food consumption rates (C, J day–1 ind–1) and body size (W, mg dry weight) in A. alburnus also varies, being dependent on the amount of food ingested. At high and low food levels, the allometric relationships were C = 18.32 (± 1.28) W0.81 (±0.02) and C = 5.07 (± 1.32) W1.04 (±0.03), respectively (Keckeis and Schiemer 1992). The slope of the allometric relationship between respiration (R), i.e. without incorporating the slope due to food searching and specific dynamic action, and body weight (R = 0.11 [± 1.86] W0.87 (± 0.02)) was found to be higher at high-food availability than that of the above-mentioned relationship between food consumption and body weight. This indicates that metabolic expenditure increases faster with body size than energy uptake rates, causing a decrease in the growth rate of A. alburnus with increasing size at higher food availability. Growth rates ranged from 5 to 8% day−1 at higher food levels, but at lower food availability they ranged from 3 to 5% day−1, which can occur independent of body size (Keckeis and Schiemer 1992).
In a comparison of growth rates over 90 days in recently hatched A. alburnus and Rutilus rutilus larvae at different levels of food supply (Keckeis and Schiemer 1990), production efficiency [PE = P (P + R)−1 × 100] was found to decrease with increasing weight in the two species. However, despite the similar energy intake and routine metabolism of the two species, A. alburnus grew slower than R. rutilus at the same age and under high food availability (Keckeis and Schiemer 1990, 1992). This interspecific difference in growth patterns can be directly attributed to differences in assimilation efficiency [AE = (P + R) × C−1 × 100], which correlates with the relative gut length of the species (Keckeis and Schiemer 1990). Because of its shorter gut length, A. alburnus is much less efficient in the conversion of consumed energy into body mass, likely due to lower power of digestion (Hofer and Nasir Uddin 1985). However, both species had similar growth when food was more restricted (Keckeis and Schiemer 1992). This finding, together with higher prey detection capacities (Wanzenböck and Schiemer 1989), indicates that A. alburnus may be well adapted to low-nutrition environments. These differences in energetic performance between the two species point to mechanisms leading to trophic-niche differentiation in their early-life history. In addition to food availability, temperature is also known to affect A. alburnus growth (Wieser et al. 1988b). Thus, the relative growth rate (% fresh weight d−1) of A. alburnus increases proportionally with temperature and decreases with increasing fish size, using R. rutilus as a model species (Wieser et al. 1988a). Understanding these energy allocation metrics is essential specially to understand A. alburnus adapting capacity given that most of the invaded range is located in southern latitudes, generally with warmer environments.
Behaviour
Activities and social patterns
Categorised as a ‘compulsory schooling’ species (Karst 1968) and an ‘obligate schooler’ (Haberlehner 1988), A. alburnus occurs almost exclusively in shoals, which commonly consist of 30–50 individuals (Holubová et al. 2020) and move through the surface layer of the water column, usually at a depth not exceeding 1.5 m (Vašek et al. 2009). Underwater observations demonstrated that adult A. alburnus shoals move rapidly (Karst 1968), positioned near the water's surface (Karst 1968; Vinyoles et al. 2008), and that high swimming speeds preclude other species (e.g. Rutilus rutilus) from joining A. alburnus shoals (Haberlehner 1988). However, A. alburnus larvae and 0 + juveniles have been observed as part of mixed shoals in various river systems within the native range, consisting of other 0 + cyprinids such as R. rutilus, chub Squalius cephalus, rudd Scardinius erythrophthalmus (Copp 1992, 1993; Gozlan et al. 1998) and Perca fluviatilis (Copp et al. 1994), with a preference for locations close to littoral areas (Černý et al. 2003).
Shoal formation acts as a predation-avoidance strategy of a small-bodied species, so A. alburnus demonstrates a preference to forming dense shoals, as observed in natural lakes (Tischler et al. 2000) but also in reservoirs (Říha 2012). The effect of fish density on shoaling formation has been investigated in the epipelagic habitat of the Římov Reservoir, Czechia (Holubová et al. 2019), where the most abundant species of the pelagic habitat corresponded to those species with the strongest shoaling behaviour (Říha 2012). The origin of these aggregations, which were mainly composed of species such as A. alburnus, may be driven by the absence of refuges in the pelagic habitat (Magurran and Pitcher 1983). Moreover, the formation of shoals has been observed to be a function of fish density in the habitat (Holubová et al. 2019). Fish aggregations tend to attract more individuals, especially when they are feeding, thus shoal size increases in a linear relationship with fish density, supporting that shoaling behaviour is partly driven by fish density in open water habitats (see experimental data in da Silva et al. 2019). Such shoaling behaviour was found to emerge at a ‘critical density’ of 20–30 individuals within 10 m distance (Makris et al. 2009; Maury 2017).
In both the native and introduced ranges, A. alburnus shoal movements are generally similar, with shoals consisting of medium- to large-sized individuals, which move in a wedge-shaped configuration to improve hydrodynamics under more slightly lotic conditions (Haberlehner 1988). Shoals were observed to swim in one direction in the centre of the river channel, whereas along the river banks circles or loop-shaped patterns of several meters in diameter were formed (Haberlehner 1988; da Silva et al. 2019). Within a shoal, A. alburnus position was highly variable, with inter-individual distances ranging from a single body length at the front of the shoal to ≈2 m separation towards the back of the shoal. Moreover, solitary adult individuals may be occasionally found together with juveniles (Hohausová et al. 2003). Nevertheless, solitary individuals can increase swimming activity to join new schools rapidly, including other species' aggregations, such as those of chubs (Genus Leuciscus), until a group of A. alburnus is found (Haberlehner 1988).
In the open and shallow waters of Rybinsk Reservoir (Russia), the highest number of 0 + juvenile A. alburnus was recorded during the day, whereas the number of older larvae recorded during darkness was much lower (Stolbunov and Kuzmina 2018). Further, the weights of older larvae at different stages of development differed significantly throughout the day, decreasing as darkness approached, thus indicating diurnal migrations and redistribution of older larvae along the reservoir. A similar diel pattern was reported for A. alburnus juveniles in a side-channel of the River Danube (Copp et al. 2005c), where relative densities of A. alburnus decreased at night but size (mean SL ± SE) increased at night (60.4 mm ± 12.17) compared with day-time size (20.8 mm ± 0.73). Presumably, a circadian cycle may also be involved in the movement of individuals that migrate between pelagic and littoral habitats. Thus, in a stratified European reservoir, larger individuals were caught in greater proportion during the day, whereas the proportion of smaller fish increased during the afternoon and night (Vašek et al. 2009).
In experiments to assess the effect of predator (Esox lucius) presence on A. alburnus behaviour, feeding and growth under two treatments, A. alburnus feeding rates were reduced by 35% and 20% when exposed to the scent of freshly-fed and starved pike, respectively (Jachner 1997). This reduction resulted from a decrease in time spent feeding, which was followed by a decrease in individual growth rates, thus supporting the hypothesis that an alarm substance, and not simply the predator's odour, is the trigger for predator-avoidance responses (Jachner 1997). In the absence of the ‘recently-fed’ scent, A. alburnus moved calmly, either in groups or individually, and without any preference between open water and vegetation, but in its presence A. alburnus preferred to move in groups and quickly towards vegetation in search of refuge and remained hidden until it needed to feed (Jachner 1996).
Migrations and movements
Although A. alburnus has been classified as non-migratory (Wheeler 1977), perhaps because more sedentary populations have been reported in some lakes and reservoirs, this potamodromous fish is known to migrate from reservoirs and medium-to-large rivers upstream into small tributaries to spawn (e.g. Lelek and Libosvárskí 1960; Peterka et al. 2004; Meulenbroek et al. 2018), such as reported in the native range for the Rímov Reservoir (Czechia) into its main tributary (Hladík and Kubečka 2003). Migrations up tributaries of several reservoirs have also been reported to occur in the species’ non-native range, where reservoirs act as a stepping-stone from which A. alburnus invade upstream areas (Matono et al. 2018). In African rivers, such upstream migrations by A. alburnus occur in desert streams close to reservoirs and in lentic and stable habitats generated by dams (Attou and Arab 2013; Clavero et al. 2015).
Changes in A. alburnus migration rates are generally related to fluctuations in water quality (e.g. physical and chemical variables: Santos et al. 2002; Lilja et al. 2003; Kotusz et al. 2006; Brodersen et al. 2008; Taylor and Cooke 2012; Benitez et al. 2015). In the case of Rímov Reservoir (Czechia), the frequency of A. alburnus moving upstream into the tributary was related to an increase in water temperature, which is an important regulatory factor in A. alburnus spawning migrations (Hladík and Kubečka 2003). During upstream migrations in the River Tundzha (Bulgaria) during April and May (spawning period), A. alburnus migration intensity was highest in mid-afternoon (14:00) at temperatures of 11–14 °C (Angelov et al. 2020). A telemetry study of four radio-tagged A. alburnus in the River Elbe during July–September 2007 documented a mean home-range area of 0.197 ± 0.125 km2, with diurnal movements of 827 ± 580 m, which was seemingly influenced by abiotic factors, mainly turbidity, water temperature and discharge (Josefovičová 2019).
During winter, many fish species move downstream to find refuge and avoid being displaced by high water velocities (Lucas et al. 1998), such as reported for the use of pools by A. alburnus in a fish pass on the River Danube (Meulenbroek et al. 2018). By using automated, passive-integrated-transponder tags, fish behaviour was examined over a wide range of sizes and species in a narrow fish pass located in Northeast England. Tagged A. alburnus were detected in the fish pass by the downstream end of the antennae, indicating a much higher level of activity from fish entering and trying to use the pass than from those successfully ascending it (Lucas et al. 1999). Most of the records compiled by downstream antennae were by the antenna closest to the downstream end of the pass, suggesting that most fish were more active where velocity (and effort) was lowest in the water column. However, the efficiencies of coarse fish passage were low, possibly because cyprinids were impeded by the high levels of turbulence and the complex spatial environment (Lucas et al. 1999). Extensive use of fish passes by A. alburnus has also been reported elsewhere, such as in the rivers Meuse in Belgium (Baras et al. 1994), Elbe in Czechia (Prchalová et al. 2011), Odra in Poland (Kotusz et al. 2006) and Danube in Austria (Schmutz et al. 1998). Alburnus alburnus have been reported to use the lower extent of the Danube fish pass as a winter refuge habitat (Meulenbroek et al. 2018). More recently, high A. alburnus abundances were reported in different fish passages in the River Segura in southeastern Spain, where A. alburnus moved along the river but also used these passages as seasonal refugia (Sánchez-Pérez et al. 2022).
Migrations of juvenile A. alburnus against the water current can be related to either foraging (Prchalová et al. 2004) or the search for winter refuges (Prignon et al. 1998; Lucas and Baras 2000; Prchalová et al. 2004, 2006), such as observed from September to November in the delta of the River Volga (Tryapitsyna 1965; Podolyako et al. 2017). Alburnus alburnus migrations upstream, dominated by 1 + and 2 + juveniles, occur in a broadly-dispersed pattern (between 10–15 m) along the left and right margins of the river sections, beginning around 06:00, peaking between 12:00–15:00 and continuing until 20:00–21:00 (Pavlov et al. 2019).
The invasion by A. alburnus of the Iberian Peninsula has been facilitated by human-induced alterations to river channel morphology and hydrology, such as river impoundment for water retention. As a result, A. alburnus displays seasonal migrations along the tributaries of various reservoirs. A study of A. alburnus in the River Guadiana (Portugal) reported size-related seasonal migrations (Matono et al. 2018). In summer, A. alburnus of up to 60 mm TL were found mostly upstream, whereas those between 120–150 mm were found downstream, and individuals between 60–120 mm were equally dispersed along the entire river (Matono et al. 2018). These findings suggest an upstream recruitment of juvenile individuals during summer and autumn, and a higher proportion of reproductive individuals downstream in spring and summer.
Pathogens and parasites
Alburnus alburnus has been found to host more than 40 species of pathogens and parasites that belong to the groups Acanthocephala, Cestoda, Crustacea, Hirudinea, Monogenea, Myxosporea, Nematoda and Protista (Borowik 1968; Baska and Molnár 1988; Koyun and Altunel 2007; Molnár et al. 2009; Koyun 2011). Bacterial (e.g. Bacillus spp.) and fungal (e.g. Branchiomyces spp.) diseases in A. alburnus have been also detected (Table 8). Whilst viral diseases in A. alburnus have received relatively little attention compared with its parasites, experimental trials have revealed that A. alburnus is a potential healthy host of the carp edema virus (CEV), i.e. no clinical signs or mortality have yet to be reported (Matras et al. 2019). Shorter periods (only 12 h) of exposure to A. alburnus in cohabitation studies were sufficient for CEV to be transmitted to other host species, thus confirming the species' potential role in virus dispersal (Way et al. 2017).
Myxosporean parasites can also infect A. alburnus, and they have been recorded in the internal organs of A. alburnus in Hungary, where plasmodia of Myxobolus shaharomae were apparent in blood vessels of the kidney, liver, testes and intestinal wall—though in most cases the plasmodia did not elicit a host reaction (Molnár et al. 2009). A three-year study carried out in Hungary on A. alburnus from Lake Balaton and from the River Danube revealed the species to be a host of four Myxobolus species, with a prevalence up to 16% (Molnár 2000).
Based on 165 specimens of A. alburnus in a broader eco-parasitological study (Chunchukova et al. 2019a), helminth community structure in A. alburnus from the River Danube comprised seven species of parasites (see Table 8), including Trematoda (four species: n = 971), Nematoda (n = 7), Cestoda (n = 4), and Acanthocephala (n = 2). In Lake Kortowskie (Poland), 14 parasite species were found in A. alburnus, including seven monogeneans (Dzika et al. 2008). Infections by helminth parasites are common in A. alburnus populations, with Ligula intestinalis and Pomphorhynchus laevis considered as important intestinal parasites and found in several populations at relatively high prevalence levels (e.g. Kirin 2001; Chunchukova et al. 2019a, 2019b). Thus, P. laevis was a core parasite species of A. alburnus in the Bulgarian section of the River Danube, where prevalence levels were highest in summer and autumn (Chunchukova et al. 2019a). In the River Marista (Bulgaria), helminth parasites were recorded in 83% of the A. alburnus individuals examined and included both L. intestinalis and P. laevis (Chunchukova et al. 2019b). Five helminth parasites were recorded in A. alburnus from two other rivers in Bulgaria, with the nematode Rhabdochoata having the highest prevalence, but no record of either L. intestinalis or P. laevis (Kirin 2001). Conversely, in Great Britain, L. intestinalis was the only helminth detected in a review of helminth parasites of freshwater fishes in Great Britain (Price and Clancy 1983), with helminth prevalence in the River Thames varying seasonally and peaking in summer (Harris and Wheeler 1974). A similar pattern was observed in Lake Enne, Turkey (Koyun et al. 2007).
A feature of cestode parasites is their accumulation of heavy metals relative to their host species and environment (Sures and Siddall 1999; Sures et al. 1999). Accumulations in A. alburnus infected with Pomphorynchus spp. (P. laevis, P. tereticollis) has been found to be significantly higher, with regard to arsenic, nickel and lead, in the parasite than in the host tissues and organs (Chunchukova et al. 2017; Chunchukova 2018; Chunchukova and Kuzmanova 2017).
Amongst the monogenean parasites that infect A. alburnus populations, Dactylogyrus spp. are common, with two species recorded as infecting two lacustrine populations in two Adriatic river basins (lakes Prespa and Ohrid, Macedonia), together with two monogenean parasites, and with overall prevalence levels at 45% (see Table 8, Stojanovski et al. 2009). A previous study of A. alburnus from Lake Prespa revealed a similar monogenean fauna, with 41% of A. alburnus infected with at least one of these parasites and with the highest prevalence (22%) for Dactylogyrus alatus (Stojanovski et al. 2003), whereas in Lake Dojran (Macedonia) prevalence was lower, with 2% of A. alburnus infected with Ligophorus sp. (Stojanovski et al. 2008). In the River Porsuk (Turkey), monthly A. alburnus samples indicated infection by three Dactylogyrus species (see Table 8), with prevalence levels, abundance, and mean intensity always highest for D. fraternus, with a prevalence of 50%, 5.2% and 2.6%, respectively (Koyun 2011). Studies on the molecular phylogeny of Dactylogyrus parasites have revealed the presence of three lineages, with A. alburnus populations infected by a single lineage that also infects other leuciscids (European minnows) as well as the European common barbel Barbus barbus (Šimková et al. 2004). Finally, two species of Gyrodactylus parasites have been recorded for A. alburnus: G. bliccensis (Matejusová et al. 2001) and G. gracilihamatus (Zietara and Lumme 2002).
Regarding digenean parasites, the majority of A. alburnus examined from Lake Dabie (Poland) were parasitised in muscle tissues by the metacercariae of either Paracoenogonimus ovatus or Posthodiplostomum cuticola, but with a low proportion, and with an intensity of infection up to 34 metacercariae (Ostrowska et al. 2019). In 22 host fish species from Lake Modrac (Bosnia), the metacercaria of P. cuticula was commonly encountered, but with the lowest prevalence recorded in A. alburnus (Adrović et al. 2011). Among others, the Opisthorchiidae and Heterophyidae Families include genera that cause fish zoonoses; however, owing to the small size of their metacercaria, these parasites cannot be visually detected in fishes. Through the application of multiple PCR methods, the Genus Metagonimus (Heterophyidae), which is one of the main potentially zoonotic trematodes present in Europe, was identified in A. alburnus specimens (Caffara et al. 2020). In addition, the most important species of zoonotic flukes transmitted in fresh waters is Opisthorchis felineus (Cech et al. 2021), which has been observed at a prevalence of 74% in A. alburnus from German fresh waters (Hering-Hagenbeck and Schuster 1996).
Ergasilid parasites tend to infect the gills of their fish hosts, with Paraergasilus longidigitus recorded in the branchial filaments of A. alburnus in Lake Enne (Turkey) at an overall prevalence of 57%, but with a peak in autumn at 74%, and with prevalence increasing with fish size (Koyun and Altunel 2007). Lernaea cyprinacea (also known as ‘anchor worm’) feeds on the host's blood and tissue was recorded in A. alburnus from Iran, where 68% of the fish examined were infected, but with the extent of the pathological damage not reported (Raissy et al. 2013).
Genetic traits
The genetic character of A. alburnus is characterised by a diploid karyotype that consists of 50 chromosomes: 14 metacentric, 14 sub-metacentric, 14 sub-telocentric and 8 telocentric (Ziegler et al. 2003). In addition, there are two giant super-numerary chromosomes of two different sizes, which can extend the possible karyotypes to 2n = 51 and 2n = 52, respectively. This remarkable genetic trait has been reported for 11% of individuals possessing one or two giant supernumerary B chromosomes of different size (Ziegler et al. 2003; Schmid et al. 2006). Detailed DNA sequencing of the B chromosomes determined this trait to be of retro-transposable origin, as a strong homology was found with the long-terminal-repeat retro-transposon from the medaka fish Oryzias latipes. Overall, these findings suggest a possible interspecific origin for the A. alburnus supernumerary chromosomes (Camacho et al. 2000; Ziegler et al. 2003).
As in other species, an important factor that influences genetic diversity in A. alburnus is its elevated capacity for hybridisation. The introduction of closely related fish species, along with habitat disturbance, has increased the incidence of interspecific hybridization and establishment of hybrid zones (Costedoat et al. 2005). Alburnus alburnus can hybridise with other cyprinid genera, including Abramis, Blicca, Leuciscus, Rutilus and Squalius (Wheeler 1978; Blachuta and Witkowski 1984; Crivelli and Dupont 1987; Berrebi et al. 1989; Maceda-Veiga et al. 2010; García-Berthou et al. 2015; Witkowski et al. 2015). However, detailed information on long-term viability and reproductive performance of hybrid individuals is still lacking.
To improve knowledge on the extent of hybridisation impact in Iberian freshwaters, the genetic profile of A. alburnus was identified for cyt b and beta-actin genes, together with Iberian endemic leuciscids, namely Spanish minnowcarp Anaecypris hispanica, Iberian roach Squalius alburnoides and Southern Iberian chub Squalius pyrenaicus (Almodóvar et al. 2012; Sousa-Santos et al. 2018). Sequencing of the mitochondrial cyt b gene yielded four haplotypes that differed by 1–5 mutations, accounting for 0.10 to 0.50% of pairwise divergence among sequences. A phylogenetic tree based on these analysed sequences revealed A. alburnus to be more closely related to A. hispanica than to the sympatric Squalius species. One A. alburnus haplotype from the River Guadiana was shared with specimens from the River Jarama (a tributary of the River Tagus in central Spain) and, together with one haplotype from Czechia, formed a well-supported clade different from the clade conformed by individuals from Croatia, Greece and Russia. On the other hand, sequencing of the nuclear beta-actin gene revealed the presence of 12 haplotypes among specimens from the River Guadiana and Croatia, with only three of them being found in homozygosity. Mitochondrial and nuclear molecular analyses were also conducted in A. alburnus specimens from the River Jarama to establish the pattern of introgression of A. alburnus with the genus Squalius (Almodóvar et al. 2012). Mitochondrial DNA analyses (cyt b and 16S) in hybrids were of A. alburnus maternal origin. Since the Internal Transcribed Spacer 1 sequences showed the same results, these regions were deemed to be of maternal type as well (Slynko and Stolbunova 2010). Regarding the beta-actin gene, the results showed that all hybrid sequences had a double peak generated by two different parental sequences, namely A. alburnus and Squalius. However, the hybrids were different in terms of the direction of gene introgression, with one hybrid proving to be of A. alburnus paternal origin and the other two hybrids proving to be of maternal A. alburnus origin (Sousa-Santos et al. 2018). More recently, a single direction on hybridisation from male A. alburnus towards females of northern Iberian chub Squalius carolitertii has been identified (Curto et al. 2022). Because there is increasing evidence supporting the theory that hybridisation can lead to adaptation through the establishment of new and optimised genotypes and morphologies (Rieseberg et al. 1999), the findings described here above indicate that introduced A. alburnus, which are widespread in the Iberia Peninsula (see Curto et al. 2022), have the potential to produce irreversible genetic swamping of rare endemic species.
Invasiveness and ecological impacts
Introduced populations of A. alburnus exhibit large and sudden increases in abundance, which is characteristic of invasive fishes (Copp et al. 2005a; Fox et al. 2011; Britton and Gozlan 2013), and this is one reason that introduced A. alburnus populations have dispersed widely in the Iberian Peninsula. The life-history traits that enabled A. alburnus to invade drainage basins of the Iberian Peninsula will vary depending on invasion stage (Ribeiro et al. 2008). In fact, small body size, an elevated capacity to adapt to local conditions and high fecundity rates have all been demonstrated to be important in population establishment (Ribeiro et al. 2008). The species dispersal between and within drainages is mostly related with its interest for recreational fisheries (Ribeiro et al. 2008), with propagule pressure (i.e. frequency and quantity of fish releases) being a key factor in the rate of spread. Alburnus alburnus is listed amongst the most frequently introduced species in both Spain and Portugal due to its common use as live bait by anglers (Banha et al. 2017). These bait bucket releases generally involve small numbers of fish (< 30 specimens), but the frequency of these releases is high (25% of anglers), representing a considerable propagule pressure (Banha et al. 2017). This elevated propagule pressure is exacerbated by demonstrated plasticity in life-history traits (Table 5) (e.g. high reproduction, see below), which may increase the establishment and spread success (Lockwood et al. 2005). The ability of A. alburnus to cope with long term environmental variability on Iberian waters (invasion stage: integration) appears to be associated with the species' dietary traits as well as the similarity in environmental conditions of the A. alburnus' native and non-native ranges and their proximity (Ribeiro et al. 2008).
Not surprisingly, A. alburnus was the second most captured fish species in basins of the Catalonia Region (northeastern Spain), where native fish populations were reduced on average by 60% relative to the period prior to invasion by A. alburnus. Local extinctions of the endemic ‘bermejuela’ Achondrostoma arcasii have coincided with the arrival of A. alburnus (Maceda-Veiga et al. 2010). Alburnus alburnus is also known to threaten species of the genus Parachondrostoma, such as P. arrigonis and P. turiense, through competition for food and habitat resources (Doadrio et al. 2011; Latorre and Almeida 2019). In the Keddara Dam (Algeria), the accidental introduction of A. alburnus has been linked to the decline of native Algerian barb Barbus setivimensis (Attou and Arab 2013). Introduced A. alburnus populations have also been linked to the decline of other endemic fishes in Spain, including the Ebro nase Parachondrostoma miegii (Almeida and Grossman 2012), and to cause a substantial shift in the behavioural patterns of this Iberian nase species (Vinyoles et al. 2009; Almeida and Grossman 2012) as well as on the endangered minnow Anaecypris hispanica (da Silva et al. 2019). Specifically, the change in behaviour has led to higher metabolic expense, reduced shelter use, and increased predation risk in A. hispanica (da Silva et al. 2019).
The role of hydrological disturbances as a facilitator of invasions by A. alburnus of temporary Mediterranean streams has examined the species' dispersal process within the River Guadiana basin (Portugal) at three different levels (Matono et al. 2018): (i) upstream and downstream movements along river sections, (ii) invasion of interconnected river sections from passive movements through upstream dams, and (iii) dispersal related to human intervention or deliberate introductions–the only factor that explains movement through large dams. Accordingly, the expansion of A. alburnus was found to be clearly associated with dam-regulated river systems (Matono et al. 2018), where A. alburnus spread appears to be related to hydrological alterations (Vinyoles et al. 2007). The lentic conditions created by water retention structures have likely facilitated the establishment of A. alburnus and its dispersal both by active movement and through passive larval dispersal (Reichard et al. 2002). Alburnus alburnus is considered by many to be an eurytopic fish species (Copp 1989, 1992; Wolter and Vilcinskas 1997; Aarts and Nienhuis 2003; Fladung et al. 2003; Zitek et al. 2004a; Lasne et al. 2007) and a good swimmer due to its low drag coefficient (Sagnes and Statzner 2009), thus enabling the species to sustain prolonged swimming performance and efficiency (Rubio-Gracia et al. 2020a). Consequently, A. alburnus can achieve high densities in a variety of freshwater environments (Masó et al. 2016), including the highly variable hydrological regimes of natural Mediterranean temporary streams (Almeida et al. 2014; Amat-Trigo et al. 2019). Alburnus alburnus have demonstrated their ability to move large distances from the rivers and reservoirs where the species was first introduced (Almeida et al. 2014). This mobility is complemented by plasticity of several biological traits that facilitate adaptation and invasion to new environments, such as high fecundity, an omnivorous diet, and a broad temperature tolerance (Fig. 3) (Chappaz et al. 1987; Latorre et al. 2016, 2018, 2020a). Indeed, temperature tolerances of A. alburnus (Kuttel et al. 2002) indicate that eggs are able to withstand water temperatures of 14–31 °C (Alabaster and Lloyd 1980), with optimal embryonic development at 21–27 °C (Alabaster and Lloyd 1980). For adults, temperatures > 20 °C are critical, with a CTMax of 38 °C reported for A. alburnus in a heated lake (Alabaster and Lloyd 1980).
As an omnivorous planktivore, A. alburnus occupies a relatively-low trophic position (Almeida et al. 2014; Latorre et al. 2016, 2018, 2020a), which an afford a fish species greater efficiency in obtaining energy from basal trophic groups (Gido and Franssen 2007). During the early stages of A. alburnus invasions, different life traits are important along the invasion stage, from recently introduced to well-established populations (Ribeiro et al. 2008). For example, faster growth rates have been found during the initial stages of invasion compared with sites where fish populations are in the establishment phase (e.g. Bøhn et al. 2004; Fobert et al. 2013; Copp et al. 2017). A similar pattern has been suggested for reproductive effort (Copp and Fox 2007). Overall, phenotypic and habitat plasticity of A. alburnus in both the native and introduced populations appears to be an important factor in its invasiveness, such as throughout highly regulated, Mediterranean-type rivers (Almeida et al. 2014; Masó et al. 2016; Matono et al. 2018; Amat-Trigo et al. 2019).
Elevated abundances of A. alburnus in reservoirs and rivers do not seem to be controlled by the region's predators, such as Lutra lutra, which is known to be an opportunistic predator. However, despite the high abundance of introduced fish species, the diet of L. lutra has been found to contain limited occurence (Miranda et al. 2006) or no evidence (Bedmar et al. 2022) of the invading species. With specific reference to A. alburnus in Iberia, no evidence was found of otter predation on A. alburnus at a large reservoir in the River Guadiana from spraint samples collected before 2001 and in 2003 as well as in 2018, despite the high A. alburnus abundance in this last period (Bedmar et al. 2022). Similarly, the efficient non-native piscivorous Sander lucioperca was not found to reduce high abundance of A. alburnus in a study of 14 different populations across Portuguese drainage basins (Ribeiro et al. 2021).
Whenever native and non-native fishes display similar ecological traits and life histories, the risk of developing ‘strong interactions’ (sensu Schumann et al. 2015) with native species increases, as demonstrated by the European cyprinid sunbleak Leucaspius delineatus in England, where it is not native (Beyer et al. 2010). Thus, in the absence of an evolutionary history of coexistence, which promotes segregation of ecological niches, interactions between A. alburnus and Anaecypris hispanica may be expected due to their close phylogenetic relationship (Sousa-Santos et al. 2018). However, these two species currently do not live in sympatry, despite there being evidence of a high overlap in their ecological niches (da Silva et al. 2019). Behavioural interference and aggression seems to be one of the main causes of native fish exclusion due to the dominance of an invasive species (Blanco-Garrido et al. 2009; Leunda 2010; Almeida et al. 2014). Some of these mechanisms may be related to indirect competition for space and food as well as changes in feeding behaviour (i.e. prey preference or feeding rate) and activity (Keller and Brown 2008; Schumann et al. 2015). Under laboratory conditions, A. alburnus presence was found to be responsible for changes in the behavioural patterns of A. hispanica, these being related mainly to an increase in the activity rate of individual fish and a decrease in their time spent within a refuge; this suggests a potential dominance of A. alburnus when coexisting in the wild with its highly endangered congener (da Silva et al. 2019). Although no direct competition was observed in the above study between these two species, their possible coexistence in the future may have a negative effect on the general behaviour pattern of A. hispanica.
Additional risk factors associated with A. alburnus include disease transmission, habitat and foodweb alteration and genetic contamination. Alburnus alburnus is a host of Ligula intestinalis in many European countries (see Section “Pathogens and parasites”). In the Iberian Peninsula, this cestode was detected in A. alburnus collected from the River Guadiana basin, including both tributaries and reservoirs (Sánchez and Alarcón-Elbal 2014), indicating that A. alburnus can carry this parasite throughout Iberian waters and affect other native fishes (Latorre and Almeida 2019). Regarding habitat alteration, A. alburnus can affect food web structure, and thus water quality, by feeding on cladocerans, copepods, and other small invertebrates (see Section “Trophic ecology”) that play an important role in freshwater ecosystems as zooplankton (Maceda-Veiga et al. 2010; Adamczuk 2016). This aspect consequently increases productivity and biomass of algae, thereby promoting eutrophication events (Horppila and Kairesalo 1992). In terms of genetic ‘contamination’, A. alburnus has been able to affect native leuciscid species through hybridisation (Blachuta and Wikowski 1984; Crivelli and Dupont 1987; Maceda-Veiga et al. 2010; see also Section “Genetic traits”). For instance, in the River Jarama an event of hybridisation has been documented with Squalius alburnoides complex and S. pyrenaicus, resulting in a certain degree of introgression (Almodóvar et al. 2012). More recently, A. alburnus hybridisation was observed with S. carolitertii in northern Portugal, expanding the hybridisation concerns to other local Squalius endemics (e.g. Malaga chub Squalius malacitanus or Torgal chub Squalius torgalensis), which present very restricted distributions (Curto et al. 2022). The aforementioned study also described wide hybridisation across several Portuguese river basins, encompassing different Squalius species, but the authors acknowledged that this impact has been poorly studied and largely overlooked (Curto et al. 2022).
In summary, the ‘broad plasticity and capacity of A. alburnus to adapt to different environmental conditions when invading new habitats makes this species a potentially successful global invader (Masó et al. 2016; Latorre et al. 2018; Attou and Arab 2019). Using the Fish Invasiveness Screening Kit (Copp et al. 2009) in Iberia, A. alburnus was found to pose a medium risk of being invasive in Catalonia (Andreu et al. 2011), but a high risk in the Iberian Peninsula as a whole (Almeida et al. 2013). More recent screenings that used the Aquatic Species Invasiveness Screening Kit (Copp et al. 2016, 2021), which includes predictions of how future climate conditions could affect a species' invasiveness, A. alburnus was classified as posing a high-risk of being invasive in Turkey (Tarkan et al. 2017b), in non-native parts of Great Britain (Dodd et al. 2019), and in both Croatia and Slovenia (Radočaj et al. 2021) under current and future climate conditions. Whereas, a medium-risk ranking was attributed to A. alburnus for the River Ob basin in Russia (Interesova et al. 2020), and a low risk ranking for Lake Marmara in Turkey (Tarkan et al. 2017a), and in both cases the risk rank was the same under current and future climate conditions. However, future climate conditions are expected to facilitate expansion of the A. alburnus' invasive range (Lehtonen 1996). Indeed, the available data suggest that the high phenotypic plasticity shown by A. alburnus outside its native range (Masó et al. 2016; Latorre et al. 2018) could favour its invasion process, colonisation, and establishment outside its natural distribution area, and in particular Iberia.
Management
Management options to control A. alburnus are highly dependent on the extent of its invaded range. In the Iberian Peninsula, it is unfeasible to eradicate A. alburnus from large water bodies, particularly given its life-history traits described here above. In areas where the species is spatially restricted to smaller, isolated reservoirs, containment options could be feasible, such as the installation of physical barriers that would reduce dispersal rates from reservoirs to lotic systems (e.g. Rischbieter 2000). However, barrier construction is not only context dependent but also runs contrary to European efforts to reconnect rivers. Currently, the legal framework in Portugal and Spain aims for local and national administrations to develop specific management plans to control and eradicate A. alburnus in those areas where it has been introduced. Alburnus alburnus is included in both Spanish (RD 630/2013) and Portuguese (DL 92/2019) catalogues (i.e. regulation lists) of invasive species. Under both legislative acts, the introduction of A. alburnus to the natural environment (usually as ‘forage’ fish) for culture, transport or trade is completely forbidden. In fact, Spanish legislation also provides management strategies for this species. However, for more than five years since its implementation, these management plans have clearly failed to control the spread of A. alburnus in Iberia (Latorre and Almeida 2019; Martelo et al. 2021). As illegal A. alburnus introductions (and possibly translocations) are likely to continue, this propagule pressure will most likely lead to further spread of the species. Since no viable eradication or control measures are known for A. alburnus, management must focus on campaigns to educate anglers about the risks posed by A. alburnus and other non-native fishes in order to prevent their translocation, enhance compliance of the existing regulations, and to increase their enforcement. These are the only preventive actions that could reduce the species spread. Moreover, monitoring programmes for invasive alien species should be conducted across the Iberia to permit evaluation of A. alburnus population trends. At the local level, particularly for riverine systems high ecological value or threatened native fish species, where A. alburnus impacts could potentially be greater, culling campaigns should be considered (Salvador Vilariño 2015).
Future research on non-native A. alburnus populations could best focus on the species' ontogeny, growth, trophic impacts, behaviour and physiology to inform impact assessments and to implement more efficient control measures. For instance, a better understanding of A. alburnus' adverse impacts on food webs using stable isotope analysis (SIA) can be particularly useful as an integrative tool to assess long-term dietary traits (e.g. Cucherousset et al. 2007, 2012). Assessing hybridisation impacts, particularly looking at genetic introgression, is a priority because of the strong evidence of A. alburnus hybridisation with native fish species. The role of A. alburnus on disease transmission to other fish species is also an important area for future research due to the severe impacts that some novel parasites and diseases can impose on naïve native species. Finally, for population control, research is needed to understand A. alburnus reproductive behaviour in order to determine whether or not there are chemical cues that could be used in bait traps, similar to what has been developed for invasive sea lampreys in the Great Lakes region (Miehls et al. 2020).
References
Aarts BGW, Nienhuis PH (2003) Fish zonations and guilds as the basis for assessment of ecological integrity of large rivers. Hydrobiologia 500(1–3):157–178. https://doi.org/10.1023/A:1024638726162
Abramiuk II, Afanasyev SA (2017) Swimming performance of juveniles of some freshwater fishes as index of transition to nekton mode of life. Hydrobiol J 53(4):37–44. https://doi.org/10.1615/Hydrobj.v53.i4.30
Adamczuk M. (2016) Past, present, and future roles of small cladoceran Bosmina longirostris (O. F. Müller, 1785) in aquatic ecosystems. Hydrobiologia 767(1):1–11 https://doi.org/10.1007/s10750-015-2495-7
Adrović A, Žujo D, Skenderović I, Marković G., Bajrić A. (2011) Distribution of Posthodiplostomum cuticola (Digenea) metacercariae in cyprinids of the Modrac Reservoir [Bosnia and Herzegovina]. V Međunarodna Konferencija “Akvakultura i Ribarstvo”—Zbornik Predavanja, pp. 319–324. http://arhiva.nara.ac.rs/bitstream/handle/123456789/986/60%20Aquaculture%20and%20Fishery%202011%20-%20Adrovic.pdf?sequence=1
Alabaster JS, Lloyd R (1980) Water quality criteria for freshwater fish. 2nd edn. Butterworths, London and Boston, p 382
Almeida D, Grossman GD (2012) Utility of direct observational methods for assessing competitive interactions between non-native and native freshwater fishes. Fish Manage Ecol 19(2):157–166. https://doi.org/10.1111/j.1365-2400.2012.00847.x
Almeida D, Ribeiro F, Leunda PM, Vilizzi L, Copp GH (2013) Effectiveness of FISK, an invasiveness screening tool for non-native freshwater fishes, to perform risk identification assessments in the Iberian Peninsula. Risk Anal 33(8):1404–1413. https://doi.org/10.1111/risa.12050
Almeida D, Stefanoudis PV, Fletcher DH, Rangel C, da Silva E (2014) Population traits of invasive bleak Alburnus alburnus between different habitats in Iberian fresh waters. Limnologica 46:70–76. https://doi.org/10.1016/j.limno.2013.12.003
Almeida D, Fletcher DH, Rangel C, García-Berthou E, da Silva E (2017) Dietary traits of invasive bleak Alburnus alburnus (Actinopterygii, Cyprinidae) between contrasting habitats in Iberian fresh waters. Hydrobiologia 795(1):23–33. https://doi.org/10.1007/s10750-016-3052-8
Almodóvar A, Nicola GG, Leal S, Torralva M, Elvira B (2012) Natural hybridization with invasive bleak Alburnus alburnus threatens the survival of Iberian endemic calandino Squalius alburnoides complex and southern Iberian chub Squalius pyrenaicus. Biol Inv 14(11):2237–2242. https://doi.org/10.1007/s10530-012-0241-x
Amat-Trigo F, Torralva M, Ruiz-Navarro A, Oliva-Paterna FJ (2019) Colonization and plasticity in population traits of the invasive Alburnus alburnus along a longitudinal river gradient in a Mediterranean river basin. Aquat Inv 14(2):310–331. https://doi.org/10.3391/ai.2019.14.2.10
Andreu J, Pino J, Rodríguez-Labajos B, Munné A. (2011) Avaluació de l’estat i el risc d'invasió per espècies exòtiques dels ecosistemes aquàtics de Catalunya. Agència Catalana de l’Aigua, Departament de Territori i Sostenibilitat, Generalitat de Catalunya, 97 https://aca-web.gencat.cat/aca/documents/ca/aigua_medi/especies_invasores/Informe_EXOAQUA_2011.pdf
Andreu-Soler A, Oliva-Paterna FJ, Verdiell D, Torralva M. (2004) Primeras citas de Alburnus Alburnus (Linnaeus, 1758) y Tinca tinca (Linnaeus, 1758) (Actinopterygii, Cyprinidae) en la cuenca del Río Segura (Murcia, Sudeste de la Península Ibérica). Anales de Biol 26:222–224 https://www.um.es/analesdebiologia/numeros/26/PDF/10-PRIMERAS.pdf
Angelov MV, Zhelev ZM, Popgeorgiev GS, Apostolos AI. (2020) Dynamics of 24-hour upstream fish migration in the lower course of Tundzha River, Southern Bulgaria, Based on a Fish-Pass Video Monitoring. Acta Zool Bulg 72(2):289–296 www.acta-zoologica-bulgarica.eu/002322
Attou F, Arab A. (2013) Impact de l'introduction d'Alburnus alburnus (Linnaeus, 1759) sur l' espèce autochtone Barbus setivimensis (Valenciennes, 1842) (poissons cyprinidés) dans le lac de barrage de Keddara (Algérie). Rev Écol (Terre Vie). 68(2):193–202 http://documents.irevues.inist.fr/bitstream/handle/2042/55965/RevuedEcologie_2013_68_2_193.pdf?sequence=1
Attou F, Arab A (2019) Biology and ecology of the accidentally introduced bleak, Alburnus alburnus (Actinopterygii: Cypriniformes: Cyprinidae), in Keddara Dam Lake. Algeria Acta Ichthyol Piscat 49(2):119–132. https://doi.org/10.3750/AIEP/02488
Babkina IB, Petlina AP, Shestakova AS. (2013) Morphological and ecological features of the bleak (Alburnus alburnus (L.)) in the Lower Tom' River. Vestn Tomsk Gos Pedagog Univ 8(136):61–69 (In Russian)
Balaban, C. (2010) Fish fauna and some biological properties of fish species of Manyas Lake. MSc Thesis, University of Balikesir, Institue of Science and Technology, Turkey: 157 pp. (In Turkish with English abstract)
Balon EK (1975) Reproductive guilds of fishes: a proposal and definition. J Fish Res Board Can 32(6):821–864. https://doi.org/10.1139/f75-110
Balon EK. (1990) Epigenesis of an epigeneticist: the development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol Rev 1:1–42. https://journal.lib.uoguelph.ca/index.php/gir/article/view/64/83
Balzani P, Gozlan RE, Haubrock PJ (2020) Overlapping niches between two co-occurring invasive fish: the topmouth gudgeon Pseudorasbora parva and the common Alburnus alburnus. J Fish Biol 97(5):1385–1392. https://doi.org/10.1111/jfb.14499
Banha F, Diniz A, Anastácio PM (2017) The role of anglers’ perceptions and habits in biological invasions: perspectives from the Iberian Peninsula. Aquat Conserv 27(1):51–64. https://doi.org/10.1002/aqc.2677
Baras E, Lambert H, Philippart JC (1994) A comprehensive assessment of the failure of Barbus barbus spawning migrations through a fish pass in the canalized River Meuse (Belgium). Aquat Living Res 7(3):181–189. https://doi.org/10.1051/alr:1994020
Baruš V, Prokeš M (1993) Fecundity of the bleak (Alburnus alburnus) in the Věstonice reservoir. Folia Zool 42(3):281–288
Baruš V, Prokeš M, Zukal J (1998) A biometric study of four populations of the bleak (Alburnus alburnus) from the Czech Republic. Folia Zool 47(2):135–144
Barzegar M, Jalali B. (2009) Crustacean parasites of fresh and brackish (Caspian Sea) water fishes of Iran. J Agric Sci Technol 11(2):161–171 https://jast.modares.ac.ir/article-23-11140-en.pdf
Baska F, Molnár K (1988) Blood stages of Sphaerospora spp. (Myxosporea) in cyprinid fishes. Dis Aquat Organ 5:23–28. https://doi.org/10.3354/dao005023
Battes KW, Palaghiţă DK (1999) Research concerning the growth, structure production of bleak population (Alburnus alburnus L.) from Bacau Dam Reservoir. Stud Cercet 4:273–282
Bedmar S, Blanco-Garrido F, Delibes M, Clavero M (2022) Temporal and spatial patterns in the shifting of otter diet to invasive prey after river damming. River Res Appl 38(8):1–10. https://doi.org/10.1002/rra.3961
Benitez JP, Nzau Matondo B, Dierckx A, Ovidio M (2015) An overview of potamodromous fish upstream movements in medium-sized rivers, by means of fish passes monitoring. Aquat Ecol 49(4):481–497. https://doi.org/10.1007/s10452-015-9541-4
Berg LS. (1933) Ryby presnykh vod SSSR i sopredel'nykh stran (Freshwater fishes of Soviet Union and adjacent countries). Akademii Nauk SSSR: 545–903 (In Russian)
Berrebi P, Dupont F, Cattaneo-Berrebi G, Crivelli AJ (1989) An isozyme study of the natural cyprinid hybrid Alburnus alburnus×Rutilus rubilio in Greece. J Fish Biol 34(2):307–313. https://doi.org/10.1111/j.1095-8649.1989.tb03311.x
Beyer K, Gozlan RE, Copp GH (2010) Social network properties within a fish assemblage invaded by non-native sunbleak Leucaspius delineatus. Ecol Model 221(17):2118–2122. https://doi.org/10.1016/j.ecolmodel.2010.06.002
Biggs J, Williams P, Whitfield M, Nicolet P, Weatherby A (2005) 15 years of pond assessment in Britain: results and lessons learned from the work of pond conservation. Aquat Cons 15(6):693–714. https://doi.org/10.1002/aqc.745
Billard R (1997) Les poissons d’eau douce des rivières de France: Identification, inventaire et répartition des 83 espèces. Delachaux et Niestlé, Paris, p 192
Bíró P (1980) First two-year growth of the bleak, Alburnus alburnus, in Lake Balaton [Hungary]. Aquac Hung 2:168–180
Bíró P (1990) Population structure, growth, P/B–ratio and egg–production of bleak (Alburnus alburnus L.) in lake Balaton. Aquac Hung 6:105–118
Bíró P, Muskó IB. (1995) Population dynamics and food of bleak (Alburnus alburnus L.) in the littoral zone of Lake Balaton, Hungary. Hydrobiologia. 310(3):139–149 https://doi.org/10.1007/BF00015532.
Blachuta J, Witkowski A. (1984) Natural hybrids Alburnus alburnus (L.)×Rutilus rutilus (L.), Alburnus alburnus (L.)×Blicca bjoerkna (L.) and Alburnus alburnus (L.)×Abramis brama (L.) from the Oder River. Acta Hydrobiol 25(26):189–203.
Blanc A, Lamoroux N (2007) Large-scale intraspecific variation in life-history traits of European freshwater fish. J Biogeogr 34(5):862–875. https://doi.org/10.1111/j.1365-2699.2006.01654.x
Blanck A, Tedesco PA, Lamouroux N (2007) Relationships between life-history strategies of European freshwater fish species and their habitat preferences. Freshwat Biol 52(5):843–859. https://doi.org/10.1111/j.1365-2427.2007.01736.x
Blanco-Garrido F, Miguel C, Prenda J. (2009) Jarabugo (Anaecypris hispanica) and freshwater blenny (Salaria fluviatilis): habitat preferences and relationship with exotic fish species in the Middle Guadiana Basin. Limnetica 28(1):139–148 https://doi.org/10.23818/limn.28.10
Hugh, C (Ed) (1911) Bleak. Encyclopædia Britannica 11th ed. Cambridge University Press, 55 pp.
Bogacka-Kapusta E, Kapusta A. (2007) The diet of roach, Rutilus rutilus (L.), and bleak, Alburnus alburnus (L.) larvae and fry in the shallow littoral zone of a heated lake. Arch Pol Fish 15(4):401–413. https://fal.infish.com.pl/index.php/FisheriesAndAquaticLife/article/view/224/224
Bohl E (1979) Diel pattern of pelagic distribution and feeding in planktivorous fish. Oecologia 44(3):368–375. https://doi.org/10.1007/BF00545241
Bohl E (1982) Food supply and prey selection in planktivorous cyprinidae. Oecologia 53(1):134–138. https://doi.org/10.1007/BF00377148
Bøhn T, Sandlund OT, Amundsen PA, Primicerio R (2004) Rapidly changing life history during invasion. Oikos 106(1):138–150. https://doi.org/10.1111/j.0030-1299.2004.13022.x
Bonisławska M, Formicki K, Korzelecka-Orkisz A, Winnicki A. (2001) Fish egg size variability: biological significance. Electron J Polish Agric Univ 4(2):14 pp. www.ejpau.media.pl/articles/volume4/issue2/fisheries/art-02.pdf
Borowik M. (1968) Dynamics of infection of various age groups of Alburnus Alburnus L. in the Zegrzyński Reservoir. Acta Parasitol Pol 15(40–50):321–332.
Bouzid W, Štefka J, Hypša V, Lek S, Scholz T, Legal L, Hassine O, Loot G (2008) Geography and host specificity: two forces behind the genetic structure of the freshwater fish parasite Ligula intestinalis (Cestoda: Diphyllobothriidae). Int J Parasitol 38(12):1465–1479. https://doi.org/10.1016/j.ijpara.2008.03.008
Bozorgnia A, Sharifi N, Youssefi MR, Barzegar M. (2018) Acipenser stellatus as a new host record for Lernaea cyprinacea Linnaeus, 1758 (Crustacea; Copepoda), a parasite of freshwater fishes in Iran. J Aquac Mar Biol 7(3):123–125 https://doi.org/10.15406/jamb.2018.07.00197
Britton JR (2007) Reference data for evaluating the growth of common riverine fishes in the UK. J Appl Ichthyol 23(5):555–560. https://doi.org/10.1111/j.1439-0426.2007.00845.x
Britton JR, Gozlan RE (2013) How many founders for a biological invasion? Predicting introduction outcomes from propagule pressure. Ecology 94(11):2558–2566. https://doi.org/10.1890/13-0527.1
Brodersen J, Ådahl E, Brönmark C, Hansson LA (2008) Ecosystem effects of partial fish migration in lakes. Oikos 117(1):40–46. https://doi.org/10.1111/j.2007.0030-1299.16118.x
Buj I, Vukić J, Šanda R, Perea S, Ćaleta M, Marčić Z, Bogut I, Povž M, Mrakovčić M. (2010) Morphological comparison of bleaks (Alburnus, Cyprinidae) from the Adriatic Basin with the description of a new species. J Vertebr Biol 59(2):129–141 https://doi.org/10.25225/fozo.v59.i2.a8.2010
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Env Manage 30(4):492–507. https://doi.org/10.1007/s00267-002-2737-0
Burnham KP, Anderson DR (2003) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York, p 512
Caffara M, Gustinelli A, Mazzone A, Fioravanti ML (2020) Multiplex PCR for simultaneous identification of the most common European Opisthorchiid and Heterophyid in fish or fish products. Food Waterborne Parasitol 19:e00081 https://doi.org/10.1016/j.fawpar.2020.e00081
Camacho JPM, Sharbel TF, Beukeboom LW (2000) B-chromosome evolution. Philos Trans R Soc Lond B Biol Sci 355(1394):163–178. https://doi.org/10.1098/rstb.2000.0556
Cano-Barbacil C, Radinger J, Argudo M, Rubio-Gracia F, Vila-Gispert A, García-Berthou E (2020) Key factors explaining critical swimming speed in freshwater fish: a review and statistical analysis for Iberian species. Sci Rep 10(1):18947. https://doi.org/10.1038/s41598-020-75974-x
Cardona L, Hereu B, Torras X, Royo P (2002) Primera cita de l’alburn (Alburnus albnurnus L.) i noves dades sobre la presencia de la madrilleta vera (Rutilus rutilus L.) a la Muga. Butll Inst Catal D’hist Nat 70:111–112
Čech M, Čech P, Kubečka J, Prchalová M, Draštík V (2008) Size selectivity in summer and winter diets of great cormorant (Phalacrocorax carbo): does it reflect season-dependent difference in foraging efficiency? Waterbirds 31(3):438–447. https://doi.org/10.1675/1524-4695-31.3.438
Čech M, Vejřík L (2011) Winter diet of great cormorant (Phalacrocorax carbo) on the River Vltava: estimate of size and species composition and potential for fish stock losses. Folia Zool 60(2):129–142. https://doi.org/10.25225/fozo.v60.i2.a7.2011
Cech G, Sándor D, Molnár K, Varga A, Caffara M, Fioravanti ML, Buchmann K, Székely C (2021) Digenean trematodes in Hungarian freshwater aquacultures. Food Waterborne Parasitol 22:e00101 https://doi.org/10.1016/j.fawpar.2020.e00101
Černý J, Copp GH, Kováč V, Gozlan RE, Vilizzi L (2003) Initial impact of the Gabčíkovo hydroelectric scheme on 0+ fish assemblages in the Slovak flood plain. River Danube River Res Appl 19(7):749–766. https://doi.org/10.1002/rra.716
Chappaz R, Brun G, Olivari G. (1987) Mise en évidence de différences de régime alimentaire dans une population d’ablettes Alburnus alburnus (L.) dans le Lac de Sainte-Croix. Conséquences sur la croissance et la fécondité. Ann Limnol 23(3):245–252 https://doi.org/10.1051/limn/1987022
Chitravadivelu K (1974) Growth, age composition, population density, mortality, production and yield of Alburnus alburnus (Linnaeus, 1758) and Rutilus rutilus (Linnaeus, 1758) in the inundation region of Danube-Žofín. Acta Carol Biol 1972:1–76
Chunchukova M, Kirin D (2020) Helminth fauna of some cyprinid fish species from lower stream of River Tundzha, Bulgaria. International May Conference On Strategic Management—IMCSM20, September 25–27, Bor, Serbia 16(1):465–473.
Chunchukova M., Kuzmanova D (2017) Arsenic content in parasite-host system: Alburnus alburnus—Pomphorhynchus laevis and the impact of the Acanthocephalan on his host. Agric Sci 9(22):43–47. http://agrarninauki.au-plovdiv.bg/wp-content/uploads/2018/11/06_22_2017.pdf
Chunchukova M, Kirin D, Shukerova S, Kuzmanova D (2017) Accumulation of lead in Barbus barbus, Alburnus alburnus and in their common parasite Pomphorhynchus tereticollis from River Danube (Vetren Area), Bulgaria. Scientific Papers, Series D. Animal Sci LX:323–326 http://animalsciencejournal.usamv.ro/pdf/2017/Art57.pdf
Chunchukova M, Kirin D, Kuzmanova D (2019a) Gastrointestinal helminth fauna and helminth communities of bleak (Alburnus alburnus, L. 1758) from lower section of Danube River, Bulgaria. Bulg J Vet Med 22(3):344–352 https://doi.org/10.15547/bjvm.2082
Chunchukova M, Kirin D, Kuzmanova D (2019b) New data for helminth communities of Alburnus alburnus (Linnaeus, 1758) from Maritsa River, Bulgaria. Sci Papers Ser D Animal Sci LXII(1):439–444 http://animalsciencejournal.usamv.ro/pdf/2019b/issue_1/Art66.pdf
Chunchukova M. (2018) Differences in accumulation of nickel in infected and uninfected with Pomphorhynchus laevis specimens of Alburnus alburnus from the freshwater ecosystem of Danube River, Bulgaria. Agric Sci 10(24):29–34 http://agrarninauki.au-plovdiv.bg/wp-content/uploads/2019/01/Agrarni-Nauki_24_2018_5.pdf
Clavero M (2011) Assessing the risk of freshwater fish introductions into the Iberian Peninsula. Freshw Biol 56(10):2145–2155. https://doi.org/10.1111/j.1365-2427.2011.02642.x
Clavero M, Esquivias J, Qninba A, Riesco M, Calzada J, Ribeiro F, Fernández N, Delibes M (2015) Fish invading deserts: non-native species in arid Moroccan rivers. Aquat Conserv 25(1):49–60. https://doi.org/10.1002/aqc.2487
Coad BW. (2006) Endemicity in the freshwater fishes of Iran. Iran J Anim Biosyst 1(1):1–13 https://doi.org/10.22067/ijab.v1i1.36733
Copp GH (1989) The habitat diversity and fish reproductive function of floodplain ecosystems. Environ Biol Fish 26(1):1–27. https://doi.org/10.1007/BF00002472
Copp GH (1992) Comparative microhabitat use of cyprinid larvae and juveniles in a lotic floodplain channel. Environ Biol Fish 33(1–2):181–193. https://doi.org/10.1007/BF00002563
Copp GH (1993) The upper River Rhône revisited: an empirical model of microhabitat use by 0+ juvenile fishes. Folia Zool 42(4):329–340
Copp GH, Peňáz M (1988) Ecology of fish spawning and nursery zones in the flood plain, using a new sampling approach. Hydrobiologia 169(2):209–224. https://doi.org/10.1007/BF00007312
Copp GH, Olivier JM, Peňáz M, Roux AL (1991) Juvenile fishes as functional describers of fluvial ecosystem dynamics: Applications on the River Rhône. France Regul River 6(2):135–145. https://doi.org/10.1002/rrr.3450060209
Copp GH, Guti G, Rovný B, Černý J (1994) Hierarchical analysis of habitat use by 0+ juvenile fish in the Hungarian/Slovak flood plain of the River Danube. Environ Biol Fish 40(4):329–348. https://doi.org/10.1007/BF00005279
Copp GH, Vranovský M, Černý J, Kováč V (2005c) Diel dynamics of young fishes and zooplankton in a lentic side-channel of the River Danube. Biol Bratisl 60(2):179–188
Copp GH, Vilizzi L, Mumford JD, Fenwick GV, Godard MJ, Gozlan RE (2009) Calibration of FISK, an invasive-ness screening tool for non-native freshwater fishes. Risk Anal 29(3):457–467. https://doi.org/10.1111/j.1539-6924.2008.01159.x
Copp GH, Vilizzi L, Tidbury HJ, Stebbing PD, Tarkan AS, Miossec L, Goulletquer P (2016) Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manage Biol Inv 7(4):343–350. https://doi.org/10.3391/mbi.2016.7.4.04
Copp GH, Britton JR, Wesley KJ, Davison PI (2017) Non-native fish dispersal as a contaminant of aquatic plant consignments–a case study from England. Manag Biol Inv 8(3):437–442. https://doi.org/10.3391/mbi.2017.8.3.17
Copp GH, Cellot B. (1988) Drift of embryonic and larval fishes, especially Lepomis gibbosus (L.), in the Upper Rhône River. J Freshwat Ecol 4(4):419–424 https://doi.org/10.1080/02705060.1988.9665193
Copp GH, Fox MG. (2007) Growth and life history traits of introduced pumpkinseed (Lepomis gibbosus) in Europe, and the relevance to invasiveness potential. In: Freshwater Bioinvaders: Profiles, Distribution, and Threats. F. Gherardi (Ed.), Springer, Berlin, pp. 289–306 https://doi.org/10.1007/978-1-4020-6029-8_15
Copp GH, Doherty S, Faulkner H, Watkins MS, Majecki J (2002) Diel drift behaviour of fish eggs and larvae, in particular barbel, Barbus barbus (L.), in an English chalk stream. Fish Manag Ecol 9(2):95–104 https://doi.org/10.1046/j.1365-2400.2002.00286.x
Copp GH, Bianco PG, Bogutskaya N, Erős T, Falka I, Ferreira MT, Fox MG, Freyhof J, Gozlan RE, Grabowska J, Kováč V, Moreno-Amich R, Naseka AM, Peňáz M, Povž M, Przybylski M, Robillard M, Russell IC, Stakėnas S, Šumer S, Vila-Gispert A, Wiesner C (2005a) To be, or not to be, a non-native freshwater fish? J Appl Lchthyol 21(4):242–262 10111/j.1439–0426.2005a.00679.x
Copp GH, Garthwaite R, Gozlan RE (2005b) Risk identification and assessment of non-native freshwater fishes: concepts and perspectives on protocols for the UK. Cefas Science Technical Report No. 129, Cefas, Lowestoft, 32 p https://doi.org/10.13140/RG.2.2.13947.05926
Copp GH, Vilizzi L, Wei H, Li S, Piria M, Al-Faisal AJ, Almeida D, Atique U, Al-Wazzan Z, Bakiu R, Bašić T, Bui TD, Canning-Clode J, Castro N, Chaichana R, Çoker T, Dashinov D, Ekmekçi FG, Erős T, Ferincz Á, Ferreira T, Giannetto D, Gilles ASJr, Głowacki Ł, Goulletquer P, Interesova E, Iqbal S, Jakubčinová K, Kanongdate K, Kim J-E, Kopecký O, Kostov V, Koutsikos N, Kozic S, Kristan P, Kurita Y, Lee H-G, Leuven RSEW, Lipinskaya T, Lukas J, Marchini A, González Martínez AI, Masson L, Memedemin D, Moghaddas SD, Monteiro J, Mumladze L, Naddafi R, Năvodaru I, Olsson KH, Onikura N, Paganelli P, Pavia RTJr, Perdikaris C, Pickholz R, Pietraszewski D, Povž M, Preda C, Ristovska M, Rosíková K, Santos JM, Semenchenko V, Senanan W, Simonović P, Smeti E, Števove B, Švolíková K, Ta KAT, Tarkan AS, Top N, Tricarico E, Uzunova E, Vardakas L, Verreyken H, Zięba G, Mendoza R (2021) Speaking their language—development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environ Modell Softw 135:104900 https://doi.org/10.1016/j.envsoft.2020.104900
Costedoat C, Pech N, Salducci MD (2005) Evolution of mosaic hybrid zone between invasive and endemic species of Cyprinidae through space and time. Biol J Linn Soc 85(2):135–155. https://doi.org/10.1111/j.1095-8312.2005.00478.x
Crivelli AJ, Dupont F (1987) Biometrical and biological features of Alburnus alburnus × Rutilus rubilio natural hybrids from Lake Mikri Prespa, northern Greece. J Fish Biol 31(6):721–733. https://doi.org/10.1111/j.1095-8312.2005.00478.x
Cucherousset J, Aymes JC, Santoul F, Céréghino R (2007) Stable isotope evidence of trophic interactions between introduced brook trout (Salvelinus fontinalis) and native brown trout (Salmo trutta) in a mountain stream of southwest France. J Fish Biol 71(Suppl D):210–223. https://doi.org/10.1111/j.1095-8649.2007.01675.x
Cucherousset J, Bouletreau S, Martino A, Roussel J-M, Santoul F (2012) Using stable isotope analyses to determine the ecological effects of non-native fishes. Fish Manage Ecol 19(2):111–119. https://doi.org/10.1111/j.1365-2400.2011.00824.x
Cucherousset J, Olden JD (2011) Ecological impacts of nonnative freshwater fishes. Fisheries 36(5):215–230 https://doi.org/10.1080/03632415.2011.574578
Curto M, Morgado-Santos M, Alexandre CM, Alves MJ, Gante HF, Gkenas C, Medeiros JP, Pinheiro P, Almeida PR, Magalhães MF, Ribeiro F (2022) Widespread hybridization between invasive bleak (Alburnus alburnus) and Iberian chubs (Squalius spp.): a neglected conservation threat. Fishes 2022(7):247 https://doi.org/10.3390/fishes7050247
Djikanovic V, Momir P, Vera N, Predrag S, Predrag C (2012) Parasitofauna of freshwater fishes in the Serbian open waters: a checklist of parasites of freshwater fishes in Serbian open waters. Rev Fish Biol Fish 22(1):297–324. https://doi.org/10.1007/s11160-011-9226-6
Doadrio I, Perea S, Garzón-Heydt P, González JL (2011) Ictiofauna continental española. Bases para su seguimiento. DG Medio Natural y Política Forestal. MARM, Madrid, 616 pp.
Doadrio I (2001) Atlas y libro rojo de los peces continentales de España. Organismo Autónomo Parques, Madrid, Spain, 358 pp.
Dodd JA, Vilizzi L, Bean CW, Davison PI, Copp GH (2019) At what spatial scale should risk screenings of translocated freshwater fishes be undertaken—River basin district or climo-geographic designation? Biol Conserv 230:122–130. https://doi.org/10.1016/j.biocon.2018.12.002
Dzika E, Kuształa M, Kozłowski J (2008) Metazoan parasite fauna of fish species from Lake Kortowskie. Arch Pol Fish 16(1):75–86. https://doi.org/10.2478/s10086-008-0006-4
El-Fiky N, Wieser W (1988) Life styles and patterns of development of gills and muscles in larval cyprinids (Cyprinidae; Teleostei). J Fish Biol 33(1):135–145. https://doi.org/10.1111/j.1095-8649.1988.tb05455.x
El-Fiky N, Hinterleitner S, Wieser W (1987) Differentiation of swimming muscles and gills, and development of anaerobic power in the larvae of cyprinid fish (Pisces, Teleostei). Zoomorphology 107(2):126–132. https://doi.org/10.1007/BF00312122
Elliott JM (1987) Population regulation in contrasting populations of trout Salmo trutta in two Lake District streams. J Anim Ecol 56(1):83–98. https://doi.org/10.2307/4801
Elvira B (1995) Native and exotic freshwater fishes in Spanish river basins. Freshwat Biol 33(1):103–108. https://doi.org/10.1111/j.1365-2427.1995.tb00390.x
Elvira B, Almodóvar A (2001) Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. J Fish Biol 59(sA):323–331 https://doi.org/10.1111/j.1095-8649.2001.tb01393.x
Entz B, Lukacsovics F (1957) Untersuchungen im Winterhalbjahr an einigen Balaton-See-Fischen zwecks Feststellung ihrer Ernährungs-, Wachstums-und Vermehrungsumstände. Annal Inst Biol Tihany Hung 24:71–86.
Erdoğan Z, Koç HT (2017) An investigation on length-weight relationships, condition and reproduction of the bleak, Alburnus alburnus (L.) population in Çaygören Dam Lake (Balikesir), Turkey. J Balikesir Univ Inst Sci Technol 19(1):39–50. http://fbed.balikesir.edu.tr/index.php/dergi/article/view/360/331
Filipe AF, Marques TA, Tiago P, Ribeiro F, Moreira Da Costa L, Cowx IG, Collares-Pereira MJ (2004) Selection of priority areas for fish conservation in Guadiana River Basin. Iber Penins Conserv Biol 18(1):189–200. https://doi.org/10.1111/j.1523-1739.2004.00620.x
Fisher R, Hogan JD (2007) Morphological predictors of swimming speed: a case study of pre-settlement juvenile coral reef fishes. J Exp Biol 210(14):2436–2443. https://doi.org/10.1242/jeb.004275
Fladung E, Scholten M, Thiel R (2003) Modelling the habitat preferences of preadult and adult fishes on a shoreline of the large, lowland Elbe River. J Appl Ichthyol 19(5):303–314. https://doi.org/10.1046/j.1439-0426.2003.00506.x
Fobert E, Zięba G, Vilizzi L, Godard MJ, Fox MG, Stakėnas S, Copp GH (2013) Predicting non-native fish dispersal under conditions of climate change: case study in England of dispersal and establishment of pumpkinseed Lepomis gibbosus in a floodplain pond. Ecol Freshwat Fish 22(1):106–116. https://doi.org/10.1111/eff.12008
Forró B, Eszterbauer E (2016) Correlation between host specificity and genetic diversity for the muscle-dwelling fish parasite Myxobolus pseudodispar: examples of myxozoan host-shift?. Folia Parasit 63:019 https://doi.org/10.14411/fp.2016.019
Fouzia A, Abdeslem A (2012) Environmental determinism of sex-ratio in the bleak, Alburnus alburnus (Linnaeus, 1758) (Cyprinidae) in Keddara dam. Alger Indian J Fish 59(4):7–10
Fox MG, Villeneuve F, Copp GH (2011) Seasonal reproductive allocation, local-scale variation and environmental influences on life history traits of introduced pumpkinseed (Lepomis gibbosus) in southern England. Fund Appl Limnol 178(3):231–243. https://doi.org/10.1127/1863-9135/2011/0178-0231
Froese R, Pauly D (Eds) (2021) FishBase. World Wide Web electronic publication. www.fishbase.org, version (08/2022).
Gagliardi A, Martinoli A, Preatoni D, Wauters LA, Tosi G (2007) From mass of body elements to fish biomass: a direct method to quantify food intake of fish eating birds. Hydrobiologia 583(1–3):213–222. https://doi.org/10.1007/s10750-006-0528-y
Gama J, Nyberg M (2017) Length-weight relationships of six freshwater fish species from Lake Kirkkojärvi. Finl Croat J Fish 75(4):155–160. https://doi.org/10.1515/cjf-2017-0020
García-Berthou E, Almeida D, Benejam L, Magellan K, Bae M-J, Casals F, Merciai R (2015) Impacto ecológico de los peces continentales introducidos en la Península Ibérica. Ecosistemas 24(1):36–42. https://doi.org/10.7818/ECOS.2015.24-1.07
Garner P (1996) Microhabitat use and diet of 0+ cyprinid fishes in a lentic, regulated reach of the River Great Ouse. England J Fish Biol 48(3):367–382. https://doi.org/10.1111/j.1095-8649.1996.tb01434.x
Gąsowska M (1974) Biometric and ecological studies on the bleak, Alburnus alburnus (Linnaeus)(Pisces, Cyprinidae) from different bodies of water in Poland, in connexion with the geographic variability of this species. Annal Zool 31(4):373–405
Gehrke PC, Harris JH (2001) Regional-scale effects of flow regulation on lowland riverine fish communities in New South Wales. Aust Regul Riv 17(4–5):369–391. https://doi.org/10.1002/rrr.648
Gerdeaux D, Anneville O, Hefti D (2006) Fishery changes during re-oligotrophication in 11 peri-alpine Swiss and French lakes over the past 30 years. Acta Oecol 30(2):161–167. https://doi.org/10.1016/j.actao.2006.02.007
Gido KB, Franssen NR (2007) Invasion of stream fishes into low trophic positions. Ecol Freshwat Fish 16(3):457–464. https://doi.org/10.1111/j.1600-0633.2007.00235.x
Golemansky V (2017) Review and check-list of coccidian parasites (Apicomplexa: Eucoccidiorida) of humans and animals in Bulgaria. Acta Zool Bulgar 69(2):151–166
Golub D, Bukva M, Dekić R (2019) Morphological characteristics and condition of bleak Alburnus alburnus (Teleostei; Cyprinidae) from the Bosna River. SKUP 10:16–28. https://doi.org/10.7251/SKPEN191001016G
Gozlan RE (2008) Introduction of non-native freshwater fish: Is it all bad? Fish Fish 9(1):106–115. https://doi.org/10.1111/j.1467-2979.2007.00267.x
Gozlan RE, Mastrorillo S, Dauba F, Tourenq JN, Copp GH (1998) Multi-scale analysis of habitat use by 0+ fishes during late summer in the River Garonne (France). Aquat Sci 60(2):99–117. https://doi.org/10.1007/s000270050028
Grift RE, Buijse AD, Van Densen WLT, Machiels MAM, Kranenbarg J, Klien Breteler JGP, Backx JJGM (2003) Suitable habitats for 0-group fish in rehabilitated floodplains along the lower river Rhine. River Res Appl 19(4):353–374. https://doi.org/10.1002/rra.711
Grimaldi E (1971) Heavy mortality in populations of bleak (Alburnus alborella) in the lakes of north Italy, caused by a gill infection due to fungi of the genus Branchiomyces. Riv Ital Piscicolt Ittiopatol 61:11–14
Grupcheva G, Golemansky V, Margaritov N (2006) Catalogus faunae bulgaricae. Protozoan Parasites Fishes 6:80
Haberlehner E (1988) Comparative analysis of feeding and schooling behaviour of the Cyprinidae Alburnus alburnus (L., 1758), Rutilus rutilus (L., 1758), and Scardinius erythrophthalmus (L., 1758) in a backwater of the Danube near Vienna. Inter Rev Ges Hydrobiol 73(5):537–546
Hansen H, Alarcón M (2019) First record of the asian fish tapeworm Schyzocotyle (Bothriocephalus) acheilognathi (Yamaguti, 1934) in Scandinavia. BioInv Rec 8(2):437–441. https://doi.org/10.3391/bir.2019.8.2.26
Harris MT, Wheeler A (1974) Ligula infestation of bleak Alburnus alburnus (L.) in the tidal Thames. J Fish Biol 6(2):181–188 https://doi.org/10.1111/j.1095-8649.1974.tb04535.x
Hering-Hagenbeck S, Schuster R (1996) A focus of opisthorchiidosis in Germany. Appl Parasitol 37(4):260–265
Herzig A (1994) Predator-prey relationships within the pelagic community of Neusiedler See. Hydrobiologia 275(1):81–96. https://doi.org/10.1007/978-94-017-2460-9_8
Hladík M, Kubečka J (2003) Fish migration between a temperate reservoir and its main tributary. Hydrobiologia 504(1–3):251–266. https://doi.org/10.1023/B:HYDR.0000008525.46939.42
Hofer R, Nasir Uddin A (1985) Digestive processes during the development of roach, Rutilus rutilus (L.). J Fish Biol 26(6):683–689 https://doi.org/10.1111/j.1095-8649.1985.tb04308.x
Hohausová E, Copp GH, Jankovský P (2003) Movement of fish between a river and its backwater: diel activity and relation to environmental gradients. Ecol Freshwat Fish 12(2):107–117. https://doi.org/10.1034/j.1600-0633.2003.00014.x
Holubová M, Blabolil P, Čech M, Vašek M, Peterka J (2020) Species-specific schooling behaviour of fish in the freshwater pelagic habitat: an observational study. J Fish Biol 97(1):64–74. https://doi.org/10.1111/jfb.14326
Holubová M, Čech M, Vašek M, Peterka J (2019) Density dependent attributes of fish aggregative behaviour. PeerJ 7:e6378 https://doi.org/10.7717/peerj.6378
Horppila J, Kairesalo T (1992) Impacts of bleak (Alburnus alburnus) and roach (Rutilus rutilus) on water quality, sedimentation and internal nutrient loading. Hydrobiologia 243–244(1):323–331. https://doi.org/10.1007/978-94-011-2745-5_33
Hristovski N, Stojanovski S, Baker RA, Petrovic Z, Rusinek O, Čakić P (2006) Parasite fauna of the fishes from Lake Prespa and life cycle of economically important and most frequently found parasite species. Monography. University "Sv. Kliment Ohridski". Faculty of Biotechnical Sciences. Bitola, North Macedonia. (In Macedonian)
Hristovski N, Stojanovski S, Talevski T, Blažeković-Dimovska D (2012) The fish parasite fauna and the fish of Lake Prespa. Monography. University “St. Kliment Ohridski”, Bitola, North Macedonia. (In Macedonian)
Interesova EA (2016) Alien fish species in the Ob River basin. Russian J Biol Inv 7(2):156–167. https://doi.org/10.1134/S2075111716020089
Interesova EA, Chakimov RM (2015) Bleak Alburnus alburnus (Cyprinidae) in the Inya River (southwestern Siberia). J Ichthyol 55(2):282–284. https://doi.org/10.1134/S0032945215020071
Interesova E, Vilizzi L, Copp GH (2020) Risk screening of the potential invasiveness of non-native freshwater fishes in the River Ob basin (West Siberian Plain, Russia). Reg Environ Change 20:64. https://doi.org/10.1007/s10113-020-01644-3
Ioganzen BG (1947) Essays on geography and genesis of Siberian ichthyofauna. Message II. Ecological-geographic description of fishes of the Ob River basin. Uchenye Zap Tomsk Gos Univ 3:43–60 ((In Russian))
Ivlev VS (1960) On the utilisation of food by planktophage fishes. B Math Biophys 22(4):371–389. https://doi.org/10.1007/BF02476721
Jachner A (1997) The response of bleak to predator odour of unfed and recently fed pike. J Fish Biol 50(4):878–886. https://doi.org/10.1111/j.1095-8649.1997.tb01980.x
Jachner A (1996) Alarm reaction in bleak (Alburnus alburnus (L.), Cyprinidae) in response to chemical stimuli from injured conspecifics. Hydrobiologia 325(2):151–155 https://doi.org/10.1007/BF00028275
Jakubas D, Mioduszewska A (2005) Diet composition and food consumption of the grey heron (Ardea cinerea) from breeding colonies in northern Poland. Eur J Wildl Res 51(3):191–198. https://doi.org/10.1007/s10344-005-0096-x
Jalali B (1998) Parasites and parasitic diseases of fresh water fishes of Iran. Iranian Fisheries Organization Press, Tehran, Iran: 562 pp (In Persian)
Josefovičová A (2019) Denní aktivita oukleje obecné Alburnus alburnus ve vztahu k potravní nabídce. [Diel activity of common bleak Alburnus alburnus in response to prey availability]. MSc Thesis, Charles University, Prague. www.nusl.cz/ntk/nusl-405249 [In Czech]
Kakacheva-Avramova D (1972) Contribution to the Helminth Fauna of Fish from Tundzha River. Izv Tsentralnata Khelmintol Lab 15:89–107 ((In Bulgarian))
Kangur A, Kangur P (1998) Diet composition and size-related changes in the feeding of pikeperch, Stizostedion lucioperca (Percidae) and pike, Esox lucius (Esocidae) in the lake Peipsi (Estonia). Ital J Zool 65(Suppl 1):255–259. https://doi.org/10.1080/11250009809386828
Kara HM (2012) Freshwater fish diversity in Algeria with emphasis on alien species. Eur J Wild Res 58(1):243–253. https://doi.org/10.1007/s10344-011-0570-6
Karst H (1968) Unterwasserbeobachtungen an sozialen Gruppierungen von Süßwasserfischen außerhalb der Laichzeit. Int Rev Ges Hydrobiol 53(4):573–599. https://doi.org/10.1002/iroh.19680530406
Keckeis H, Schiemer F (1990) Consumption, growth and respiration of bleak, Alburnus alburnus ( L.), and roach, Rutilus rutilus ( L.), during early ontogeny. J Fish Biol 36(6):841–851 https://doi.org/10.1111/j.1095-8649.1990.tb05632.x
Keckeis H, Schiemer F (1992) Food consumption and growth of larvae and juveniles of three cyprinid species at different food levels. Environ Biol Fish 33(1–2):33–45. https://doi.org/10.1007/BF00002551
Keith P, Allardi J (2001) Atlas des Poissons d’Eau Douce de France. Muséum National d’Histoire Naturelle, Paris, p 387
Keller K, Brown C (2008) Behavioural interactions between the introduced plague minnow Gambusia holbrooki and the vulnerable native australian ornate rainbowfish Rhadinocentrus ornatus, under experimental conditions. J Fish Biol 73(7):1714–1729. https://doi.org/10.1111/j.1095-8649.2008.02045.x
Kennard MJ, Arthington AH, Pusey BJ, Harch BD (2005) Are alien fish a reliable indicator of river health? Freshwat Biol 50(1):174–193. https://doi.org/10.1111/j.1365-2427.2004.01293.x
Ketmaier V, Finamore F, Largiadèr C, Milone M, Bianco PG (2009) Phylogeography of bleaks Alburnus spp. (Cyprinidae) in Italy, based on cytochrome b data. J Fish Biol 75(5):997–1017 https://doi.org/10.1111/j.1095-8649.2009.02357.x
Khalko VV (2018) Sexual dimorphism of physiological and biochemical state of trunk muscle in bleak (Alburnus alburnus L.) (Cypriniformes, Cyprinidae) spawners of the Rybinsk Reservoir in the prespawning period. Inland Water Biol 11(2):214–218 https://doi.org/10.1134/S1995082918020074
Kiabi BH, Abdoli A, Naderi M (1999) Status of the fish fauna in the South Caspian Basin of Iran. Zool Middle East 18(1):57–65 https://doi.org/10.1080/09397140.1999.10637782
Kirin DA (2003) Biodiversity and ecological evaluation of the helminth communities of Barbus cyclolepis and Alburnus alburnus from Arda River. Bulgaria Exp Pathol Parasitol 6(11):44–50
Kirin D, Buchvarov G, Kuzmanov N (2002) Biological diversity and ecological evaluation of the fresh water ecosystems of the Arda River. J Environ Protect Ecol 3(2):449–456 http://www.jepe-journal.info/vol-3-no-2/%CF%83%CE%B5%CE%BB449-456.pdf?attredirects=0.
Kirin AD (2001) Biodiversity of the helminth communities of Leuciscus cephalus and Alburnus alburnus from Kardzhali Reservoir. Comptes Rendus l'Acad Bulgare Sci 54(11):11–97 http://adsabs.harvard.edu/full/2001CRABS..54k..97K
Kolomin Yu.M (2006) The find of two species of Cyprinidae fishes: bleak Alburnus alburnus and verkhovka Leucaspius delineatus in reservoirs of North Kazakhstan region. In: Rybokhozyaistvennye issledovaniya v Respublike Kazakhstan [Fishery Studies in Kazakhstan Republic], Almaty, Bastau, 203–206 (In Russian)
Kompowski A (2000) Growth rate of bleak, Alburnus alburnus (l., 1758) in Międzyodrze waters. Acta Ichthyol Piscat 30(1):37–51. https://www.aiep.pl/volumes/2000/0_1/pdf/30_1_03.pdf
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen–Geiger climate classification updated. Meteorol Z 15(3):259–263. https://doi.org/10.1127/0941-2948/2006/0130
Kotusz J, Witkowski A, Baran M, Błachuta J (2006) Fish migrations in a large lowland river (Odra R., Poland)—based on fish-pass observations. Folia Zool. 55(4):386–398.
Koutrakis ET, Tsikliras AC (2003) Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). J Appl Ichthyol 19(4):258–260. https://doi.org/10.1046/j.1439-0426.2003.00456.x
Koval VP (1950) A new species of Allocreadium from fish in the Dnieper River. Dopovidi Akademiyi Nauk Ukrayins’koi RSR, Seriya b 5:359–362 ([In Ukrainian.])
Koyun M (2011) Seasonal distribution and ecology of some Dactylogyrus species infecting Alburnus alburnus and Carassius carassius (Osteichthyes: Cyprinidae) from Porsuk River. Turkey Afr J Biotechnol 10(7):1154–1159. https://doi.org/10.4314/ajb.v10i7
Koyun M, Altunel FN (2007) Metazoan parasites of bleak (Alburnus alburnus), crucian carp (Carassius carassius) and golden carp (Carassius auratus) in Enne Dam Lake. Turkey Int J Zool Res 3(2):94–100. https://doi.org/10.3923/ijzr.2007.94.100
Koyun M, Karadavut U (2010) Sex-related growth performance of bleak (Alburnus alburnus). Int J Agric Biol 12(4):629–631 http://www.fspublishers.org/Issue.php?no_download=published_papers/54536_..pdf&issue_id=1162
Koyun M, Bulut S, Alas A, Solak K (1997) An investigation on Argulus foliaceus L. seen some fishes in Kütahya Region. IX National Aquatic Products Symposium (17–19 September 1997, Isparta), Greece, pp. 245–253.
Koyun M, Altunel FN, Oktener A (2007) Paraergasilus longidigitus Yin, 1954 (Copepoda: Poecilostomatoida) infestations in the bleak, Alburnus alburnus Lin., 1758 from Enne Dam Lake. Turkiye Parazitoloji Dergisi 31(2):158–161 https://cms.galenos.com.tr/Uploads/Article_22833/TPD-31-158.pdf
Kratochvíl M, Vašek M, Peterka J, Draštík V, Čech M, Jůza T, Muška M, Matěna J, Kubečka J (2014) Towards a better understanding of small scale distribution of littoral age-0 fish in a deep-valley reservoir: day or night surveys? Hydrobiologia 728(1):125–139. https://doi.org/10.1007/s10750-014-1812-x
Kuchta R, Řehulková E, Francová K, Scholz T, Morand S, Šimková A (2020) Diversity of monogeneans and tapeworms in cypriniform fishes across two continents. Int J Parasitol 50(10–11):771–786. https://doi.org/10.1016/j.ijpara.2020.06.005
Kudlai O, Oros M, Kostadinova A, Georgieva S (2017) Exploring the diversity of Diplostomum (Digenea: Diplostomidae) in fishes from the River Danube using mitochondrial DNA barcodes. Parasite Vector 10(1):592. https://doi.org/10.1186/s13071-017-2518-5
Kuttel S, Armin P, Wüest A (2002) Rhône Revitalisierung. Temperaturpräferenzen und –limiten von Fischarten Schweizerischer Fliessgewässer. Publ Num 1:1–34 www.rhone-thur.eawag.ch/temperaturpraeferenzen1.pdf
Lasne E, Bergerot B, Lek S, Laffaille P (2007) Fish zonation and indicator species for the evaluation of the ecological status of rivers: example of the Loire basin (France). River Res Appl 23(8):877–890. https://doi.org/10.1002/rra.1030
Latli A, Michel LN, Lepoint G, Kestemont P (2019) River habitat homogenisation enhances trophic competition and promotes individual specialisation among young of the year fish. Freshwat Biol 64(3):520–531. https://doi.org/10.1111/fwb.13239
Latorre D, Masó G, Hinckley A, Rubio-Gracia F, Vila-Gispert A, Almeida D (2016) Inter-population plasticity in dietary traits of invasive bleak Alburnus alburnus (Linnaeus, 1758) in Iberian fresh waters. J Appl Ichthyol 32(6):1252–1255. https://doi.org/10.1111/jai.13186
Latorre D, Masó G, Hinckley A, Verdiell-Cubedo D, Tarkan AS, Vila-Gispert A, Copp GH, Cucherousset J, da Silva E, Fernández-Delgado C, García-Berthou E, Miranda R, Oliva-Paterna FJ, Ruiz-Navarro A, Serrano JM, Almeida D (2018) Inter-population variability in growth and reproduction of invasive bleak Alburnus alburnus (Linnaeus, 1758) across the Iberian Peninsula. Mar Freshwat Res 69(8):1326–1332. https://doi.org/10.1071/MF17092
Latorre D, García-Berthou E, Rubio-Gracia F, Galobart C, Almeida D, Vila-Gispert A (2020a) Captive breeding conditions decrease metabolic rates and alter morphological traits in the endangered Spanish toothcarp. Aphanius Iberus Int Rev Hydrobiol 105(5–6):119–130. https://doi.org/10.1002/iroh.201902014
Latorre D, Masó G, Hinckley A, Verdiell-Cubedo D, Castillo-García G, González-Rojas AG, Black-Barbour EN, Vila-Gispert A, García-Berthou E, Miranda R, Oliva-Paterna FJ (2020b) Interpopulation variability in dietary traits of invasive bleak Alburnus alburnus (Actinopterygii, Cyprinidae) across the Iberian Peninsula. Water 12(8):2200. https://doi.org/10.3390/w12082200
Latorre D, Almeida D (2019) Alburno—Alburnus alburnus. In: Enciclopedia Virtual de los Vertebrados Españoles. López P, Martín J, García-Berthou E (Eds) Museo Nacional de Ciencias Naturales, Madrid. www.vertebradosibericos.org/
Lehtonen H (1996) Potential effects of global warming on northern European freshwater fish and fisheries. Fish Manage Ecol 3(1):59–71. https://doi.org/10.1111/j.1365-2400.1996.tb00130.x
Lelek A, Libosvárskí J (1960) Vískít ryb v rybím prechodu na rece Dyji pri Breclavi [The occurrence of fish in a fish ladder in Dyje River near Breclav]. Folia Zool 9:293–308
Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S (2008) Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol 6(12):E322. https://doi.org/10.1371/journal.pbio.0060028
Leunda P (2010) Impacts of non-native fishes on Iberian freshwater ichthyofauna: current knowledge and gaps. Aquat Inv 5(3):239–262. https://doi.org/10.3391/ai.2010.5.3.03
Leunda PM, Oscoz J, Miranda R (2006) Length–weight relationships of fishes from tributaries of the Ebro River. Spain J Appl Ichthyol 22(4):299–300. https://doi.org/10.1111/j.1439-0426.2006.00737.x
Levy A, Doadrio I, Almada VC (2009) Historical biogeography of European leuciscins (Cyprinidae): evaluating the Lago Mare dispersal hypothesis. J Biogeogr 36(1):55–65. https://doi.org/10.1111/j.1365-2699.2008.01969.x
Libois R, Ghalmi R, Brahimi A (2015) Insight into the dietary habits of the Eurasian otter, Lutra lutra, in the East of Algeria (El-Kala National Park). Ecol Medit 41(2):85–91. http://hdl.handle.net/2268/192800
Lilja J, Keskinen T, Marjomäki TJ, Valkeajärvi P, Kar-jalainen J (2003) Upstream migration activity of cyprinids and percids in a channel, monitored by a horizontal split-beam echosounder. Aquat Living Resour 16(3):185–190. https://doi.org/10.1016/S0990-7440(03)00016-0
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228. https://doi.org/10.1016/j.tree.2005.02.004
Lounaci-Daoudi, D, Lounaci A, Arab A (2016) Freshwater fish fauna of Algeria. The fish fauna of inland waters of Great-Kabylia. Adv Environ Biol 10(12):74–83. http://www.aensiweb.net/AENSIWEB/aeb/aeb/2016/December/74-83.pdf
Lucas MC, Baras E (2000) Methods for studying spatial behaviour of freshwater fishes in the natural environment. Fish Fish 1(4):283–316. https://doi.org/10.1046/j.1467-2979.2000.00028.x
Lucas MC, Mercer T, Batley E, Frear P, Peirson G, Duncan A, Kubekca J (1998) Spatio–temporal variations in the distribution and abundance of fish in the Yorkshire Ouse system. Sci Total Env 210–211(211):437–455. https://doi.org/10.1016/S0048-9697(98)00030-8
Lucas MC, Mercera T, Armstrong JD, McGintyc S, Rycroftd P (1999) Technical note use of a flat-bed passive integrated transponder antenna array to study the migration and behaviour of lowland river fishes at a fish pass. Fish Res 44(2):183–191. https://doi.org/10.1016/S0165-7836(99)00061-2
Lujić J, Kostić D, Bjelić-Čabrilo O, Popović E, Miljanović B, Marinović Z, Marković G (2013) Ichthyofauna composition and population parameters of fish species from the special nature reserve “Koviljsko-Petrovaradinski Rit” (Vojvodina, Serbia). Turk J Fish Aquat Sci 13(4):665–673. https://doi.org/10.4194/1303-2712-v13_4_12
Maceda-Veiga A, de Sostoa A, Solorio-Ornelas E, Monroy M, Vinyoles D, Caiola N, Casals F, García-Berthou E, Munne A (2010) Distribution of alien bleak Alburnus alburnus (Linnaeus, 1758) in the northeastern Iberian Mediterranean watersheds: past and present. In: Kühn K (ed) Settele J, Penev L, Georgiev T, Grabaum R, Grobelnik V, Hammen V, Klotz S and M. Atlas of Biodiversity Risk. Pensoft Publishers, Sofia, pp 44–145
Mackay I, Mann KH (1969) Fecundity of two cyprinid fishes in the River Thames, Reading. England J Fish Res Board Can 26(11):2795–2805. https://doi.org/10.1139/f69-276
Magurran AE, Pitcher TJ (1983) Foraging, timidity and shoal size in minnows and goldfish. Behav Ecol Sociobiol 12(2):147–152. https://doi.org/10.1007/BF00343206
Makris NC, Ratilal P, Jagannathan S, Gong Z, Andrews M, Bertsatos I, Godø O, Nero R, Jech JM (2009) Critical population density triggers rapid formation of vast oceanic fish shoals. Science 323(5922):1734–1737. https://doi.org/10.1126/science.1169441
Mann RHK (1996) Environmental requirements of European non-salmonid fish in rivers. Hydrobiologia 323(3):223–235. https://doi.org/10.1007/BF00007848
Mann RHK (1997) Temporal and spatial variations in the growth of 0-group roach (Rutilus rutilus), in the River Great Ouse: in relation to water temperature and food availability. Regul Rivers Res Manag 13:277–285. https://doi.org/10.1002/(SICI)1099-1646(199705)13:3%3c277::AID-RRR455%3e3.0.CO;2-7
Mann RHK, Penczak T (1984) The efficiency of a new electrofishing technique in determining fish numbers in a large river in Central Poland. J Fish Biol 24(2):173–185. https://doi.org/10.1111/j.1095-8649.1984.tb04788.x
Mann RHK (1991) Growth and production. Chapter 16, pp. pp.456–482. In: Winfield IJ, Nelson JS (eds) Cyprinid Fishes. Systematics, Biology and Exploitation. Chapman & Hall, London.
Marčeta B (2014) Length-weight relationship of Alburnus alburnus from Slovenia, sampled from 20.4.2010 to 14.10.2014 (n = 4862, length range from 1.5 cm to 27.2 cm, specimen condition: mixed). http://biosweb.org
Marchetti MP, Moyle PB (2001) Effects of flow regime on fish assemblages in a regulated California stream. Ecol Appl 11(2):530–539. https://doi.org/10.1890/1051-0761(2001)011[0530:EOFROF]2.0.CO;2
Martelo J, da Costa LM, Ribeiro D, Gago J, Magalhães MF, Gante HF, Alves MJ, Cheoo G, Gkenas C, Banha F, Gama M, Anastácio PM, Tiago PM, Ribeiro F (2021) Evaluating the range expansion of recreational non-native fishes in Portuguese freshwaters using scientific and citizen science data. BioInv Rec 10(2):378–389 10. 3391/bir.2021.10.2.16.
Masó G, Latorre D, Tarkan AS, Vila-Gispert A, Almeida D (2016) Inter-population plasticity in growth and reproduction of invasive bleak, Alburnus alburnus (Cyprinidae, Actinopterygii), in northeastern Iberian Peninsula. Folia Zool 65(1):10–14 https://doi.org/10.25225/fozo.v65.i1.a3.2016
Matejusová I, Gelnar M, McBeath AJA, Collins CM, Cunningham CO (2001) Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int J Parasitol 31(7):738–745. https://doi.org/10.1016/S0020-7519(01)00176-X
Matono P, da Silva J, Ilhéu M (2018) How does an invasive cyprinid benefit from the hydrological disturbance of Mediterranean temporary streams?. Diversity 10(2):47. https://doi.org/10.3390/D10020047
Matras M, Stachnik M, Borzym E, Maj-Paluch J, Reichert M (2019) Potential vector species of carp edema virus (CEV). J Fish Dis 42(7):959–964. https://doi.org/10.1111/jfd.13000
Maury O (2017) Can schooling regulate marine populations and ecosystems?. Progr Oceanogr 156:91–103. https://doi.org/10.1016/j.pocean.2017.06.003
Mehner T, Diekmann M, Brämick U, Lemcke R (2005) Composition of fish communities in German lakes as related to lake morphology, trophic state, shore structure and human-use intensity. Freshwat Biol 50(1):70–85. https://doi.org/10.1111/j.1365-2427.2004.01294.x
Mérő TO (2014) Diet in pike (Esox lucius) in northwestern Vojvodina (Serbia). Natura Croatica 23(1):27–34
Meulenbroek P, Drexler S, Nagel C, Geistler M, Waidbacher H (2018) The importance of a constructed near-nature-like Danube fish by-pass as a lifecycle fish habitat for spawning, nurseries, growing and feeding: a long-term view with remarks on management. Mar Freshwat Res 69(12):1857–1869. https://doi.org/10.1071/MF18121
Miehls S, Sullivan P, Twohey M, Barber J, McDonald R (2020) The future of barriers and trapping methods in the sea lamprey (Petromyzon marinus) control program in the Laurentian Great Lakes. Rev Fish Biol Fish 30(1):1–24. https://doi.org/10.1007/s11160-019-09587-7
Mills CA (1991) Reproduction and life history. Chapter 17, pp. 483–508. In: Winfield IJ, Nelson JS (eds) Cyprinid Fishes. Systematics, Biology and Exploitation. Chapman & Hall, London
Miranda R, Oscoz J, Leunda PM, Escala MC (2006) Weight–length relationships of cyprinid fishes of the Iberian Peninsula. J Appl Ichthyol 22(4):297–298. https://doi.org/10.1111/j.1439-0426.2006.00646.x
Molnár K, Eszterbauer E, Szekely C, Dan A, Harrach B (2002) Morphological and molecular biological studies on intramuscular Myxobolus spp. of cyprinid fish. J Fish Dis 25(11):643–652 https://doi.org/10.1046/j.1365-2761.2002.00409.x
Molnár K, Eszterbauer E, Marton S, Cech G, Székely C (2009) Myxobolus erythrophthalmi sp. n. and Myxobolus shaharomae sp. n. (Myxozoa: Myxobolidae) from the internal organs of rudd, Scardinius erythrophthalmus (L.), and bleak, Alburnus alburnus (L.). J Fish Dis 32(3):219–231 https://doi.org/10.1111/j.1365-2761.2008.00976.x
Molnár K (2000) Survey on Myxobolus infection of the bleak (Alburnus alburnus L.) in the River Danube and in Lake Balaton. Acta Vet Hungar 48(4):421–432 https://doi.org/10.1556/004.48.2000.4.5
Moyle PB, Light T (1996) Biological invasions of fresh water: empirical rules and assembly theory. Biol Conserv 78(1–2):149–161. https://doi.org/10.1016/0006-3207(96)00024-9
Muñoz-Mas R, Gil-Martínez E, Oliva-Paterna FJ, Belda EJ, Martínez-Capel F (2019) Tree-based ensembles unveil the microhabitat suitability for the invasive bleak (Alburnus alburnus L.) and pumpkinseed (Lepomis gibbosus L.): Introducing XGBoost to eco-informatics. Ecol Inform 53:100974 https://doi.org/10.1016/j.ecoinf.2019.100974
Nanami A (2007) Juvenile swimming performance of three fish species on an exposed sandy beach in Japan. J Exp Mar Biol Ecol 348(1–2):1–10. https://doi.org/10.1016/j.jembe.2007.02.016
Navodaru I, Buuse AD, Staras M (2002) Effects of hydrology and water quality on the fish community in Danube Delta lakes. Int Rev Hydrobiol 87(2–3):329–348. https://doi.org/10.1002/1522-2632(200205)87:2/3%3C329::AID-IROH329%3E3.0.CO;2-J
Nedić Z, Skenderović I, Adrović A (2018) Study of some ectoparasites of fishes from the Sava River as part of water management in Bosnia and Herzegovina. TEM J 7(2):391–397 https://doi.org/10.18421/TEM72-21
Nocita A (2007) La fauna ittica del bacino dell’Arno. Biol Ambient 21(2):97–105
Novokhatskaya O, Ieshko EP (2010) Findings of the myxosporidia Myxobolus improvisus and Henneguya cutanea in dace (Leuciscus leuciscus) and bleak (Alburnus alburnus) from Lake Syamozero. Zool Zh 89(4):495–497
Nunn AD, Harvey JP, Cowx IG (2007) The food and feeding relationships of larval and 0+ year juvenile fishes in lowland rivers and connected waterbodies. I. Ontogenetic shifts and interspecific diet similarity. J Fish Biol 70(3):726–742 https://doi.org/10.1111/j.1095-8649.2007.01334.x
Oesmann S (2003) Vertical, lateral and diurnal drift patterns of fish larvae in a large lowland river, the Elbe. J Appl Ichthyol 19(5):284–293. https://doi.org/10.1046/j.1439-0426.2003.00503.x
Ogle DH (2016) FSA: Fisheries stock analysis. R package version 0.8.10.
Ostrowska K, Wiśniewski G, Piasecki W (2019) Spatial distribution of skin and muscle metacercariae (Digenea) of roach, Rutilus rutilus, and bleak, Alburnus alburnus (Actinopterygii: Cypriniformes: Cyprinidae), from an estuary lake in Central Europe. Acta Ichthyol Piscat 49(4):421–429. https://doi.org/10.3750/AIEP/02823
Ozaktas T, Taskin B, Gozen AG (2012) High level multiple antibiotic resistance among fish surface associated bacterial populations in non-aquaculture freshwater environment. Water Res 46(19):6382–6390. https://doi.org/10.1016/j.watres.2012.09.010
Özuluğ M, Freyhof J (2007) Rediagnosis of four shemayas from western Turkey and description of two new species (Teleostei: Cyprinidae). Ichthyol Expl Freshw 18:233–246
Pander J, Geist J (2010) Seasonal and spatial bank habitat use by fish in highly altered rivers–a comparison of four different restoration measures. Ecol Freshwat Fish 19(1):127–138. https://doi.org/10.1111/j.1600-0633.2009.00397.x
Pavlov DS, Kostin VV, Zvezdin AO, Prozorov DA, Podolyako SA (2019) Rheoreaction of some juvenile cyprinids during autumn contranatant migration. J Ichthyol 59(6):938–945. https://doi.org/10.1134/S0032945219060109
Pazooki J, Masoumian M (2012) Synopsis of the parasites in Iranian freshwater fishes. Iran J Fish Sci 11(3):570–589 http://hdl.handle.net/1834/11522
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen–Geiger climate classification. Hydrol Earth Syst Sci Discuss 4(2):439–473 https://hal.archives-ouvertes.fr/hal-00298818/document
Pehlivanov L, Stefanov T, Mihov S, Biserkov V, Vassilev M, Apostolou A, Velkov B (2011) Recent ichthyofauna in the wetlands along the Bulgarian section of the Danube. Sci Annal Danube Delta Inst 17:83–88
Peltonen H, Rita H, Ruuhijärvi J (1996) Diet and prey selection of pikeperch (Stizostedion lucioperca (L.)) in Lake Vesijärvi analysed with a logit model. Ann Zool Fennici 33(3/4):481–487. https://www.jstor.org/stable/23736093
Penczak T, Galicka W, Glowacki Ł, Koszalinski H, Kruk A, Zięba G, Kostrzewa J, Marszał L (2004) Fish assemblage changes relative to environmental factors and time in the Warta River, Poland, and its oxbow lakes. J Fish Biol 64(2):483–501. https://doi.org/10.1111/j.0022-1112.2004.00316.x
Perea S, Böhme M, Zupančič P, Freyhof J, Šanda R, Özuluğ M, Abdoli A, Doadrio I (2010) Phylogenetic relationships and biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both mitochondrial and nuclear data. BMC Evol Biol 10(1):1–27. https://doi.org/10.1186/1471-2148-10-265
Pérez-Bote JL, Roso R, Pula HJ, Díaz F, López MT (2004) Primeras citas de la lucioperca, Sander (= Stizostedion) lucioperca (Linnaeus, 1758) y del alburno, Alburnus alburnus (Linnaeus,1758) en las cuencas extremeñas de los ríos Tajo y Guadiana, SO de la Península Ibérica. Anales Biol 26:93–100. https://www.um.es/analesdebiologia/numeros/26/PDF/10-PRIMERAS.pdf
Peterka J, Vašek M, Kubečka J, Hladík M, Hohausová E (2004) Drift of juveniles after riverine spawning of fishes from the Římov reservoir. Czech Republic Int J Ecohydrol Hydrobiol 4(4):459–468
Pettersson LB, Brönmark C (1999) Energetic consequences of an inducible morphological defence in crucian carp. Oecologia 121(1):12–18. https://doi.org/10.1007/s004420050901
Pinder AC (2001) Keys to larval and juvenile stages of coarse fishes from fresh waters in the British Isles. Freshwater Biological Association. The Ferry House, Far Sawrey, Ambleside, Cumbria, UK. Scientific Publication No. 60: 136 pp.
Podolyako SA, Fedorovich VV, Litvinov KV (2017) Fishes of the astrakhan nature reserve: an updated checklist with comments of recent records. Zoosyst Ross 26(1):182–195 https://doi.org/10.31610/zsr/2017.26.1.182
Politou C-Y, Economidis PS, Sinis AI (1993) Feeding biology of bleak, Alburnus alburnus, in Lake Koronia, northern Greece. J Fish Biol 43(1):33–43. https://doi.org/10.1111/j.1095-8649.1993.tb00408.x
Politou CY (1993) Biology and dynamics of the fish Alburnus alburnus (L., 1758) in Lake Koronia. University of Thessaloniki, Thessaloniki, Hellas. PhD Thesis: 134 pp. (In Greek with English abstract)
Prchalová M, Vetesnik L, Slavík O (2006) Migrations of juvenile and subadult fish through a fishpass during late summer and fall. Folia Zool 55(2):162–166
Prchalová M, Vetesnik L, Slavík O (2004) Patterns of cyprinid migration through a fish pass. In: Proceedings of 5th International Symposium of Ecohydraulics Aquatic Habitats: Analysis and Restoration. Madrid: Int. Assoc. Hydro-Environ Eng Res pp. 68–74
Prchalová M, Horký P, Slavík O, Vetešník L, Halačka K (2011) Fish occurrence in the fishpass on the lowland section of the River Elbe, Czech Republic, with respect to water temperature, water flow and fish size. Folia Zool 60(2):104–114 https://doi.org/10.25225/fozo.v60.i2.a4.2011
Price PW, Clancy KM (1983) Patterns in number of helminth parasite species in freshwater fishes. J Parasitol 69(3):449–454. https://doi.org/10.2307/3281352
Prigioni C, Balestrieri A, Remonti L, Gargaro A, Priore G (2006) Diet of the Eurasian otter (Lutra lutra) in relation to freshwater habitats and alien fish species in southern Italy. Ethol Ecol Evol 18(4):307–320 https://doi.org/10.1080/08927014.2006.9522698
Prignon C, Micha JC, Gillet A (1998) Biological and environmental characteristics of fish-passage at the Tailfer dam on the Meuse River, Belgium. In: Jungwirth M, Schmutz S and Weiss S (Eds): Fish migration and fish bypasses. Fishing News Books. Oxford: Blackwell Science. Ltd, pp. 69–84.
R Development Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org/
Radočaj T, Špelić I, Vilizzi L, Povž M, Piria M (2021) Identifying threats from introduced and translocated non-native freshwater fishes in Croatia and Slovenia under current and future climatic conditions. Global Ecol Conserv 27:e01520 https://doi.org/10.1016/j.gecco.2021.e01520
Rafikov RR, Boznak EI (2021) Biological characteristics of bleak Alburnus alburnus (Cyprinidae) from the cooling pond in the Pechora River Basin. J Ichthyol 61(4):646–649. https://doi.org/10.1134/S0032945221030085
Raikova-Petrova G, Liev I, Petrov M (2009) Growth rate and fecundity of bleak (Alburnus alburnus L.) in the Sand-Pit Lake Chepintsi (Bulgaria). Biotechnology & Biotechnological Equipment, 23/2009 Se. Special Edition: pp. 212–216 https://doi.org/10.1080/13102818.2009.10818403
Raissy M, Sohrabi HR, Rashedi M, Ansari M (2013) Investigation of a parasitic outbreak of Lernaea cyprinacea Linnaeus (Crustacea: Copepoda) in cyprinid fish from Choghakhor Lagoon. Iran J Fish Sci 12(3):680–688. https://www.sid.ir/en/VEWSSID/J_pdf/101220130315.pdf
Rask M, Appelberg M, Hesthagen T, Tammi J, Beier U, Lappalainen A (2000) Fish status survey of Norway lakes – species composition, distribution, effects of environmental changes. TemaNord 2000:508. Nordic Council of Ministers, Copenhagen 2000.
Reichard M, Jurajda P, Ondračková M (2002) The effect of light intensity on the drift of young-of-the-year cyprinid fishes. J Fish Biol 61(4):1063–1066. https://doi.org/10.1111/j.1095-8649.2002.tb01866.x
Reshetnikov AN, Golubtsov AS, Zhuravlev VB, Lomakin SL, Rezvyi AS (2017) Range expansion of rotan Perccottus glenii, sunbleak Leucaspius delineatus, and bleak Alburnus alburnus in the Ob River basin. Contemp Probl Ecol 10(6):612–620. https://doi.org/10.1134/S1995425517060105
Reynolds SJ, Hinge MDC (1996) Foods brought to the nest by breeding Kingfishers Alcedo atthis in the New Forest of southern England. Bird Study 43(1):96–102. https://doi.org/10.1080/00063659609460999
Ribeiro F, Benigno E, Collares-Pereira MJ, Moyle PB (2008) Life-history traits of non-native fishes in Iberian watersheds across several invasion stages: a first approach. Biol Inv 10(1):89–102. https://doi.org/10.1007/s10530-007-9112-2
Ribeiro D, Gkenas C, Gago J, Ribeiro F (2021) Variation in diet patterns of the invasive top predator Sander lucioperca (Linnaeus, 1758) across Portuguese basins. Water 13:2053. https://doi.org/10.3390/w13152053
Ricker WE (1975) Computation and interpretation of biological statistics of fish population. Bulletin (Fisheries Research Board of Canada), 191. Department of the Environment, Fisheries and Marine Service, Ottawa, 382 pp
Rieseberg LH, Archer MA, Wayne RK (1999) Transgressive segregation, adaptation and speciation. Heredity 83:363–372. https://doi.org/10.1038/sj.hdy.6886170
Říha M, Hladík M, Mrkvička T, Prchalová M, Čech M, Draštík V, Frouzová J, Jůza T, Kratochvíl M, Peterka J, Vašek M, Kubečka J (2013) Post-spawning dispersal of tributary fish species to a reservoir system. Folia Zool 62(1):1–13 https://doi.org/10.25225/fozo.v62.i1.a1.2013
Říha M (2012) Dynamic of fish spatial distribution in reservoirs. PhD Thesis, University of South Bohemia České Budějovice, Czech Republic. https://theses.cz/id/56cob2/Dynamic_of_fish_tisk_zkraceny2.pdf
Rinchard J, Kestemont P. (1996) Comparative study of reproductive biology in single- and multiple-spawner cyprinid fish. I. Morphological and histological features. J Fish Biol 49(5):883–894 https://doi.org/10.1111/j.1095-8649.1996.tb00087.x
Rischbieter C (2000) Structures to prevent the spread of nuisance fish from Lake Davis. California North Am J Fish Manag 20(3):784–790. https://doi.org/10.1577/1548-8675(2000)020%3c0784:STPTSO%3e2.3.CO;2
Romanov VI, Interesova EZ, Dyldin YV, Babkina IB, Karmanova OG, Vorobiev DS (2017) An annotated list and current state of ichthyofauna of the Middle Ob River basin. Int J Environ Stud 74(5):818–830 https://doi.org/10.1080/00207233.2017.1288547
Rosenthal BM, Dunams-Morel D, Ostoros G, Molnár K (2016) Coccidian parasites of fish encompass profound phylogenetic diversity and gave rise to each of the major parasitic groups in terrestrial vertebrates. Infect Genet Evol 40:219–227. https://doi.org/10.1016/j.meegid.2016.02.018
Roux A-L, Copp GH (1996) Fish populations in rivers. In: Petts GE, Amoros C (eds) Fluvial Hydrosystems. Chapman & Hall, London, pp 167–183
Rubio-Gracia F, García-Berthou E, Guasch H, Zamora L, Vila-Gispert A (2020a) Size-related effects and the influence of metabolic traits and morphology on swimming performance in fish. Curr Zool 66(5):493–503. https://doi.org/10.1093/cz/zoaa013
Rubio-Gracia F, García-Berthou E, Latorre D, Moreno-Amich R, Srean P, Luo Y, Vila-Gispert A (2020b) Differences in swimming performance and energetic costs between an endangered native toothcarp (Aphanius iberus) and an invasive mosquitofish (Gambusia holbrooki). Ecol Freshw Fish 29(2):230–240. https://doi.org/10.1111/eff.12509
Ruiz-Olmo J, Jiménez J (2009) Diet diversity and breeding of top predators are determined by habitat stability and structure: a case study with the Eurasian otter (Lutra lutra L.). Eur J Wildl Res 55(2):133–144 https://doi.org/10.1007/s10344-008-0226-3
Russell IC, Cook AC, Ives MJ, Davison PI (2022) The diet of two sympatric great cormorant Phalacrocorax carbo subspecies wintering at freshwater fishery sites in England and Wales. Ardea 109(3):443–456. https://doi.org/10.5253/arde.v109i2.a15
Sagnes P, Statzner B (2009) Hydrodynamic abilities of riverine fish: a functional link between morphology and velocity use. Aquat Living Resour 22(1):79–91. https://doi.org/10.1051/alr/2009008
Salvador Vilariño, V (2015) Diagnóstico de la situación de las especies exóticas invasoras dentro del ámbito del proyecto LIFE11 NAT ES/699 MedWetRivers. Sociedad Pública de Infraestructuras y Medio Ambiente de Castilla y León S.A (SOMACYL).
Sánchez JM, Alarcón-Elbal PM (2014) Parasitación por el plerocercoide Ligula intestinalis (Linnaeus, 1758) (Cestoda: Pseudophyllidea) en alburno, Alburnus alburnus (Linnaeus, 1758) capturado en el Río Albarragena de la Provincia de Badajoz, SO España. XIX Congreso Laboratorio Veterinario Avedila, Zaragoza.
Sánchez-González JR, Arbonés A, Casals F (2020) Variation over time of length–weight relationships and condition factors for four exotic fish species from a restored shallow lake in NE Iberian Peninsula. Fishes 5(1):7. https://doi.org/10.3390/fishes5010007
Sánchez-Pérez A, Torralva M, Zamora-Marín JM, Bravo-Córdoba FJ, Sanz-Ronda FJ, Oliva-Paterna FJ (2022) Multispecies fishways in a Mediterranean river: Contributions as migration corridors and compensatory habitat for fish. Sci Total Env 830:154613 https://doi.org/10.1016/j.scitotenv.2022.154613
Santos JM, Ferreira MT, Godinho FN, Bochechas J (2002) Performance of fish lift recently built at the Touvedo Dam on the Lima River. Portugal J Appl Ichthyol 18(2):118–123. https://doi.org/10.1046/j.1439-0426.2002.00309.x
Scharbert A, Borcherding J (2013) Relationships of hydrology and life-history strategies on the spatio-temporal habitat utilisation of fish in European temperate river floodplains. Ecol Indic 29:348–360. https://doi.org/10.1016/j.ecolind.2013.01.009
Schmid M, Ziegler CG, Steinlein C, Nanda I, Schartl M (2006) Cytogenetics of the bleak (Alburnus alburnus), with special emphasis on the B chromosomes. Chromosome Res 14(3):231–242. https://doi.org/10.1007/s10577-006-1038-5
Schmutz S, Giefing C, Wiesner C (1998) The efficiency of a nature-like bypass channel for pike-perch (Stizostedion lucioperca) in the Marchfeldkanalsystem. Hydrobiologia 371:355–360. https://doi.org/10.1007/978-94-011-5090-3_40
Schröder T (1979) Aspekte der Ökologie von Frühentwicklungsstadien einiger Fischarten in Altrhein und Labor. Diplomarbeit, W. Goethe Universität, Frankfurt am Main, p 100
Schumann D, Hoback W, Koupal K (2015) Complex interactions between native and invasive species: investigating the differential displacement of two topminnows native to Nebraska. Aquat Inv 10(3):339–346. https://doi.org/10.3391/ai.2015.10.3.09
Shukerova S, Kirin D, Hanzelová V (2010) Endohelminth communities of the perch, Perca fluviatilis (Perciformes, Percidae) from Srebarna Biosphere Reserve. Bulg Helminthol 47(2):99–104. https://doi.org/10.2478/s11687-010-0016-9
da Silva J, Matono P, Barata EN, Bernardo JM, Costa AM, Ilhéu M (2019) Behavioural interactions between the endangered native fish Saramugo, Anaecypris hispanica, and the invasive bleak, Alburnus alburnus. Limnetica 38(2):517–533 https://doi.org/10.23818/limn.38.30
Šimková A, Morand S, Jobet E, Gelnar M, Verneau O (2004) Molecular phylogeny of congeneric monogenean parasites (Dactylogyrus): a case of intrahost speciation. Evolution 58(5):1001–1018. https://doi.org/10.1111/j.0014-3820.2004.tb00434.x
Slynko YV, Stolbunova VV (2010) Elimination of the parental ITS1 region of rDNA in the first generation of interspecific hybrids between the bream Abramis brama (L.) and roach Rutilus rutilus (L.). Dokl Biol Sci 430(1):31–33 https://doi.org/10.1134/S0012496610010114
Šmejkal M, Baran R, Blabolil P, Vejřík L, Prchalová M, Bartoň D, Mrkvička T, Kubečka J (2017) Early life-history predator-prey reversal in two cyprinid fishes. Sci Rep 7:6924. https://doi.org/10.1038/s41598-017-07339-w
Sousa-Santos C, Matono P, da Silva J, Ilheú M (2018) Evaluation of potential hybridization between native fishes and the invasive bleak, Alburnus alburnus (Actinopterygii: Cypriniformes: Cyprinidae). Acta Ichthyol Piscat 48(2):109–122. https://doi.org/10.3750/AIEP/02395
Stavrescu-Bedivan MM, Aioanei FT, Scăeţeanu GV (2017) Length-weight relationships and condition factor of 11 fish species from the Timiş River. West Rom Agric For 63(4):281–285
Stefanov T (2007) Fauna and distribution of fishes in Bulgaria. In: Fet V, Popov A. (Eds) Biogeography and ecology of Bulgaria. Monographiae Biologicae, Vol. 82. Springer, Dordrecht https://doi.org/10.1007/978-1-4020-5781-6_5
Stojanovski S, Hristovski N, Caki P, Hristovski M, Baker RA (2003) Fauna of monogenean trematode parasites in bleak Alburnus alburnus belvica Karaman, 1924 (Pisces: Cyprinidae) from the Lake Prespa (Macedonia). In: Proceedings of the 2nd Congress of Ecologists of the Republic of Macedonia with International Participation, Ohrid. Special Issues of Macedonian Ecological Society, Vol. 6, Skopje.
Stojanovski S, Hristovsk N, Cakic P, Nedeva I, Karaivanova E, Atanasov G (2009) Monogenean trematods—Parasites of some cyprinid fishes from lakes Ohrid and Prespa (Macedonia). Biotechnol Biotec Eq 23(Suppl 1):360–364 https://doi.org/10.1080/13102818.2009.10818439
Stojanovski S, N, Cakic P, Cvetkovic A, Atanassov G, Smiljkov S, (2008) Fauna of monogenean trematodes-parasites of cyprinid fish from Lake Dojran (Macedonia). Nat Monten 7(3):389–398
Stolbunov IA, Kuzmina VV (2018) Diurnal dynamics of activity of peptidases at early ontogenesis of bleak Alburnus alburnus (L.) from coastal shallows of the Rybinsk Reservoir. Inland Water Biol 11(2):227–230 https://doi.org/10.1134/S1995082918010182
Stolbunov IA, Kutuzova OR, Krylov AV (2017) Impact of heron (Ardea cinerea L. and A. alba L.) habitat on coastal juvenile fish assemblages in Rybinsk Reservoir. Inl Water Biol 10(4):427–435 https://doi.org/10.1134/S1995082917030166
Sures B, Siddall R (1999) Pomphorhynchus laevis: the intestinal acanthocephalan as a lead sink for its fish host, chub (Leuciscus cephalus). Exp Parasitol 93(2):66–72. https://doi.org/10.1006/expr.1999.4437
Sures B, Siddall R, Taraschewski H (1999) Parasites as accumulation indicators of heavy metal pollution. Parasitol Today 15(1):16–21. https://doi.org/10.1016/S0169-4758(98)01358-1
Tandjir L, Djebar AB (2010) L’ichtyofaune de l’Oued Kebir (Oum Toub, Skikda, Algerie). Sci Technol C 32:51–58
Tarkan AS, Vilizzi L, Top N, Ekmekçi FG, Stebbing PD, Copp GH (2017b) Identification of potentially invasive freshwater fishes, including translocated species, in Turkey using the Aquatic Species Invasiveness Screening Kit (AS-ISK). Int Rev Hydrobiol 102(1–2):47–56. https://doi.org/10.1002/iroh.201601877
Tarkan AS, Sarı HM, İlhan A, Kurtul I, Vilizzi L (2017a) Risk screening of non-native and translocated freshwater fish species in a Mediterranean-type shallow lake: Lake Marmara (West Anatolia). Zool Middle East 63(1):48–57 https://doi.org/10.1080/09397140.2017.1269398
Taylor MK, Cooke SJ (2012) Meta-analyses of the effects of river flow on fish movement and activity. Environ Rev 20(4):211–219. https://doi.org/10.1139/a2012-009
Terent’eva NN, Mukhachev IS (2006) Ecological and fishery role of new species in the Ob River basin. IX Congress of Hydrobiological Society, Russian Academy of Sciences, Abstracts of Papers, Tolyatti 2:188 ((In Russian))
Tirelli T, Gamba M, Pessani D (2012) Support vector machines to model presence/absence of Alburnus alburnus alborella (Teleostea, Cyprinidae) in north-western Italy: Comparison with other machine learning techniques. C R Biol 335(10–11):680–686. https://doi.org/10.1016/j.crvi.2012.09.001
Tischler G, Gassner H, Wanzenböck J (2000) Sampling characteristics of two methods for capturing age-0 fish in pelagic lake habitats. J Fish Biol 57(6):1474–1487. https://doi.org/10.1006/jfbi.2000.1410
Treer T, Šprem N, Torcu-Koc H, Sun Y, Piria M (2008) Length–weight relationships of freshwater fishes of Croatia. J Appl Ichthyol 24(5):626–628. https://doi.org/10.1111/j.1439-0426.2008.01084.x
Tryapitsyna LN (1965) Specific distribution and biology of fishes in avandelta of the Volga River. Fishery in Avandelta of the Volga River. Trans Astrakhan Sanc 10:315–458 ((In Russian))
European Union (2000) Directive 2000/60/Ec of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Commun 22.12.2000) L 327/1:1–73 https://www.eea.europa.eu/policy-documents/directive-2000-60-ec-of
Vašek M, Kubečka J (2004) In situ diel patterns of zooplankton consumption by subadult/adult roach Rutilus rutilus, bream Abramis brama, and bleak Alburnus alburnus. Folia Zool 53(2):203–214
Vašek M, Kubečka J, Čech M, Draštík V, Matěna J, Mrkvička T, Peterka J, Prchalová M (2009) Diel variation in gillnet catches and vertical distribution of pelagic fishes in a stratified european reservoir. Fish Res 96(1):64–69. https://doi.org/10.1016/j.fishres.2008.09.010
Velasco JC, Lizana M, Román J, Delibes M, Fernández J (2005) Guía de los peces, anfibios, reptiles y mamíferos de Castilla y León. Nayade, Medina del Campo, Spain, p 271
Verreycken H, Van Thuyne G, Belpaire C (2011) Length–weight relationships of 40 freshwater fish species from two decades of monitoring in Flanders (Belgium). J Appl Ichthyol 27(6):1416–1421. https://doi.org/10.1111/j.1439-0426.2011.01815.x
Vilches A, Miranda R, Arizaga J (2012) Fish prey selection by the common kingfisher Alcedo atthis in Northern Iberia. Acta Ornithol 47(2):167–175. https://doi.org/10.3161/000164512X662278
Vilizzi L, Copp GH (2017) Global patterns and clines in the growth of common carp Cyprinus carpio. J Fish Biol 91(1):3–40. https://doi.org/10.1111/jfb.13346
Vinni M, Horppila J, Olin M, Ruuhijärvi J, Nyberg K (2000) The food, growth and abundance of five co-existing cyprinids in lake basins of different morphometry and water quality. Aquat Ecol 34(4):421–431. https://doi.org/10.1023/A:1011404721775
Vinyoles D, Robalo JI, De Sostoa A, Almodóvar A, Elvira B, Nicola GG, Fernández-Delgado C, Santos CS, Doadrio I, Sardá Palomera F, Almada VC (2007) Spread of the alien bleak Alburnus alburnus (Linnaeus, 1758) (Actinopterygii, Cyprinidae) in the Iberian Peninsula: the role of reservoirs. Graellsia 63(1):101–110
Vinyoles D, García E, Guadalupe J, Capelli I (2008) La regressió de la madrilla (Parachondrostoma miegii) a Catalunya: possibles interaccions comportamentals amb l’alburn (Alburnus alburnus). Technical report, University of Barcelona, Spain: 133 pp. http://agricultura.gencat.cat/web/.content/06-medi-natural/pesca-continental/enllacos-documents/estudis-diagnosis/fitxers-binaris/regressio-madrilla-chondostoma-miegii-catalunya-possibles-interaccions-comportamentals-alburn-alburnus-alburnus.pdf
Vinyoles D, Puigcerver M, Rodríguez-Tejeiro JD (2009) Interacciones comportamentales entre un pez autóctono (Parachondrostoma miegii) y un invasor (Alburnus alburnus) en condiciones experimentales.” III Congreso Nacional Sobre Especies Exóticas Invasoras. Zaragoza, Spain.
Vøllestad LA (1985) Resource partitioning of roach Rutilus rutilus and bleak Alburnus alburnus in two eutrophic lakes in SE Norway. Ecography 8(2):88–92. https://doi.org/10.1111/j.1600-0587.1985.tb01157.x
Vøllestad L, Skurdal J, Qvenild T (1986) Habitat use, growth, and feeding of pike (Esox lucius L.) in four Norwegian lakes. Arch Hydrobiol 108(1):107–117.
Voutilainen A, Seppänen E, Huuskonen H (2011) A methodological approach to measuring the oxygen consumption profile of six freshwater fish species: Implications for determination of the standard metabolic rate. Mar Freshw Behav Physiol 44(4):239–250 https://doi.org/10.1080/10236244.2011.622090
von Wagner JR, Fischer F, Gautier L (1903) Traité de chimie industrielle, 4th ed. Masson & Co., Paris, France, Vol. 2, pp. 64–65.
Wanzenböck J, Schiemer F (1989) Prey detection in cyprinids during early development. Can J Fish Aquat Sci 46(6):995–1001. https://doi.org/10.1139/f89-129
Way K, Haenen O, Stone D, Adamek M, Bergmann SM, Bigarré L, Diserens N, El-Matbouli M, Gjessing MC, Jung-Schroers V, Leguay E, Matras M, Olesen NJ, Panzarin V, Piačková V, Toffan A, Vendramin N, Veselý T, Waltzek T (2017) Emergence of carp edema virus (CEV) and its significance to European common carp and koi Cyprinus carpio. Dis Aquat Organ 126(2):155–166. https://doi.org/10.3354/dao03164
Welcomme RL (1988) International introductions of inland aquatic species. FAO Fisheries Technical Paper 294, Food and Agriculture Organization of the United Nations (FAO) 318.
Wheeler AC (1977) The origin and distribution of the freshwater fishes of the British Isles. J Biogeogr 4:1–24. https://doi.org/10.2307/3038124
Wheeler A (1978) Hybrids of bleak, Alburnus alburnus, and chub, Leuciscus cephalus in English rivers. J Fish Biol 13(4):467–473. https://doi.org/10.1111/j.1095-8649.1978.tb03456.x
Wieser W (1991) Limitations of energy acquisition and energy use in small poikilotherms: evolutionary implications. Funct Ecol 5(2):234–240. https://doi.org/10.2307/2389261
Wieser W, Forstner H, Medgyesy N, Hinterleitner S (1988a) To switch or not to switch: partitioning of energy between growth and activity in larval cyprinids (Cyprinidae: Teleostei). Funct Ecol 2(4):499–507. https://doi.org/10.2307/2389393
Wieser W, Forstner H, Schiemer F, Mark W (1988b) Growth rates and growth efficiencies in larvae and juveniles of Rutilus rutilus and other cyprinid species: effects of temperature and food in the laboratory and in the field. Can J Fish Aquat Sci 45(6):943–950. https://doi.org/10.1139/f88-116
Williams WP (1967) The growth and mortality of four species of fish in the River Thames at Reading. J Anim Ecol 36(3):695–720. https://doi.org/10.2307/2821
Williams WP (1965) The population density of four species of freshwater fish, roach (Rutilus rutilus (L.)), bleak (Alburnus alburnus (L.)), dace (Leuciscus leuciscus (L.)) and perch (Perca fluviatilis L.) in the River Thames at Reading. J Anim Ecol 35(1):173–185 https://doi.org/10.2307/2374
Winnicki A, Korzelecka A (1997) Morphomechanical aspects of the development of the bleak (Alburnus alburnus L.). Acta Ichthyol Pisc 27(2):17–27.
Witkowski A, Kotusz J, Wawer K, Stefaniak J, Popiolek M, Blachuta J (2015) A natural hybrid of Leuciscus leuciscus (L.) and Alburnus alburnus (L.) (Osteichthyes: Cyprinidae) from the Bystrzyca River (Poland). Ann Zool 65(2):287–293 https://doi.org/10.3161/00034541ANZ2015.65.2.010
Wolter C, Bischoff A (2001) Seasonal changes of fish diversity in the main channel of the large lowland River Oder. Regul Riv 17(4–5):595–608. https://doi.org/10.1002/rrr.645
Wolter C, Vilcinskas A (1997) Characterization of the typical fish community of inland waterways of the north-eastern lowlands in Germany. Regul Riv 13(4):335–343. https://doi.org/10.1002/(SICI)1099-1646(199707)13:4%3C335::AID-RRR443%3E3.0.CO;2-G
Xavier R, Severino R, Pérez-Losada M, Gestal C, Freitas R, Harris DJ, Veríssimo A, Rosado D, Cable J (2018) Phylogenetic analysis of apicomplexan parasites infecting commercially valuable species from the north-east atlantic reveals high levels of diversity and insights into the evolution of the group. Parasit Vector 11(1):63. https://doi.org/10.1186/s13071-018-2645-7
Yadrenkina EN (2012) Distribution of alien fish species in lakes within the temperate climatic zone of Western Siberia. Russian J Biol Inv 3(2):145–157. https://doi.org/10.1134/S2075111712020117
Yevseyeva AA, Bolbotov GA, Kirichenko OI (2019) An annotated list of fish-like vertebrates and fishes of upper Irtysh River basin (Eastern Kazakhstan) with comments on their taxonomy and zoogeography. Acta Biol Sib 5(4):156–174 https://doi.org/10.14258/abs.v5.i4.7180
Yurakova TV, Petlina AP (2001) Structure of ichthyocenes of the Lower Tom River tributaries. In: Sovremennye problemy gidrobiologii Sibiri [Modern problems in hydrobiology of Siberia]. Tomsk Tomsk Gos Univ 105–106 (In Russian)
Zardoya R, Doadrio I (1999) Molecular evidence on the evolutionary and biogeographical patterns of European cyprinids. J Molec Evol 49:227–237. https://doi.org/10.1007/PL00006545
Ziegler CG, Lamatsch DK, Steinlein C, Engel W, Schartl M, Schmid M (2003) The giant b chromosome of the cyprinid fish Alburnus alburnus harbours a retrotransposon-derived repetitive DNA sequence. Chromosom Res 11(1):23–25. https://doi.org/10.1023/A:1022053931308
Zietara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea, Gyrodactylidae). Evolution 56(12):2445–2458. https://doi.org/10.1111/j.0014-3820.2002.tb00170.x
Zinov’ev EA, Baklanov MA (2007) Unusual elements in fish fauna in reservoirs of Chelyabinsk and Kurgan oblasts. Vestnik Permskogo Univ Ser Biol 5(10):53–56 ((In Russian))
Zitek A, Schmutz S, Unfer G, Ploner A (2004a) Fish drift in a Danube sidearm system: I. Site, inter-and intraspecific patterns. J Fish Biol 65(5):1319–1338 https://doi.org/10.1111/j.0022-1112.2004.00533.x
Zitek A, Schmutz S, Ploner A (2004b) Fish drift in a Danube sidearm-system: II. Seasonal and diurnal patterns. J Fish Biol 65(5):1339–1357 https://doi.org/10.1111/j.0022-1112.2004.00534.x
Zivkov M (1974) Dynamics of abundance of fish populations in Batak Reservoir. I. Age composition and growth of chub population (Leuciscus cephalus L.). Izv Zool Inst Sofia 10:203–216 ([In Bulgarian with English abstract.])
Zogaris S, Chatzinikolaou Y, Koutsikos N, Economou AN, Oikonomou E, Michaelides G, Hadjisterikotis E, Beaumont WR, Ferreira MT (2012) Freshwater fish assemblages in Cyprus with emphasis on the effects of dams. Acta Ichthyol Piscat 42(3):165–175 www.aiep.pl/volumes/2010/3_3/pdf/02_1221_F1.pdf
Acknowledgements
This research was funded by the European project LIFE INVASAQUA (LIFE17 GIE/ES/000515). The participation of G.H. Copp was supported by Cefas' Science Excellence fund, and F. Rubio-Gracia benefited from a pre-doctoral fellowship from the University of Girona (IFUdG17). Carlos Cano-Barbacil benefited from a pre-doctoral fellowship from the Spanish Ministry of Science (ref. BES-2017-081999). Filipe Ribeiro is supported by Foundation for Science and Technology through an individual contract (CEEC/0482/2020) and by the MARE strategic plan (UIDB/04292/2020) and through the project LA/P/0069/2020 granted to the Associate Laboratory ARNET. We thank I. Woodgate for assistance with bibliographic searches during the early stages of this study. J.M. Z.-M. was supported by a postdoctoral research grant funded by the Spanish Ministry of Science and Innovation and by the European Union NextGeneration EU/PRTR (FJC2021-046923-I).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Age and growth modelling
Data on A. alburnus growth were retrieved from both primary and secondary (cf. fide) literature sources. A necessary condition for inclusion of a literature source was that it provided mean length-at-age (LAA) values for the population under study. Whenever mean LAA values were provided for only one or a few age classes (e.g. as representative of the population from which fish were sampled), these were still included into the global database for the sake of completeness (cf. Vilizzi and Copp 2017). For these analyses (and in other relevant parts of the present study), LAA data originally given as total length (TL, mm) were converted to standard length (SL, mm) using the formula SL = 0.875 × TL (www.fishbase.org).
The latitude and longitude of the water body where each A. alburnus population was sampled were recorded, except for those ‘large’ rivers for which no specific indication was provided of the sampling location(s). Sections of rivers or sampling locations therein were considered as separate water bodies (cf. A. alburnus populations). For each water body, the corresponding habitat was labelled as either ‘lentic’ (i.e. natural lakes and man-made reservoirs) or ‘lotic’ (water courses). Based on the waterbody latitude and longitude, the corresponding Köppen-Geiger climate class and type (Peel et al. 2007) were identified with reference to a regular 0.5 degree latitude/longitude grid for the period 1951–2000 (Kottek et al. 2006: http://koeppen-geiger.vu-wien.ac.at/data/Koeppen-Geiger-ASCII.zip).
Growth models were based on the Beverton-Holt parameterisation of the von Bertalanffy growth function (VBGF: Ricker 1975):
where SL∞ is the asymptotic SL, K the instantaneous growth rate or Brody’s growth coefficient (years−1), and t0 the age of the fish at 0 mm SL. Following Vilizzi and Copp (2017), VBGF-based comparisons in growth of ide populations between ranges, habitats, climates classes and climate D types (see Table) were made by fitting eight models in total: i) a general model with separate parameter estimates for each population; ii) three models with one parameter in common amongst populations; iii) three models with two parameters in common amongst populations; and iv) one common model with the same parameter estimates for all populations. Both the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) were computed to select the best-fitting model, with preference given to BIC in case of major disparity of outcomes for reasons of model parsimony (i.e. fewer parameters), otherwise to AIC for ‘biological meaningfulness’ (Burnham and Anderson 2003). Fitting of growth models was in R × 64 v3.6.3 (R Development Core Team 2021) using packages FSA and nlstools (Ogle 2016) with 1000 bootstrap confidence interval estimates of the parameters (and with additional code written by LV). Note that growth models based on the A. alburnus' range of distribution (i.e. native vs non-native) could not be fitted because of the low number of populations (hence, LAA data points) from the non-native range (see Table).
See Tables
6,
7,
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Latorre, D., Masó, G., Cano-Barbacil, C. et al. A review and meta-analysis of the environmental biology of bleak Alburnus alburnus in its native and introduced ranges, with reflections on its invasiveness. Rev Fish Biol Fisheries 33, 931–975 (2023). https://doi.org/10.1007/s11160-023-09767-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-023-09767-6